Pathological Abnormalities Observed on Ultrasonography among Fishermen Associated with Male Genital Schistosomiasis (MGS) along the South Lake Malawi Shoreline in Mangochi District, Malawi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Population and Sampling
2.2. Study Data Collection
2.3. Ultrasonography Examination of Urogenital Organs
2.3.1. Preparations for the Procedure
2.3.2. Outline of the Ultrasonographic Procedure
Urinary Bladder and Kidneys
Prostate
Seminal Vesicles
Scrotum
2.4. Disinfection and Patient Information after the Completion of the Procedure
2.5. Statistical Analyses
2.6. Ethical Considerations
3. Results
3.1. Demographic Information and Diagnostic Results
3.2. Baseline Results of the Ultrasonography Exanimations
3.2.1. Urinary Bladder and Kidneys
3.2.2. Prostate
3.2.3. Seminal Vesicles
3.2.4. Scrotum
3.3. Follow-Up Ultrasonography Examinations
3.3.1. One-Month Follow-Up
3.3.2. Three-Months Follow-Up
3.3.3. Six-Months Follow-Up
3.3.4. Twelve-Months Follow-Up
4. Discussion
4.1. Genital Consequences of Schistosomiasis on Ultrasonography
4.2. Pathological Abnormalities Associated with MGS in Malawian Fishermen
4.3. Pathological Abnormalities after Treatment
4.4. Study Limitations and Ultrasonographic Diagnostic Challenges in MGS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Age (Years) | Baseline | 1-Month | 3-Months | 6-Months | 12-Months |
|---|---|---|---|---|---|
| 19 | Bladder thickening, severe hydronephrosis | Lost to follow-up | |||
| 22 | Bladder thickening, prostate nodule | Did not show up | No abnormality | Inadequate bladder filling, not reported for repeat scan | Lost to follow-up |
| 24 | Enlarged asymmetrical seminal vesicles | Inadequate bladder filling, not reported for repeat scan | Lost to follow-up | ||
| 29 | Left hydrocele | Lost to follow-up | |||
| 30 | Left hydrocele | Did not show up | Did not show up | Did not show up | No abnormality |
| 49 | Bilateral hydroceles | Bilateral hydroceles | Bilateral hydroceles | Bilateral hydroceles | Lost to follow-up |
| 49 | Testicular nodule, mild bilateral hydroceles | Bilateral hydroceles | Lost to follow-up | ||
| 51 | Enlarged prostate | Did not show up | Did not show up | No abnormality | Lost to follow-up |
| 54 | Bilateral hydroceles | Lost to follow-up | |||
| 69 | Enlarged prostate, bilateral hydroceles | Lost to follow-up |
| Age (Years) | Baseline | 1-Month | 3-Months | 6-Months | 12-Months |
|---|---|---|---|---|---|
| 25 | No abnormality | No abnormality | Did not show up | Left hydrocele | Lost to follow-up |
| 33 | No abnormality | Did not show up | Left hydrocele | Did not show up | No abnormality |
| 33 | No abnormality | Did not show up | Did not show up | Enlarged, asymmetrical seminal vesicles | Lost to follow-up |
| 39 | No abnormality | Did not show up | Did not show up | Left hydrocele | Lost to follow-up |
| 44 | No abnormality | Left hydrocele | Left hydrocele | No abnormality | Lost to follow-up |
| 53 | No abnormality | Did not show up | Did not show up | Did not show up | Bladder thickening, left kidney mass |
| MGS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 130) | 1-Month (n = 29) | 3-Months (n = 32) | 6-Months (n = 38) | 12-Months (n = 17) | |||||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | ||
| USS | Positive | 4 | 5 | 2 | 1 | 1 | 2 | 0 | 4 | 0 | 0 |
| Negative | 14 | 107 | 1 | 25 | 3 | 26 | 1 | 33 | 2 | 15 | |
References
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- WHO. Schistosomiasis Geneva, Switzerland: World Health Organization. 2018. Available online: http://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis (accessed on 20 February 2018).
- Feldmeier, H.; Leutscher, P.; Poggensee, G.; Harms, G. Male genital schistosomiasis and haemospermia. Trop. Med. Int. Health 1999, 2, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Leutscher, P.; Ramarokoto, C.-E.; Reimert, C.; Feldmeier, H.; Esterre, P.; Vennervald, B.J. Community-based study of genital schistosomiasis in men from Madagascar. Lancet 2000, 355, 117–118. [Google Scholar] [CrossRef]
- Madden, F.C. Two rare manifestations of Bilharziosis. Lancet 1911, 178, 754–755. [Google Scholar] [CrossRef]
- WHO. Manual of Diagnostic Ultrasound, 2nd ed.; World Health Organization and World Federation for Ultrasound in Medicine and Radiology: Geneva, Switzerland, 2011. [Google Scholar]
- Ramarakoto, C.E.; Leutscher, P.D.C.; van Dam, G.; Christensen, N.O. Ultrasonographical findings in the urogenital organs in women and men infected with Schistosoma haematobium in northern Madagascar. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 767–773. [Google Scholar] [CrossRef]
- WHO. Ultrasound in Schistosomiasis: A Practical Guide to the Standard Use of Ultrasonography for Assessment of Schistosomiasis-Related Morbidity: Second International Workshop, October 22–26 1996, Niamey, Niger; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Shebel, H.M.; Elsayes, K.M.; Abou El Atta, H.M.; Elguindy, Y.M.; El-Diasty, T.A. Genitourinary schistosomiasis: Life cycle and radiologic-pathologic findings. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2012, 32, 1031–1046. [Google Scholar] [CrossRef]
- Fender, D.; Hamdy, F.C.; Neal, D.E. Transrectal ultrasound appearances of schistosomal prostato-seminovesiculitis. Br. J. Urol. 1996, 77, 166–167. [Google Scholar] [CrossRef]
- Al-Saeed, O.; Sheikh, M.; Kehinde, E.O.; Makar, R. Seminal vesicle masses detected incidentally during transrectal sonographic examination of the prostate. J. Clin. Ultrasound JCU 2003, 31, 201–206. [Google Scholar] [CrossRef]
- de Cassio Saito, O.; de Barros, N.; Chammas, M.C.; Oliveira, I.R.S.; Cerri, G.G. Ultrasound of tropical and infectious diseases that affect the scrotum. Ultrasound Q. 2004, 20, 12–18. [Google Scholar] [CrossRef]
- Vilana, R.; Corachan, M.; Gascon, J.; Valls, E.; Bru, C. Schistosomiasis of the male genital tract: Transrectal sonographic findings. J. Urol. 1997, 158, 1491–1493. [Google Scholar] [CrossRef]
- Richter, J. Evolution of schistosomiasis-induced pathology after therapy and interruption of exposure to schistosomes: A review of ultrasonographic studies. Acta Trop. 2000, 77, 111–131. [Google Scholar] [CrossRef]
- Kayuni, S.; Lampiao, F.; Makaula, P.; Juziwelo, L.; Lacourse, E.J.; Reinhard-Rupp, J.; Leutscher, P.D.C.; Stothard, J.R. A systematic review with epidemiological update of male genital schistosomiasis (MGS): A call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiol. Control 2019, 4, e00077. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.H.; Condemine, C.; Christiansen, R.; LaCourse, E.J.; Makaula, P.; Stanton, M.C.; Juziwelo, L.; Kayuni, S.; Stothard, J.R. Biomphalaria pfeifferi snails and intestinal schistosomiasis, Lake Malawi, Africa, 2017–2018. Emerg. Infect. Dis. 2019, 25, 613–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makaula, P.; Sadalaki, J.R.; Muula, A.S.; Kayuni, S.; Jemu, S.; Bloch, P. Schistosomiasis in Malawi: A systematic review. Parasites Vectors 2014, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Kayuni, S.A.; Corstjens, P.L.A.M.; Lacourse, E.J.; Bartlett, K.E.; Fawcett, J.; Shaw, A.; Makaula, P.; Lampiao, F.; Juziwelo, L.; de Dood, C.J.; et al. How can schistosome circulating antigen assays be best applied for diagnosing male genital schistosomiasis (MGS): An appraisal using exemplar MGS cases from a longitudinal cohort study among fishermen on the south shoreline of Lake Malawi. Parasitology 2019, 146, 1785–1795. [Google Scholar] [CrossRef] [Green Version]
- Kayuni, S.A.; LaCourse, E.J.; Makaula, P.; Lampiao, F.; Juziwelo, L.; Fawcett, J.; Shaw, A.; Alharbi, M.H.; Verweij, J.J.; Stothard, J.R. Case report: Highlighting male genital schistosomiasis (MGS) in fishermen from the southwestern shoreline of Lake Malawi, Mangochi District. Am. J. Trop. Med. Hyg. 2019, 101, 1331–1335. [Google Scholar] [CrossRef]
- Martino, P.; Galosi, A.B.; Bitelli, M.; Consonni, P.; Fiorini, F.; Granata, A.; Gunelli, R.; Liguori, G.; Palazzo, S.; Pavan, N.; et al. Practical recommendations for performing ultrasound scanning in the urological and andrological fields. Arch. Ital. Urol. Androl. 2014, 86, 56–78. [Google Scholar] [CrossRef] [Green Version]
- Kukula, V.A.; MacPherson, E.E.; Tsey, I.H.; Stothard, J.R.; Theobald, S.; Gyapong, M. A major hurdle in the elimination of urogenital schistosomiasis revealed: Identifying key gaps in knowledge and understanding of female genital schistosomiasis within communities and local health workers. PLoS Negl. Trop. Dis. 2019, 13, e0007207. [Google Scholar] [CrossRef]
- Yirenya-Tawiah, D.R.; Ackumey, M.M.; Bosompem, K.M. Knowledge and awareness of genital involvement and reproductive health consequences of urogenital schistosomiasis in endemic communities in Ghana: A cross-sectional study. Reprod. Health 2016, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Alves, W.; Woods, R.; Gelfand, M. The distribution of bilharzia ova in the male genital tract. Cent. Afr. J. Med. 1955, 1, 166–168. [Google Scholar]
- Gelfand, M.; Ross, C.M.D.; Blair, D.M.; Castle, W.M.; Webber, M.C. Schistosomiasis of the male pelvic organs: Severity of infection as determined by digestion of tissue and histologic methods in 300 cadavers. Am. J. Trop. Med. Hyg. 1970, 19, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Bustinduy, A.L.; King, C.H. Schistosomiasis. In Manson’s Tropical Diseases, 23rd ed.; Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; Elsevier Health Sciences: Beijing, China, 2014; pp. 698–725. [Google Scholar]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Richens, J. Genital manifestations of tropical diseases. Sex. Transm. Infect. 2004, 80, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ekenze, S.O.; Modekwe, V.O.; Nzegwu, M.A.; Ekpemo, S.C.; Ezomike, U.O. Testicular schistosomiasis mimicking malignancy in a child: A case report. J. Trop. Pediatrics 2015, 61, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Rambau, P.F.; Chandika, A.; Chalya, P.L.; Jackson, K. Scrotal swelling and testicular atrophy due to schistosomiasis in a 9-year-old boy: A case report. Case Rep. Infect. Dis. 2011, 2011, 787961. [Google Scholar] [CrossRef] [Green Version]
- Knopp, S.; Becker, S.L.; Ingram, K.J.; Keiser, J.; Utzinger, J. Diagnosis and treatment of schistosomiasis in children in the era of intensified control. Expert Rev. Anti-Infect. 2013, 11, 1237–1258. [Google Scholar] [CrossRef]
- WHO. Assessing the Efficacy of Anthelminthic Drugs against Schistosomiasis and Soil-Transmitted Helminthiases; World Health Organization: Geneva, Switzerland, 2013; p. 29. [Google Scholar]



| Variable | n | Median | Range | Interquartile Range (IQR) | Number of Positive Cases | Prevalence (%) |
|---|---|---|---|---|---|---|
| Age | 130 | 32.0 | 19.0–70.0 | 18.0 | - | - |
| Duration of stay in village (years) | 120 | 22.0 | 0.2–70.0 | 24.5 | - | - |
| Weight (kgs) | 115 | 59.0 | 43.0–75.4 | 9.0 | - | - |
| Eggs in urine (filtration, 10 mL) | 129 | 1.0 | 0.1–186.0 | 5.8 | 27 | 20.9 |
| POC-CCA | 129 | - | - | - | 6 | 4.9 |
| Eggs in semen (mL) | 81 | 2.9 | 0.4–9.3 | 4.6 | 10 | 12.3 |
| Seminal real-time PCR (Ct-value) | 57 | 26.4 | 18.9–36.6 | 10.5 | 16 | 28.1% |
| Organ | Total Scans | Number of Abnormal Scans | Percentage (%) |
|---|---|---|---|
| Urinary Bladder * | 106 | 2 | 1.9% |
| Prostate | 126 | 3 | 2.4% |
| Seminal vesicles | 117 | 1 | 0.9% |
| Testis # | 129 | 1 | 0.8% |
| Epididymis ǂ | 129 | 1 | 0.8% |
| Scrotum α | 129 | 6 | 4.7% |
| Participant | Age (Years) | Eggs in Urine (per 10 mL) | Eggs in Semen (per mL) | Real-Time PCR (Ct-Value) | Abnormalities Observed |
|---|---|---|---|---|---|
| 1 | 19 | 0 | 0 | N/D # | Irregular bladder wall and severe polypoid thickness, with bilateral hydronephrosis |
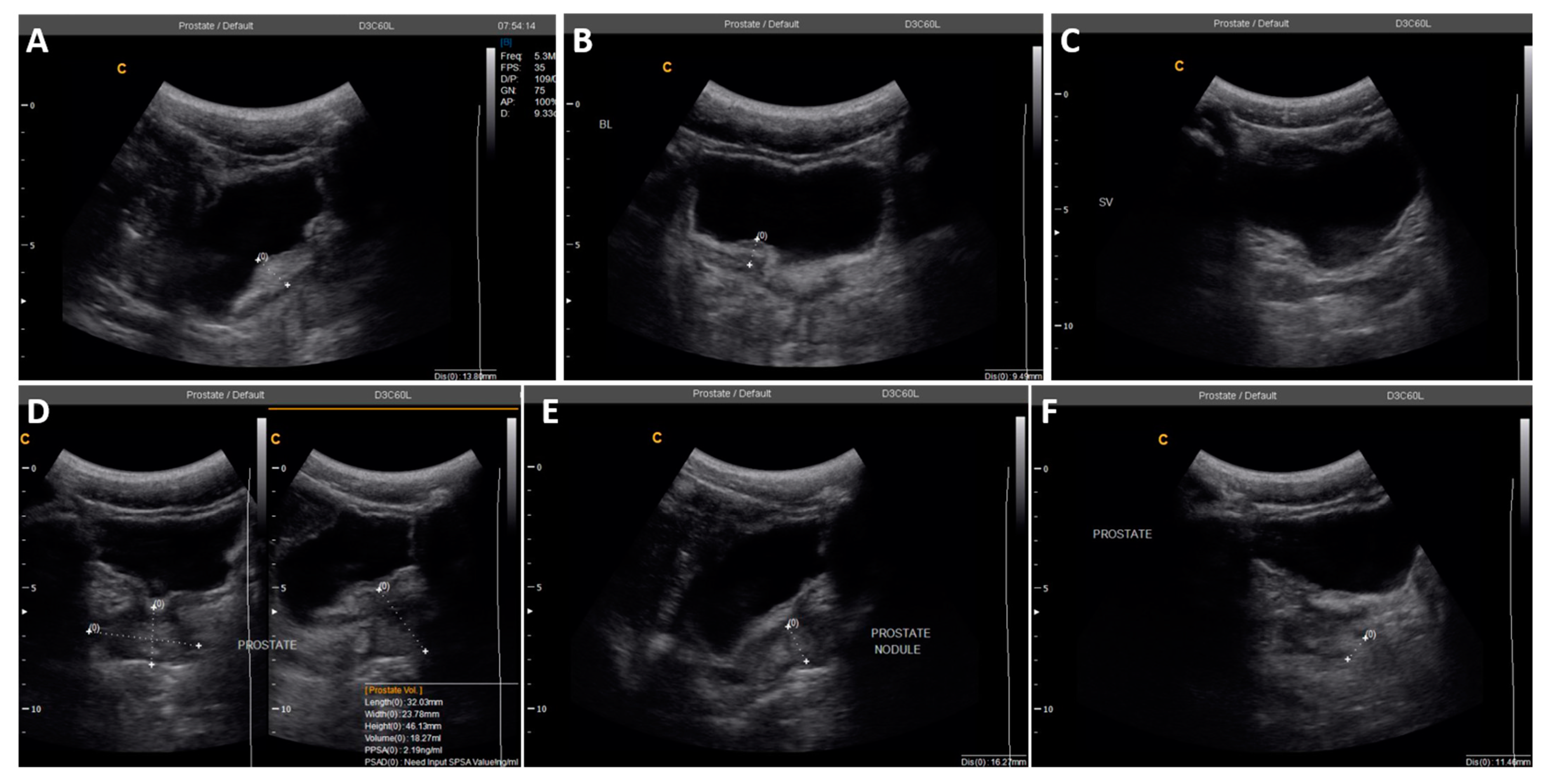
| 2 | 22 | 0 | 0 | 25.4 | Irregular bladder wall with severe focal thickness, irregular prostate with hyperechoic nodule (Figure 3) |
| 3 | 49 | 1 | 6 | N/D # | Left testicular nodule and mild bilateral hydroceles |
| 4 | 69 | 0 | N/A ǂ | N/A ǂ | Severely enlarged prostate (volume = 61.3 mL) and right epididymis, with bilateral hydrocele |
| Age (Years) | Baseline | 1-Month Follow-Up | ||
|---|---|---|---|---|
| Test Results | Abnormalities Observed | Test Results | Abnormalities Observed | |
| 49 | Eggs in urine, semen; no real-time PCR done | Left testicular nodule, mild bilateral hydroceles | No eggs in urine or semen; negative PCR | Bilateral hydroceles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayuni, S.A.; Al-Harbi, M.H.; Makaula, P.; Injesi, B.; Mainga, B.; Lampiao, F.; Juziwelo, L.; LaCourse, E.J.; Stothard, J.R. Pathological Abnormalities Observed on Ultrasonography among Fishermen Associated with Male Genital Schistosomiasis (MGS) along the South Lake Malawi Shoreline in Mangochi District, Malawi. Trop. Med. Infect. Dis. 2022, 7, 169. https://doi.org/10.3390/tropicalmed7080169
Kayuni SA, Al-Harbi MH, Makaula P, Injesi B, Mainga B, Lampiao F, Juziwelo L, LaCourse EJ, Stothard JR. Pathological Abnormalities Observed on Ultrasonography among Fishermen Associated with Male Genital Schistosomiasis (MGS) along the South Lake Malawi Shoreline in Mangochi District, Malawi. Tropical Medicine and Infectious Disease. 2022; 7(8):169. https://doi.org/10.3390/tropicalmed7080169
Chicago/Turabian StyleKayuni, Sekeleghe A., Mohammad H. Al-Harbi, Peter Makaula, Boniface Injesi, Bright Mainga, Fanuel Lampiao, Lazarus Juziwelo, E. James LaCourse, and J. Russell Stothard. 2022. "Pathological Abnormalities Observed on Ultrasonography among Fishermen Associated with Male Genital Schistosomiasis (MGS) along the South Lake Malawi Shoreline in Mangochi District, Malawi" Tropical Medicine and Infectious Disease 7, no. 8: 169. https://doi.org/10.3390/tropicalmed7080169
APA StyleKayuni, S. A., Al-Harbi, M. H., Makaula, P., Injesi, B., Mainga, B., Lampiao, F., Juziwelo, L., LaCourse, E. J., & Stothard, J. R. (2022). Pathological Abnormalities Observed on Ultrasonography among Fishermen Associated with Male Genital Schistosomiasis (MGS) along the South Lake Malawi Shoreline in Mangochi District, Malawi. Tropical Medicine and Infectious Disease, 7(8), 169. https://doi.org/10.3390/tropicalmed7080169






