Larvicidal Activities against Aedes aegypti of Supernatant and Pellet Fractions from Cultured Bacillus spp. Isolated from Amazonian Microenvironments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Bacteria Isolation
2.2. Morphological and Molecular Characterization
2.3. Fractionation of Bacterial Cultures
2.4. Mosquito Rearing
2.5. Screening for Larvicidal Activity
2.6. Bioassays with Fractionated Metabolites
2.7. Determination of Lethal Concentrations (LC50) and (LC90)
3. Results
3.1. Bacteria Strain Isolation and Characterization
3.2. Larvicidal Activity of Isolated Bacterial Strains
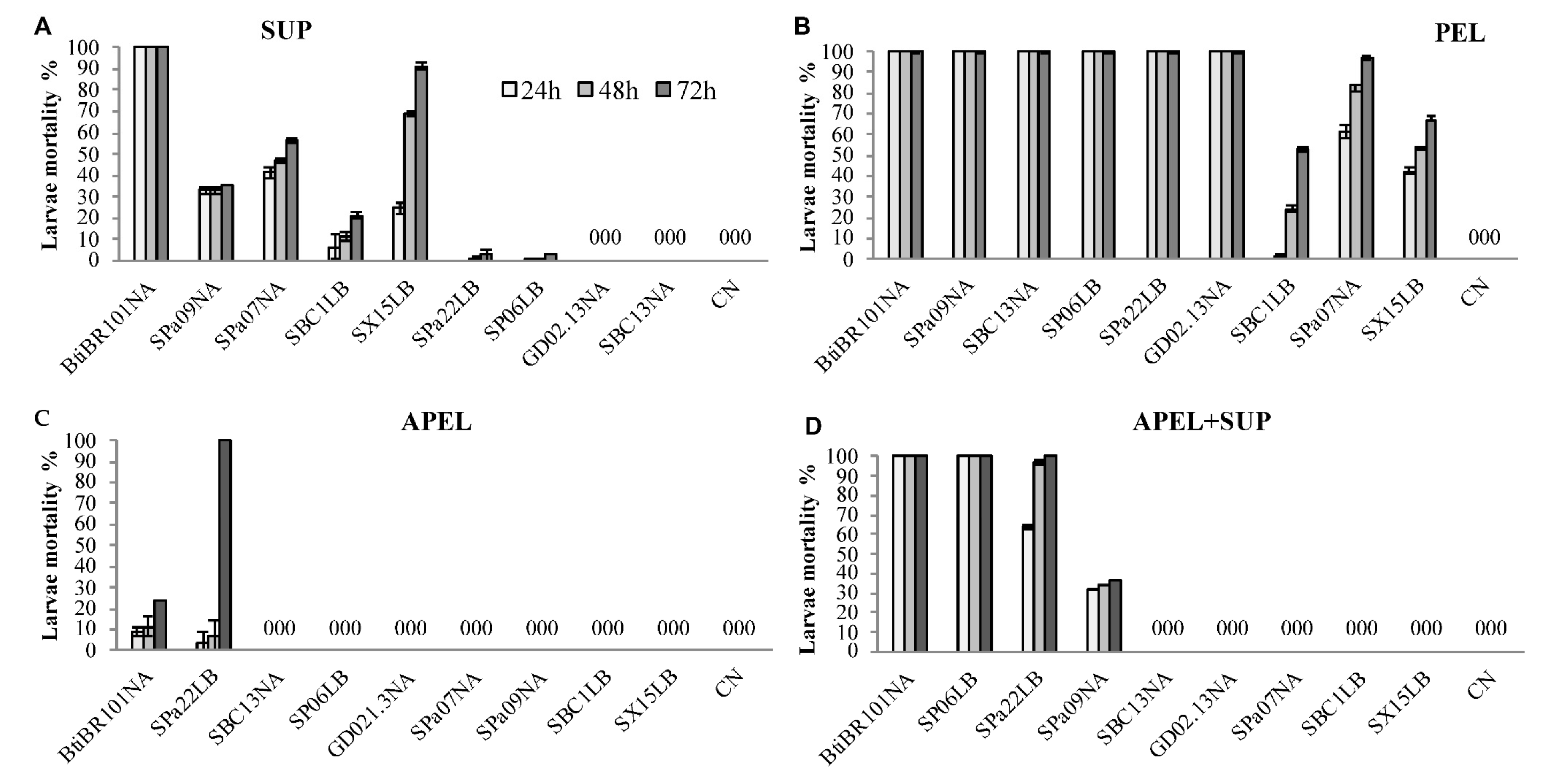
3.3. Larvicidal Activity of Fractionated Bacterial Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira-De-Brito, A.; Ribeiro, I.P.; De Miranda, R.M.; Fernandes, R.S.; Campos, S.S.; Da Silva, K.A.B.; De Castro, M.G.; Bonaldo, M.C.; Brasil, P.; Lourenço-De-Oliveira, R. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Memórias Inst. Oswaldo Cruz 2016, 111, 655–658. [Google Scholar] [CrossRef]
- Aragão, C.F.; Cruz, A.C.R.; Neto, J.P.N.; Monteiro, H.A.D.O.; Da Silva, E.V.P.; Da Silva, S.P.; Andrade, A.T.D.S.; Tadei, W.P.; Pinheiro, V.C.S. Circulation of Chikungunya virus in Aedes aegypti in Maranhão, Northeast Brazil. Acta Trop. 2018, 186, 1–4. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef]
- Nóbrega, M.; Araújo, E.L.D.L.; Wada, M.Y.; Leite, P.L.E.; Dimech, G.S.; Percio, J. Surto de síndrome de Guillain-Barré possivelmente relacionado à infecção prévia pelo vírus Zika, Região Metropolitana do Recife, Pernambuco, Brasil, 2015. Epidemiol. Serv. Saude Rev. Sist. Unico Saude Bras. 2018, 27, e2017039. [Google Scholar] [CrossRef]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2018, 3, e000530. [Google Scholar] [CrossRef]
- Cattarino, L.; Rodriguez-Barraquer, I.; Imai, N.; Cummings, D.A.T.; Ferguson, N.M. Mapping global variation in dengue transmission intensity. Sci. Transl. Med. 2020, 12, eaax4144. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; De Oliveira, W.K.; Cavalcanti, L. Zika virus outbreak in Brazil. J. Infect. Dev. Ctries. 2016, 10, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, S.P.; Sikka, V.; Chattu, V.K.; Popli, R.K.; Galwankar, S.C.; Kelkar, D.; Sawicki, S.G.; Papadimos, T. The emergence of zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint working Group (JWG). J. Glob. Infect. Dis. 2016, 8, 3–15. [Google Scholar] [CrossRef]
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.V.; Talbot, B. RADAM-LAC Research Team Arbovirus vectors of epidemiological concern in the Americas: A scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes mosquitoes and Aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef]
- Lereclus, D.; Agaisse, H.; Grandvalet, C.; Salamitou, S.; Gominet, M. Regulation of toxin and virulence gene transcription in Bacillus thuringiensis. Int. J. Med. Microbiol. 2000, 290, 295–299. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Walker, T.; Neill, S.L.O. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Lazarte, J.N.; Lopez, R.P.; Ghiringhelli, P.D.; Berón, C.M. Bacillus wiedmannii biovar thuringiensis: A specialized mosquitocidal pathogen with plasmids from diverse origins. Genome Biol. Evol. 2018, 10, 2823–2833. [Google Scholar] [CrossRef]
- Evans, H.C.; Elliot, S.L.; Barreto, R.W. Entomopathogenic fungi and their potential for the management of Aedes aegypti (Diptera: Culicidae) in the Americas. Memórias Inst. Oswaldo Cruz 2018, 113, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Raoult, D.; Fenollar, F.; Mediannikov, O. Insecticidal activity of bacteria from larvae breeding site with Natural Larvae Mortality: Screening of Separated Supernatant and Pellet Fractions. Pathogens 2020, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Arantes, O.M.N.; Vilas Bôas, L.A.; Vilas Bôas, G.T.T. Bacillus thuringiensis: Estratégias no controle biológico. In Biotecnologia: Avanços na Agricultura e na Agroindústria; Coleção Biotecnologia; Agropecuária: Caxias do Sul, Brazil, 2002; pp. 269–293. ISBN 85-7061-188-9. [Google Scholar]
- Galardo, A.K.R.; Zimmerman, R.; Galardo, C.D. Larval control of Anopheles (Nyssorhinchus) darlingi using granular formulation of Bacillus sphaericus in abandoned gold-miners excavation pools in the Brazilian Amazon Rainforest. Rev. Soc. Bras. Med. Trop. 2013, 46, 172–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreira, F.A.D.S.; Arcos, A.N.; Sampaio, R.T.D.M.; Rodrigues, I.B.; Tadei, W.P. Effect of Bacillus sphaericus Neide on Anopheles (Diptera: Culicidae) and associated insect fauna in fish ponds in the Amazon. Rev. Bras. Èntomol. 2015, 59, 234–239. [Google Scholar] [CrossRef][Green Version]
- Short, S.M.; Van Tol, S.; MacLeod, H.J.; Dimopoulos, G. Hydrogen cyanide produced by the soil bacterium Chromobacterium sp. Panama contributes to mortality in Anopheles gambiae mosquito larvae. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.L.R.; Schwalm, F.U.; Silva, C.E.; Da Costa, M.; Heermann, R.; Da Silva, O.S. Larvicidal and Growth-Inhibitory Activity of Entomopathogenic Bacteria Culture Fluids Against Aedes aegypti (Diptera: Culicidae). J. Econ. Èntomol. 2017, 110, 378–385. [Google Scholar] [CrossRef]
- Lovett, B.; Bilgo, E.; Diabate, A.; Leger, R.S. A review of progress toward field application of transgenic mosquitocidal entomopathogenic fungi. Pest Manag. Sci. 2019, 75, 2316–2324. [Google Scholar] [CrossRef]
- Malhi, Y.; Roberts, J.T.; Betts, R.A.; Killeen, T.J.; Li, W.; Nobre, C.A. Climate Change, Deforestation, and the Fate of the Amazon. Science 2008, 319, 169–172. [Google Scholar] [CrossRef]
- World Health Organization. Informal Consultation on the Development of Bacillus sphaericus as Microbial Larvicide; WHO: Geneva, Switzerland, 1985; 24p. [Google Scholar]
- Polanczyk, R.A. Estudos de Bacillus thuringiensis Berliner Visando ao Controle de Spodoptera frugiperda; Universidade de Sao Paulo: Sao Paulo, Brazil, 2004. [Google Scholar]
- Fukatsu, T.; Nikoh, N. Two Intracellular Symbiotic Bacteria from the Mulberry Psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 1998, 64, 3599–3606. [Google Scholar] [CrossRef]
- Sawada, H.; Ieki, H.; Oyaizu, H.; Matsumoto, S. Proposal for Rejection of Agrobacterium tumefaciens and Revised Descriptions for the genus Agrobacterium and for Agrobacterium radiobacter and Agrobacterium rhizogenes. Int. J. Syst. Bacteriol. 1993, 43, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; De Oliveira, C.D.; Pinheiro, W.D.; Tadei, W.P.; James, A.A.; Marinotti, O. 16S rRNA gene sequences from bacteria associated with adult Anopheles darlingi (Diptera: Culicidae) mosquitoes. J. Med. Èntomol. 2008, 45, 172–175. [Google Scholar] [CrossRef]
- Huang, X. CAP3: A DNA Sequence Assembly Program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Soares-Da-Silva, J.; Queirós, S.G.; De Aguiar, J.S.; Viana, J.L.; Neta, M.D.R.; Silva, M.; Pinheiro, V.C.; Polanczyk, R.A.; Carvalho-Zilse, G.A.; Tadei, W.P. Molecular characterization of the gene profile of Bacillus thuringiensis Berliner isolated from Brazilian ecosystems and showing pathogenic activity against mosquito larvae of medical importance. Acta Trop. 2017, 176, 197–205. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis. J. Pharm. Sci. 1971, 60, 1432. [Google Scholar] [CrossRef]
- Robertson, J.L.; Savin, N.E.; Preisler, H.K. Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ayres, M.; Ayres, M., Jr.; Ayres, D.L.; Santos, A.A. BioEstat Versão 5.3: Aplicações Estatísticas Nas Áreas Das Ciências Biológicas e Médicas; Sociedade Civil Mamirauá: Belém, Pará, Brasil; MCT/CNPQ: Brasília, Brasil, 2007. [Google Scholar]
- Makoni, M. Malaria fighters’ latest chemical weapon may not last long. Science 2020, 369, 1153. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, L. Brevibacillus laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species. Insects 2013, 4, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Marche, M.G.; Mura, M.E.; Falchi, G.; Ruiu, L. Spore surface proteins of Brevibacillus laterosporus are involved in insect pathogenesis. Sci. Rep. 2017, 7, srep43805. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Chaabouni, I.; Guesmi, A.; Cherif, A. Secondary Metabolites of Bacillus: Potentials in Biotechnology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 347–366. [Google Scholar]
- Malovichko, Y.V.; Nizhnikov, A.A.; Antonets, K.S. Repertoire of the Bacillus thuringiensis Virulence Factors Unrelated to Major Classes of Protein Toxins and Its Role in Specificity of Host-Pathogen Interactions. Toxins 2019, 11, 347. [Google Scholar] [CrossRef]
- Falqueto, S.A.; Pitaluga, B.F.; de Sousa, J.R.; Targanski, S.K.; Campos, M.G.; Mendes, T.A.D.O.; da Silva, G.F.; Silva, D.H.S.; Soares, M.A. Bacillus spp. metabolites are effective in eradicating Aedes aegypti (Diptera: Culicidae) larvae with low toxicity to non-target species. J. Invertebr. Pathol. 2021, 179, 107525. [Google Scholar] [CrossRef] [PubMed]
- Valtierra-De-Luis, D.; Villanueva, M.; Lai, L.; Williams, T.; Caballero, P. Potential of Cry10Aa and Cyt2Ba, two minority δ-endotoxins produced by Bacillus thuringiensis ser. israelensis, for the control of Aedes aegypti larvae. Toxins 2020, 12, 355. [Google Scholar] [CrossRef]

| Municipality | Collection Site | GPS Coordinates | Description | Sample Type | Date | |
|---|---|---|---|---|---|---|
| Coari | 1 | Sítio do Gordo | 4°06′45.5″ S 63°07′44.0″ W | artificial lake | water | 02/2017 |
| Manaus | 2 | Ramal do 7 (Brasileirinho) | 3°01′42.2″ S 59°52′15.6″ W | primary forest | soil | 03/2017 |
| 3 | Campus-UFAM | 3°05′48.8″ S 59°58′23.6″ W | residual forest | soil | 04/2017 | |
| 4 | Bosque da Ciência-INPA | 3°06′01.3″ S 59°59′06.6″ W | residual forest | soil | 02/2017 | |
| 5 | Casa 15-INPA | 3°05′47.2″ S 59°59′09.5″ W | residual forest | soil | 05/2017 | |
| 6 | Sitio Portela 1 | 3°02′47.0″ S 59°52′54.4″ W | fish tank | water | 02/2017 | |
| 7 | Sitio Dona Chagas | 3°02′33.5″ S 59°53′15.6″ W | fish tank | water | 06/2017 | |
| 8 | Sítio Portela 2 | 3°02′47.5″ S 59°52′56.8″ W | fish tank | water | 02/2017 | |
| Parintins | 9 | Parananema | 2°40′30.9″ S 56°45′59.2″ W | primary forest | soil | 03/2017 |
| 10 | Aninga | 2°39′07.6″ S 56°46′50.3″ W | natural pond | water | 03/2017 | |
| 11 | Lagoa Francesa | 2°37′34.7″ S 56°43′37.3″ W | natural pond | water | 04/2017 | |
| 12 | Areial | 2°39′39.2″ S 56°46′07.6″ W | creek | water | 03/2017 | |
| 13 | Macurany | 2°40′33.6″ S 56°45′30.2″ W | primary forest | soil | 04/2017 | |
| 14 | Parananema | 2°40′30.9″ S 56°45′59.2″ W | creek | water | 02/2017 | |
| 15 | Macurany (Sítio Fanuel) | 2°39′06.7″ S 56°43′29.0″ W | natural pond | water | 04/2017 | |
| Strain | Isolated from | GenBank acc. N°.; Species | Cumulative Mortality | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| BR101 | Positive control | Bacillus thuringiensis var.israelensis | 100% | - | - |
| Spa09 | Soil | MT052636; Bacillus sp. | 100% | - | - |
| SBC13 | Soil | MT052634; Bacillus sp. | 100% | - | - |
| SP06 | Soil | MT052669; Bacillus sp. | 100% | - | - |
| SPO2 | Water | MT052609; Bacillus safensis | 100% | - | - |
| SPO5 | Water | MT052624; Bacillus sp. | 100% | ||
| SX02 | Water | MT052611; Bacillus sp. | 100% | ||
| APR6I | Water | MT052596; Bacillus sp. | 100% | - | - |
| APR10I | Water | MT052598; Bacillus sp. | 100% | - | - |
| GD02.13 | Water | MT163315; Bacillus sp. | 100% | - | - |
| SX06 | Water | MT052643; Bacillus megaterium | 100% | ||
| SX08 | Water | MT052649; Bacillus velezensis | 100% | ||
| Spa07 | Soil | MT052647; Brevibacillus halotolerans | 60% | 100% | - |
| Spa03 | Soil | MT052618; Bacillus sp. | 90% | 97% | 100% |
| LFP2 | Water | MT052629; Bacillus subtilis | 10% | 80% | 90% |
| Spa22 | Soil | MT052639; Bacillus safensis | 47% | 80% | 83% |
| Spa04 | Soil | MT052633; Brevibacillus halotolerans | 70% | 70% | 80% |
| SMP1.2 | Soil | MT052614; Bacillus sp. | 56% | 63% | 67% |
| SBC2 | Soil | MT052620; Bacillus subtilis | 27% | 57% | 67% |
| SBC1 | Soil | MT052667; Bacillus sp. | 33% | 53% | 63% |
| Spa14 | Soil | MT052651; Bacillus sp. | 37% | 57% | 57% |
| SX15 | Water | MT163316; Bacillus safensis | - | 67% | 67% |
| Strain | PEL | Strain | APEL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||||
| CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | ||
| Bti | (0.008)bc | (0.058)a | (0.008)a | (0.023)a | (0.003)a | (0.012)a | Bti | - | - | - | - | - | - |
| SPa09 | (0.006)c | (0.089)a | (0.006)a | (0.026)a | (0.005)a | (0.015)a | SPa09 | - | - | - | - | - | - |
| SPa22 | (0.013)ab | (0.113)a | (0.004)ab | (0.023)a | (0.005)a | (0.015)a | SPa22 | (0.009)a | (0.073)a | (0.006)a | (0.025)a | (0.007)a | (0.018)a |
| GD02.13 | (0.006)c | (0.148)a | (0.005)ab | (0.017)a | (0.003)a | (0.012)a | GD02.13 | - | - | - | - | - | - |
| SP06 | (0.009)bc | (0.083)a | (0.007)ab | (0.029)a | (0.007)a | (0.018)a | SP06 | - | - | - | - | - | - |
| SBC13 | (0.007)c | (0.009)a | (0.004)b | (0.019)a | (0.005)a | (0.012)a | SBC13 | - | - | - | - | - | - |
| SPa07 | (0.009)bc | (0.124)a | (0.005)ab | (0.021)a | - | - | SPa07 | - | - | - | - | - | - |
| SX15 | (0.010)bc | (0.088)a | (0.005)ab | (0.022)a | - | - | SX15 | - | - | - | - | - | - |
| BC1 | - | - | - | - | (0.023)a | (0.256)a | BC1 | - | - | - | - | - | - |
| Strain | SUP | Strain | APEL+SUP | ||||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||||
| CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | CL50 | CL90 | ||
| Bti | (0.009)a | (0.048)a | (0.008)a | (0.029)a | (0.004)a | (0.004)a | Bti | (0.005)a | (0.037)a | (0.005)a | (0.018)a | (0.004)a | (0.011)a |
| SPa09 | - | - | - | - | - | - | SPa09 | - | - | - | - | - | - |
| SPa22 | - | - | - | - | - | - | SPa22 | (0.007)a | (0.031)a | (0.007)a | (0.018)a | (0.003)a | (0.012)a |
| GD02.13 | - | - | - | - | - | - | GD02.13 | - | - | - | - | - | - |
| SP06 | - | - | - | - | - | - | SP06 | (0.008)a | (0.046)a | (0.006)a | (0.023)a | (0.003)a | (0.016)a |
| SBC13 | - | - | - | - | - | - | SBC13 | - | - | - | - | - | - |
| SPa07 | (0.007)a | (0.056)a | (0.006)a | (0.020)a | (0.004)a | (0.004)a | SPa07 | - | - | - | - | - | - |
| SX15 | (0.006)a | (0.068)a | (0.006)a | (0.068)a | (0.005)a | (0.005)a | SX15 | - | - | - | - | - | - |
| BC1 | - | - | - | - | - | - | BC1 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katak, R.M.; Rocha, E.M.; Oliveira, J.C.; Muniz, V.A.; Oliveira, M.R.; Ferreira, F.A.S.; Silva, W.R.; Roque, R.A.; de Souza, A.Q.L.; Souza-Neto, J.A.; et al. Larvicidal Activities against Aedes aegypti of Supernatant and Pellet Fractions from Cultured Bacillus spp. Isolated from Amazonian Microenvironments. Trop. Med. Infect. Dis. 2021, 6, 104. https://doi.org/10.3390/tropicalmed6020104
Katak RM, Rocha EM, Oliveira JC, Muniz VA, Oliveira MR, Ferreira FAS, Silva WR, Roque RA, de Souza AQL, Souza-Neto JA, et al. Larvicidal Activities against Aedes aegypti of Supernatant and Pellet Fractions from Cultured Bacillus spp. Isolated from Amazonian Microenvironments. Tropical Medicine and Infectious Disease. 2021; 6(2):104. https://doi.org/10.3390/tropicalmed6020104
Chicago/Turabian StyleKatak, Ricardo M., Elerson M. Rocha, Juan C. Oliveira, Veranilce A. Muniz, Marta R. Oliveira, Francisco A. S. Ferreira, William R. Silva, Rosemary A. Roque, Antonia Q. L. de Souza, Jayme A. Souza-Neto, and et al. 2021. "Larvicidal Activities against Aedes aegypti of Supernatant and Pellet Fractions from Cultured Bacillus spp. Isolated from Amazonian Microenvironments" Tropical Medicine and Infectious Disease 6, no. 2: 104. https://doi.org/10.3390/tropicalmed6020104
APA StyleKatak, R. M., Rocha, E. M., Oliveira, J. C., Muniz, V. A., Oliveira, M. R., Ferreira, F. A. S., Silva, W. R., Roque, R. A., de Souza, A. Q. L., Souza-Neto, J. A., Terenius, O., Marinotti, O., & Tadei, W. P. (2021). Larvicidal Activities against Aedes aegypti of Supernatant and Pellet Fractions from Cultured Bacillus spp. Isolated from Amazonian Microenvironments. Tropical Medicine and Infectious Disease, 6(2), 104. https://doi.org/10.3390/tropicalmed6020104








