The COVID-19 Pandemic: Disproportionate Thrombotic Tendency and Management Recommendations
Abstract
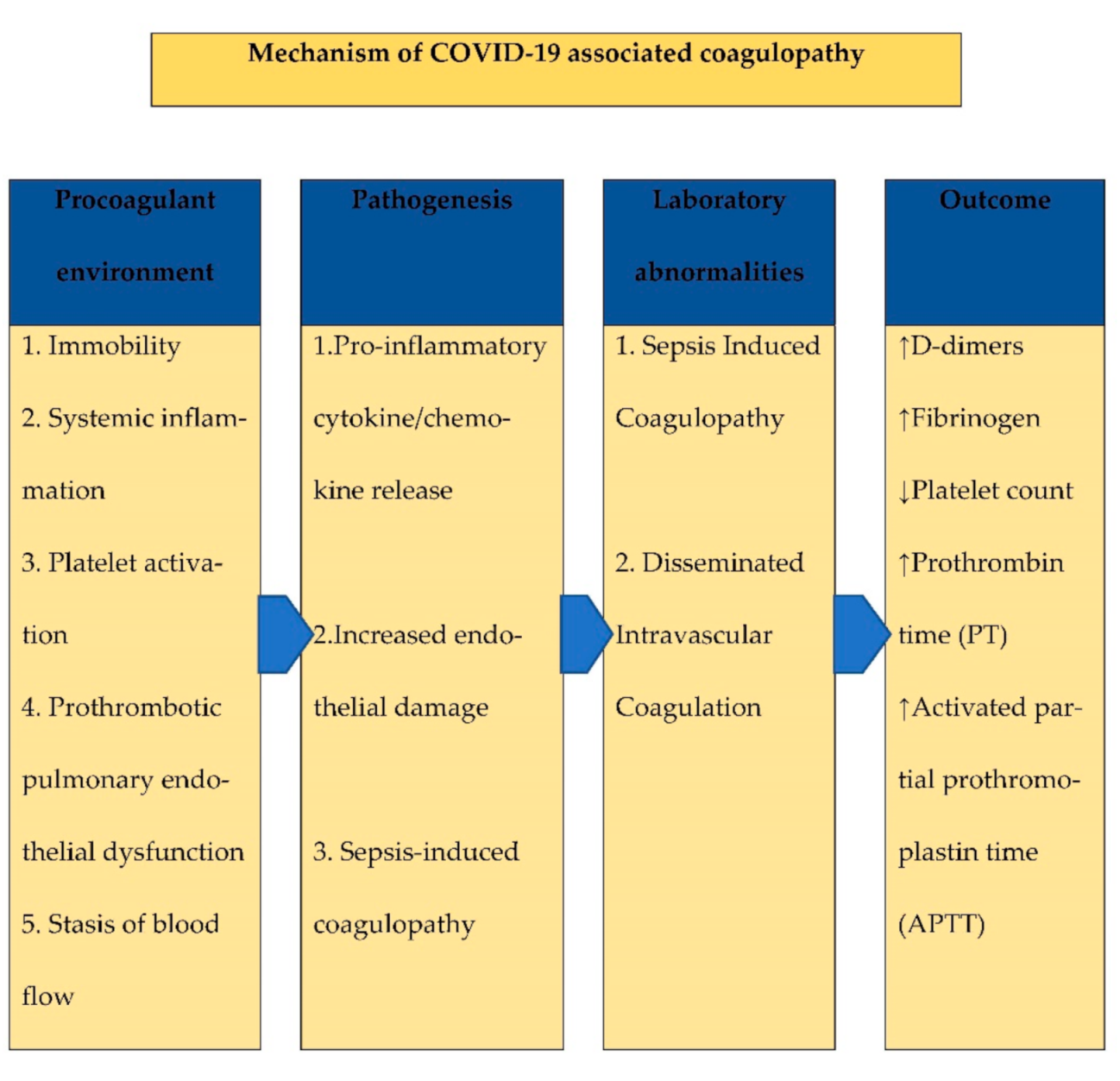
1. Background
2. Possible Pathophysiology of Coagulopathy
3. Biomarkers of Hemostasis
4. Potential Role of Complement Inhibition in COVID-19
5. Role for Antivirals and Immunomodulatory Agents to Reduce the Development of Immunothrombosis
6. D-Dimer in COVID-19 and Coagulations Disturbances
7. COVID-19, Elevated Troponin and Thrombotic Disease
8. Venous Thromboembolism
9. Management of VTE in Patients with COVID-19
10. Outpatient Management with Mild COVID-19
11. Management of Hospitalized Patients with Moderate or Severe COVID-19 without DIC
12. Hospitalized Patients with Moderate or Severe COVID-19 and with Suspected or Confirmed DIC
13. Patients with COVID-19 Presenting with Acute Coronary Syndrome (ACS)
14. Extended (Post-Discharge) VTE Prophylaxis
15. Role for Empiric Therapeutic Anticoagulation without a Diagnosis of VTE
16. Managing the Risk of Hospital-Associated VTE
17. COVID-19 and Interventional Therapies for VTE
18. Additional Considerations
19. Management of Bleeding That Occurs in COVID-19
20. Management of Patients with Thromboembolic Disease without COVID-19
21. International Travel and COVID-19
22. Public Health Considerations Related to Care for Thrombotic Disease
- As daily routines continue to be disrupted, many will experience dietary changes (especially in daily intake of green vegetables, which are the major sources of vitamin K in Western diets) that can affect treatment with vitamin K antagonists. As the quarantine measures become even more restrictive, changes in physical activity, diet and vitamin K intake are likely to impact INR values further.
- The COVID-19 pandemic has devastated the economy of many countries, with the United Nations estimating that COVID-19 could cost the world economy more than $1 trillion in 2020 [75,76]. This will negatively impact the ability of many patients to receive treatment for thrombotic diseases. Socioeconomic disadvantages are linked to higher rates of VTE and adverse outcomes [77,78].
23. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Chen, D.; Jiang, Q.; Yuan, Z. High intensities of population movement were associated with high incidence of COVID-19 during the pandemic. Epidemiol. Infect. 2020, 148, e177. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, T. COVID-19: Towards understanding of pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.M.E.; et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet Lond. Engl. 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Libby, P.; Simon, D.I. Inflammation and Thrombosis. Circulation 2001, 103, 1718–1720. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021, 88, 15–27. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020, 58, 1131–1134. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Brodsky, R.A. Severe COVID-19 infection and thrombotic microangiopathy: Success does not come easily. Br. J. Haematol. 2020, 189, e227–e230. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.D.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio 2018, 9, e01753-18. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, G.; Song, N.; Li, P.; Chen, Y.; Guo, Y.; Li, J.; Du, L.; Jiang, S.; Guo, R.; et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 2018, 7. [Google Scholar] [CrossRef]
- Fletcher-Sandersjöö, A.; Bellander, B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb. Res. 2020, 194, 36–41. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, Y.; Harley, K.T.; Tran, M.-H. Atypical Hemolytic Uremic Syndrome: A Brief Review. Hematol. Rep. 2017, 9, 7053. [Google Scholar] [CrossRef]
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Olive, L.; Marx, W.; O’Neil, A.; Athan, E.; Carvalho, A.; Maes, M.; Walder, K.; et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2020, 264, 118617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, Y.; Xiao, H.; Yang, J.; Toulon, P.; Zhang, Z. Use of D-dimer in oral anticoagulation therapy. Int. J. Lab. Hematol. 2018, 40, 503–507. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tu, W.-J.; Cao, J.; Yu, L.; Hu, X.; Liu, Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020, 46, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; De Bruyne, B.; Pijls, N.H.J.; Desai, M.; Oldroyd, K.G.; Park, S.-J.; Reardon, M.J.; Wendler, O.; Woo, J.; Yeung, A.C.; et al. Rationale and design of the Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) 3 Trial: A comparison of fractional flow reserve-guided percutaneous coronary intervention and coronary artery bypass graft surgery in patients with multivessel coronary artery disease. Am. Heart J. 2015, 170, 619–626. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Ahmad, T.; Binder, L.G.; Hucker, W.J.; Kumbhani, D.J.; Maddox, T.M.; Marine, J.E.; Morris, P.B. 2019 Methodology for Creating Expert Consensus Decision Pathways: A Report of the American College of Cardiology. J. Am. Coll. Cardiol. 2019, 74, 1138–1150. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Januzzi, J.L. Troponin and BNP Use in COVID-19. Available online: http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2020%2f03%2f18%2f15%2f25%2ftroponin-and-bnp-use-in-covid19 (accessed on 19 September 2020).
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [PubMed]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, F.; Chehade, S.; Lazo-Langner, A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020, 192, 152–160. [Google Scholar] [CrossRef]
- Artifoni, M.; Danic, G.; Gautier, G.; Gicquel, P.; Boutoille, D.; Raffi, F.; Néel, A.; Lecomte, R. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: Incidence and role of D-dimer as predictive factors. J. Thromb. Thrombolysis 2020, 50, 211–216. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Witt, D.M.; Nieuwlaat, R.; Clark, N.P.; Ansell, J.; Holbrook, A.; Skov, J.; Shehab, N.; Mock, J.; Myers, T.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018, 2, 3257–3291. [Google Scholar] [CrossRef]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Tal, S.; Spectre, G.; Kornowski, R.; Perl, L. Venous Thromboembolism Complicated with COVID-19: What Do We Know So Far? Acta Haematol. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Fontana, P.; Casini, A.; Robert-Ebadi, H.; Glauser, F.; Righini, M.; Blondon, M. Venous thromboembolism in COVID-19: Systematic review of reported risks and current guidelines. Swiss Med. Wkly. 2020, 150, w20301. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems during the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.D.; Schellong, S.M.; Tapson, V.F.; Monreal, M.; Samama, M.-M.; Nicol, P.; Vicaut, E.; Turpie, A.G.G.; Yusen, R.D. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: A randomized trial. Ann. Intern. Med. 2010, 153, 8–18. [Google Scholar] [CrossRef]
- Cohen, A.T.; Harrington, R.A.; Goldhaber, S.Z.; Hull, R.D.; Wiens, B.L.; Gold, A.; Hernandez, A.F.; Gibson, C.M. APEX Investigators Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. N. Engl. J. Med. 2016, 375, 534–544. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Lipardi, C.; Xu, J.; Peluso, C.; Spiro, T.E.; De Sanctis, Y.; Barnathan, E.S.; Raskob, G.E. Modified IMPROVE VTE Risk Score and Elevated D-Dimer Identify a High Venous Thromboembolism Risk in Acutely Ill Medical Population for Extended Thromboprophylaxis. TH Open Companion J. Thromb. Haemost. 2020, 4, e59–e65. [Google Scholar] [CrossRef]
- Cohen, A.T.; Spiro, T.E.; Büller, H.R.; Haskell, L.; Hu, D.; Hull, R.; Mebazaa, A.; Merli, G.; Schellong, S.; Spyropoulos, A.C.; et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N. Engl. J. Med. 2013, 368, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Ageno, W.; Albers, G.W.; Elliott, C.G.; Halperin, J.L.; Hiatt, W.R.; Maynard, G.A.; Steg, P.G.; Weitz, J.I.; Suh, E.; et al. Rivaroxaban for Thromboprophylaxis after Hospitalization for Medical Illness. N. Engl. J. Med. 2018, 379, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Mumoli, N.; Prisco, D.; Fontanella, A.; Di Minno, M.N.D. Efficacy and safety of extended thromboprophylaxis for medically ill patients. A meta-analysis of randomised controlled trials. Thromb. Haemost. 2017, 117, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, M.; Weitz, J.I. Broadening the Categories of Patients Eligible for Extended Venous Thromboembolism Treatment. Thromb. Haemost. 2020, 120, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.T.; Spiro, T.E.; Spyropoulos, A.C.; Desanctis, Y.H.; Homering, M.; Büller, H.R.; Haskell, L.; Hu, D.; Hull, R.; Mebazaa, A.; et al. D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalized patients: A subanalysis of the randomized controlled MAGELLAN trial. J. Thromb. Haemost. 2014, 12, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Obi, A.T.; Tignanelli, C.J.; Jacobs, B.N.; Arya, S.; Park, P.K.; Wakefield, T.W.; Henke, P.K.; Napolitano, L.M. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 317–324. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.a.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.a.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Darzi, A.J.; Karam, S.G.; Charide, R.; Etxeandia-Ikobaltzeta, I.; Cushman, M.; Gould, M.K.; Mbuagbaw, L.; Spencer, F.A.; Spyropoulos, A.C.; Streiff, M.B.; et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: A systematic review and meta-analysis. Blood 2020, 135, 1788–1810. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef]
- Reza, N.; Dudzinski, D.M. Pulmonary Embolism Response Teams. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 27. [Google Scholar] [CrossRef]
- Barnes, G.D.; Kabrhel, C.; Courtney, D.M.; Naydenov, S.; Wood, T.; Rosovsky, R.; Rosenfield, K.; Giri, J. National PERT Consortium Research Committee Diversity in the Pulmonary Embolism Response Team Model: An Organizational Survey of the National PERT Consortium Members. Chest 2016, 150, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Rosovsky, R.; Zhao, K.; Sista, A.; Rivera-Lebron, B.; Kabrhel, C. Pulmonary embolism response teams: Purpose, evidence for efficacy, and future research directions. Res. Pract. Thromb. Haemost. 2019, 3, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Sista, A.K.; Weinberg, I.; Kearon, C.; Kumbhani, D.J.; Desai, N.D.; Piazza, G.; Gladwin, M.T.; Chatterjee, S.; Kobayashi, T.; et al. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e774–e801. [Google Scholar] [CrossRef]
- Stockmann, H.; Krannich, A.; Schroeder, T.; Storm, C. Therapeutic temperature management after cardiac arrest and the risk of bleeding: Systematic review and meta-analysis. Resuscitation 2014, 85, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Thachil, J.; Di Nisio, M.; Mathew, P.; Kurosawa, S.; Gando, S.; Kim, H.K.; Nielsen, J.D.; Dempfle, C.-E.; Levi, M.; et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J. Thromb. Haemost. 2013, 11, 761–767. [Google Scholar] [CrossRef]
- Aujesky, D.; Roy, P.-M.; Verschuren, F.; Righini, M.; Osterwalder, J.; Egloff, M.; Renaud, B.; Verhamme, P.; Stone, R.A.; Legall, C.; et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: An international, open-label, randomised, non-inferiority trial. Lancet 2011, 378, 41–48. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Parpia, S.; Stewart, C.; Rudd-Scott, L.; Julian, J.A.; Levine, M. Warfarin dose assessment every 4 weeks versus every 12 weeks in patients with stable international normalized ratios: A randomized trial. Ann. Intern. Med. 2011, 155, 653–659. [Google Scholar] [CrossRef]
- Parker, S.; Mahomed, O. Hypoxia and Thrombosis in COVID-19: New Considerations for Air Passengers. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Engbers, M.J.; Blom, J.W.; Cushman, M.; Rosendaal, F.R.; van Hylckama Vlieg, A. Functional Impairment and Risk of Venous Thrombosis in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 2003–2008. [Google Scholar] [CrossRef]
- Kabrhel, C.; Varraso, R.; Goldhaber, S.Z.; Rimm, E.; Camargo, C.A. Physical inactivity and idiopathic pulmonary embolism in women: Prospective study. BMJ 2011, 343, d3867. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Raymond, N.; Hill, S.; Nowitz, M.; Hughes, R. eThrombosis: The 21st Century variant of venous thromboembolism associated with immobility. Eur. Respir. J. 2003, 21, 374–376. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Stay Physically Active during Self-Quarantine. Available online: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/technical-guidance/stay-physically-active-during-self-quarantine (accessed on 19 August 2020).
- Organisation for Economic Cooperation and Development OECD. Economic Outlook; Interim Report March 2020. Available online: https://www.oecd-ilibrary.org/economics/oecd-economic-outlook/volume-2019/issue-2_7969896b-en (accessed on 19 August 2020).
- United Nation. News Coronavirus Update: COVID-19 Likely to Cost Economy $1 Trillion during 2020, Says UN Trade Agency. Available online: https://news.un.org/en/story/2020/03/1059011 (accessed on 21 September 2020).
- Kort, D.; van Rein, N.; van der Meer, F.J.M.; Vermaas, H.W.; Wiersma, N.; Cannegieter, S.C.; Lijfering, W.M. Relationship between neighborhood socioeconomic status and venous thromboembolism: Results from a population-based study. J. Thromb. Haemost. JTH 2017, 15, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Isma, N.; Merlo, J.; Ohlsson, H.; Svensson, P.J.; Lindblad, B.; Gottsäter, A. Socioeconomic factors and concomitant diseases are related to the risk for venous thromboembolism during long time follow-up. J. Thromb. Thrombolysis 2013, 36, 58–64. [Google Scholar] [CrossRef] [PubMed]
| Study and References | Levels in Non-Severe Patients (Confidence Interval | Levels in Severe Patients (Confidence Interval) | Significance Level (p Value) | Comments |
|---|---|---|---|---|
| Huang et al. (2020) [15] | 0.5 mg/L | 2.4 mg/L | p = 0.0042 | ICU patients had significantly higher levels of D-dimer than non-ICU patients |
| Tang et al. (2020) [17] | 0.61 (0.35−1.29) | 2.12 (0.77−5.27) | p < 0.001 | Overall mortality was 11.5%, the non-survivors revealed significantly higher D-dimer levels |
| Zhou et al. (2020) [25] | 0.6 (0.3−1) | 5.2 (1.5−21.1) | p < 0.0001 | D-dimer levels > 1 μg/mL can help with early identification of patients with poor prognosis |
| Zhang et al. (2020) [27] | 0.41 mg/L (0.15–0.69) | 4.76 mg/L (2.99–11.9) | p < 0.001 | D-dimer levels > 2.0 μg/mL on admission can predict in-hospital mortality in patients with COVID-19 and could be a therapeutic marker |
| Guan et al. (2020) [28] | 43.2% with >0.5 mg/L | 59.6% with >0.5 mg/L | N/A | D-dimer levels higher in those requiring ICU admission and invasive ventilation; statistical analysis not performed |
| Tu et al. (2020) [29] | Median 0.66 g/mL | Median 3.306 g/mL | p < 0.001 | D-dimer levels were significantly higher in non-survivors |
| Status of Patient | Management Recommendations |
|---|---|
| Mild COVID-19 |
|
| Moderate to severe COVID-19 without DIC |
|
| Moderate to severe COVID-19 with DIC |
|
| Bleeding in COVID 19 | Uncommon but managed as per local guideline |
| Thromboembolic disease without COVID-19 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, S.; Islam, A.; Rafiq, S.; Laher, I. The COVID-19 Pandemic: Disproportionate Thrombotic Tendency and Management Recommendations. Trop. Med. Infect. Dis. 2021, 6, 26. https://doi.org/10.3390/tropicalmed6010026
Karim S, Islam A, Rafiq S, Laher I. The COVID-19 Pandemic: Disproportionate Thrombotic Tendency and Management Recommendations. Tropical Medicine and Infectious Disease. 2021; 6(1):26. https://doi.org/10.3390/tropicalmed6010026
Chicago/Turabian StyleKarim, Sabina, Amin Islam, Shafquat Rafiq, and Ismail Laher. 2021. "The COVID-19 Pandemic: Disproportionate Thrombotic Tendency and Management Recommendations" Tropical Medicine and Infectious Disease 6, no. 1: 26. https://doi.org/10.3390/tropicalmed6010026
APA StyleKarim, S., Islam, A., Rafiq, S., & Laher, I. (2021). The COVID-19 Pandemic: Disproportionate Thrombotic Tendency and Management Recommendations. Tropical Medicine and Infectious Disease, 6(1), 26. https://doi.org/10.3390/tropicalmed6010026








