Clinical Features and Mortality Associated with Severe Malaria in Adults in Southern Mauritania
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Site
2.2. Patients
2.3. Statistical Analysis
2.4. Research Ethics
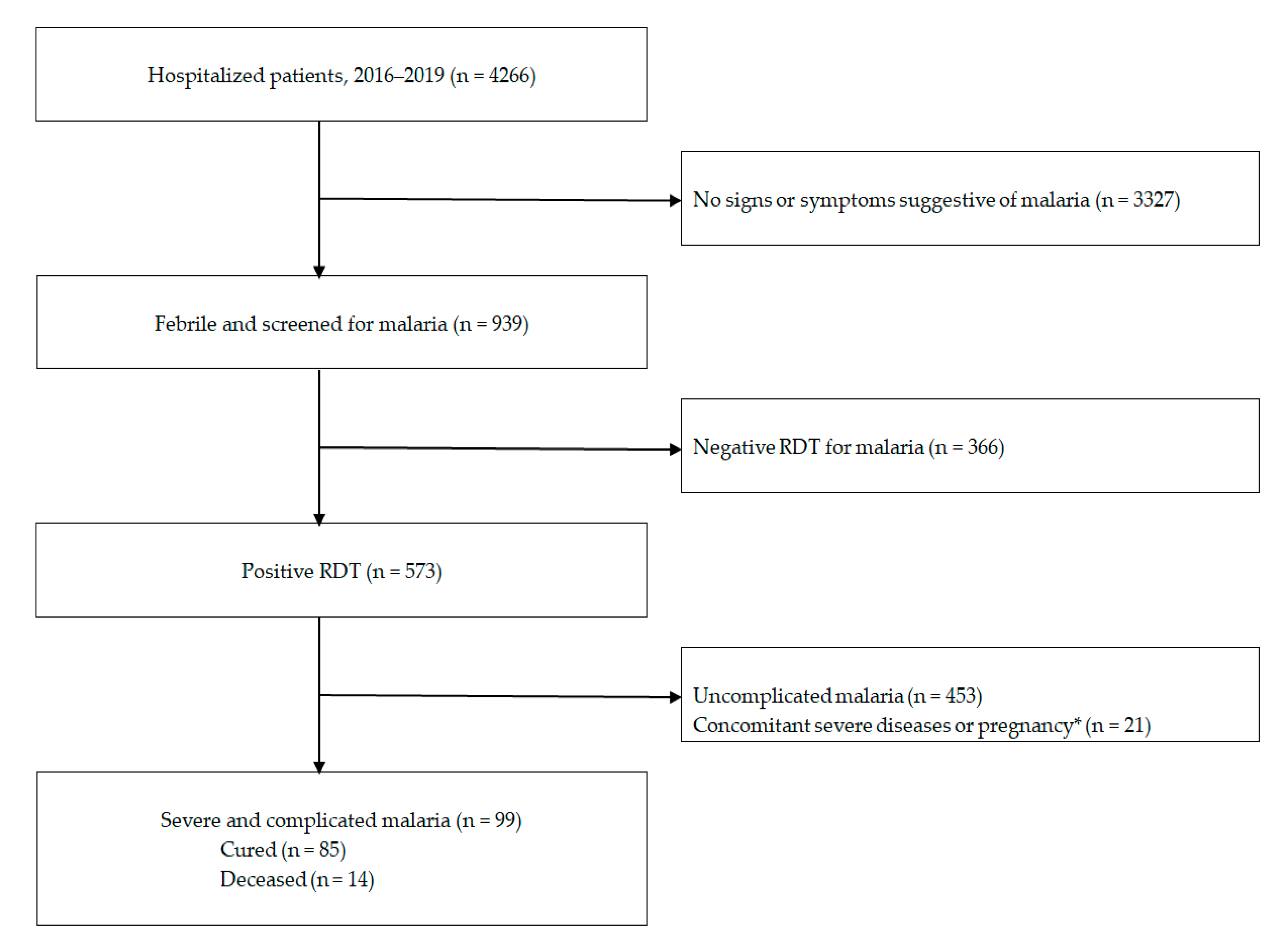
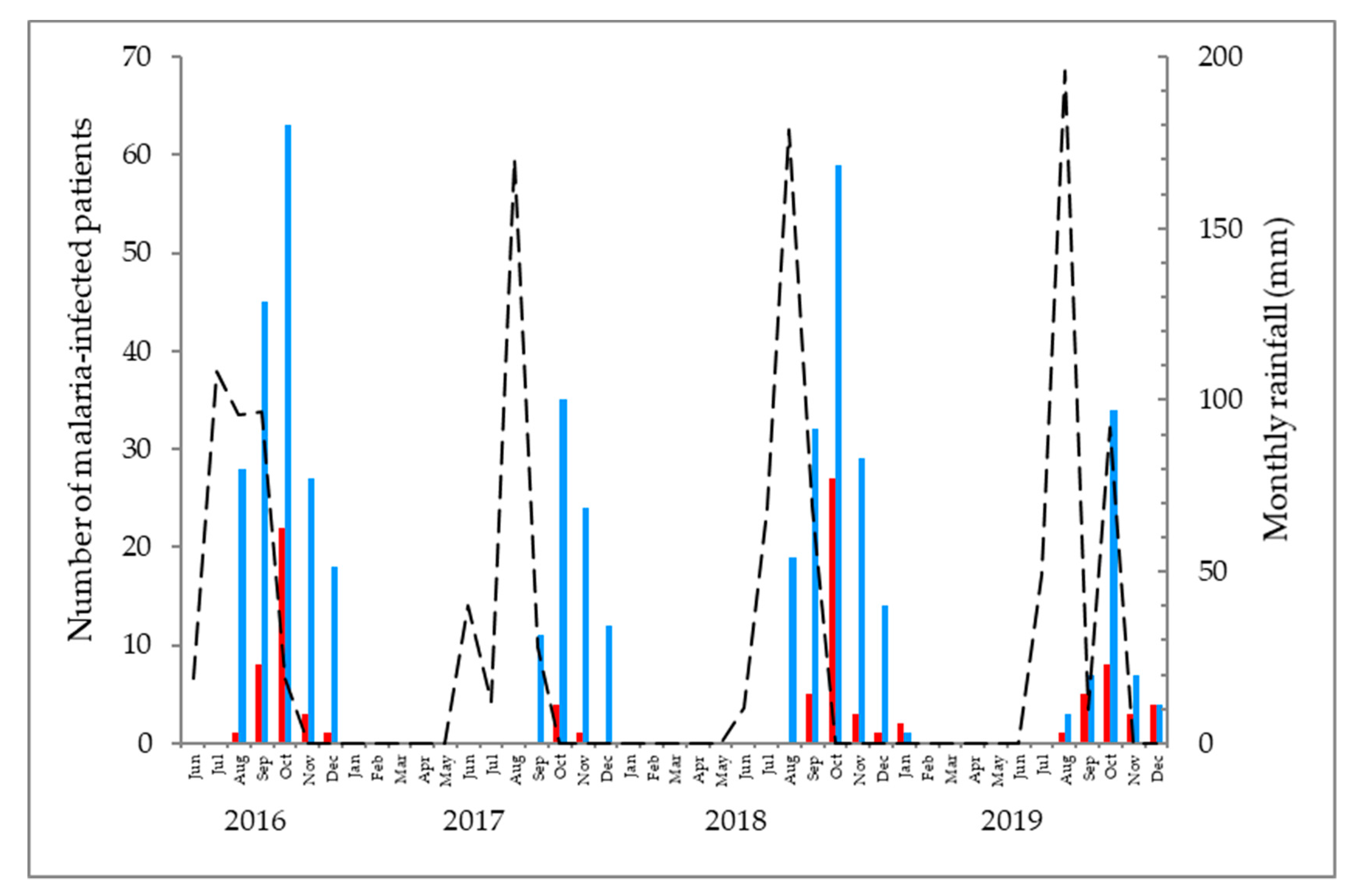
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ould Ahmedou Salem, M.S.; Basco, L.K.; Ouldabdallahi, M.; Lekweiry, K.M.; Konaté, L.; Faye, O.; Ould Mohamed Salem Boukhary, A. Malaria-associated morbidity during the rainy season in Saharan and Sahelian zones in Mauritania. Acta Trop. 2015, 152, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ouldabdallahi, M.; Alew, I.; Ould Ahmedou, M.S.; Ba, M.D.D.; Boukhary, A.O.M.S.; Ould Khairy, M.L.; Abdel Aziz, M.B.; Ringwald, P.; Basco, L.K.; Niang, S.D.; et al. Efficacy of artesunate-amodiaquine for the treatment of acute uncomplicated falciparum malaria in southern Mauritania. Malar. J. 2014, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Mint Lekweiry, K.; Ould Ahmedou, M.S.; Basco, L.K.; Briolant, S.; Hafid, J.; Boukhary, A.O.M.S. Malaria in Mauritania: Retrospective and prospective overview. Malar. J. 2015, 14, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deida, J.; Tahar, R.; Khalef, Y.O.; Lekweiry, K.M.; Hmeyade, A.; Khairy, M.L.O.; Simard, F.; Bogreau, H.; Basco, L.; Boukhary, A.O.M.S. Oasis Malaria, Northern Mauritania. Emerg. Infect. Dis. 2019, 25, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Baruch, D.I.; Marsh, K.; Doumbo, O.K. The pathogenic basis of malaria. Nature 2002, 415, 673–679. [Google Scholar] [CrossRef] [PubMed]
- WHO. Severe Malaria. Trop. Med. Int. Health 2014, 19 (Suppl. 1), 7–131. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K. Malaria—A neglected disease? Parasitology 1992, 104 (Suppl. 1), S53–S69. [Google Scholar] [CrossRef]
- Boushab, B.M.; Fall-Malick, F.Z.; Savadogo, M.; Basco, L.K. Acute kidney injury in a shepherd with severe malaria: A case report. Int. J. Nephrol. Renov. Dis. 2016, 9, 249–251. [Google Scholar] [CrossRef] [Green Version]
- Office National de la Statistique (ONS). Recensement Général de la Population et de l’Habitat (RGPH), 2013. Monographie de la Ville de Kiffa. Available online: http://www.ons.mr/index.php/publications/operations-statistiques/16-rgph-2013 (accessed on 18 November 2020).
- Office National de la Météorologie, Nouakchott, Mauritania. Available online: http://fr.meteo-mauritanie.org (accessed on 18 November 2020).
- Rassoul, E.O.M. Evaluation de la Qualité de la Prise en Charge du Paludisme Grave à L’hôpital Régional de Kaédi, Mauritanie en 2000 et 2002. Ph.D. Thesis, Faculté de Médecine, de Pharmacie et D’odonto-Stomatologie, Université de Bamako, Bamako, Mali, 2006. Available online: http://www.keneya.net/fmpos/theses/2006/med/pdf/06M137.pdf (accessed on 18 November 2020).
- Boushab, M.B.; Fall-Malick, F.Z.; Savadogo, M.; Sow, M.S.; Basco, L. Paludisme Grave à Aïoun: Étude Rétropective à Propos de 64 Cas. Rev. Malienne. Infectiol. Microbiol. 2016, 7, 63–66. [Google Scholar]
- Mauritanian Ministry of Health. Guide Clinique et Thérapeutique à L’usage du Personnel des Centres de Santé de la Mauritanie, 2nd ed.; Agencia Española de Cooperación Internacional Para el Desarrollo (AECID): Madrid, Spain, 2013; pp. 1–244. Available online: http://www.sante.gov.mr/?wpfb_dl=140) (accessed on 18 November 2020).
- Dean, A.G.; Arner, T.G.; Sangam, S.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Zubieta, J.C.; Sullivan, K.M.; Smith, D.C. Epi Info, a Database and Statistics Program for Public Health Professionals. Centres for Disease Control, Atlanta, GA, USA. 2011. Available online: https://www.cdc.gov/epiinfo/index.html (accessed on 18 November 2020).
- Makani, J.; Matuja, W.; Liyombo, E.; Snow, R.W.; Marsh, K.; Warrell, D.A. Admission diagnosis of cerebral malaria in adults in an endemic area of Tanzania: Implications and clinical description. Qjm 2003, 96, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Idro, R.; Bitarakwate, E.; Tumwesigire, S.; John, C.C. Clinical manifestations of severe malaria in the highlands of southwestern Uganda. Am. J. Trop. Med. Hyg. 2005, 72, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Pita, L.A.; Jiménez, M.R.; Murillo, A.S.; Capote, R.M.; Fonseca, M.E.R. Severe malarial infection in adults aged over 18 years: One-year experience. Rev. Cuba. Med. Trop. 2006, 58, 219–225. [Google Scholar]
- Achan, J.; Tibenderana, J.; Kyabayinze, D.; Mawejje, H.; Mugizi, R.; Mpeka, B.; Talisuna, A.; D’Alessandro, U. Case Management of Severe Malaria—A Forgotten Practice: Experiences from Health Facilities in Uganda. PLoS ONE 2011, 6, e17053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaméogon, T.M.; Kyelem, C.G.; Ouédraogo, S.M.; Diallo, O.J.; Moyenga, L.; Poda, G.E.A.; Guiguemdé, T.R. Case management and diagnosis of severe malaria in adults and the application of national guidelines in Burkina Faso. Bull. Soc. Pathol. Exot. 2011, 104, 284–287. [Google Scholar]
- Wade, K.A.; Sene, B.E.J.; Niang, E.M.; Diallo, A.; Diatta, B. Epidemiology and prognostic value of organ failure during severe malaria in the Principal Military Teaching Hospital of Dakar, Senegal. Méd. Santé Trop. 2012, 22, 422–424. [Google Scholar] [CrossRef]
- Camponovo, F.; Bever, C.A.; Galactionova, K.; Smith, T.; Penny, M.A. Incidence and admission rates for severe malaria and their impact on mortality in Africa. Malar. J. 2017, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- WHO. World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (accessed on 18 November 2020).
- Griffin, J.T.; Hollingsworth, T.D.; Reyburn, H.; Drakeley, C.J.; Riley, E.M.; Ghani, A.C. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142657. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, T.M.; Abdeen, M.T.; Ahmed, I.S.; Hamdan, H.Z.; Magzoub, M.; Adam, I. Severe Plasmodium falciparum and Plasmodium vivax malaria among adults at Kassala Hospital. Malar. J. 2013, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Bouyou-Akotet, M.K.; Offouga, C.L.; Mawili-Mboumba, D.P.; Essola, L.; Madoungou, B.; Kombila, M. Falciparum Malaria as an Emerging Cause of Fever in Adults Living in Gabon, Central Africa. BioMed Res. Int. 2014, 2014, 351281. [Google Scholar] [CrossRef] [Green Version]
- Boyce, R.; Reyes, R.; Keeler, C.; Matte, M.; Ntaro, M.; Mulogo, E.; Siedner, M.J. Anemia was an Uncommon Complication of Severe Malaria in a High-Transmission Rural Area of Western Uganda. Am. J. Trop. Med. Hyg. 2018, 98, 683–691. [Google Scholar] [CrossRef]
- Diallo, S.M.; Bogreau, H.; Mze, N.P.; Ould Ahmedou Salem, M.S.; Khairy, M.L.O.; Parola, P.; Basco, L.; Ould Mohamed Salem Boukhary, A. Malaria epidemiology in Kobeni department, southeastern Mauritania from 2015 to 2017. Infect. Dis. Poverty 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruebush, T.K.; Kern, M.K.; Campbell, C.C.; Oloo, A.J. Self-treatment of malaria in a rural area of western Kenya. Bull. World Health Organ. 1995, 73, 229–236. [Google Scholar] [PubMed]
- McCombie, S.C. Treatment seeking for malaria: A review of recent research. Soc. Sci. Med. 1996, 43, 933–945. [Google Scholar] [CrossRef]
- Diop, S.A.; Attinsounon, C.A.; Fortes-Déguénonvo, L.; Diallo, V.M.P.C.; Seydi, M. Therapeutic itinerary of severe malaria in adults admitted to a teaching hospital in Dakar, Senegal. J. Infect. Dev. Ctries. 2014, 8, 1353–1355. [Google Scholar] [CrossRef] [Green Version]
- Awuah, R.B.; Asante, P.Y.; Biney, A.A.E.; Kushitor, M.K.; Agyei, F.; de Graft Aikins, A. Factors associated with treatment-seeking for malaria in urban poor communities in Accra, Ghana. Malar. J. 2018, 17, 168. [Google Scholar] [CrossRef]
- Hopkins, H.; Talisuna, A.; Frcp, C.J.M.W.; Staedke, S.G. Impact of home-based management of malaria on health outcomes in Africa: A systematic review of the evidence. Malar. J. 2007, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Okwundu, C.I.; Nagpal, S.; Musekiwa, A.; Sinclair, D. Home- or community-based programmes for treating malaria. Cochrane Database Syst. Rev. 2013, 5, CD009527. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/malaria/publications/atoz/9789241549127/en/ (accessed on 18 November 2020).
- Dondorp, A.; Nosten, F.; Stepniewska, K.; Day, N.; White, N.; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) Group. Artesunate versus quinine for treatment of severe falciparum malaria: A randomised trial. Lancet 2005, 366, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, T.M.; Elmardi, K.A.; Elhassan, A.H.; Omer, M.B.; Elhag, M.S.; Desogi, M.A.; Siddig, M.F.; Adam, I. Comparison of artesunate and quinine in the treatment of severe Plasmodium falciparum malaria at Kassala hospital, Sudan. J. Infect. Dev. Ctries. 2014, 8, 611–615. [Google Scholar] [CrossRef] [Green Version]

| Clinical Features | Number of Patients (%) | p-Value | ||
|---|---|---|---|---|
| On Admission | Cured (n = 85) | Deceased (n = 14) | ||
| Impairment of consciousness | 81 (81.8) | 67 (78.8) | 14 (100) | NS * |
| Multiple convulsions (>2 episodes/24 h) | 70 (70.7) | 56 (65.9) | 14 (100) | 0.009 |
| Cardiovascular collapse | 61 (61.6) | 49 (57.6) | 12 (85.7) | NS * |
| Respiratory distress (acidotic breathing) | 43 (43.4) | 33 (38.8) | 10 (71.4) | 0.04 |
| Severe anaemia (haemoglobin ≤ 80 g/L) | 36 (36.4) | 27 (31.8) | 9 (64.3) | 0.03 |
| Haemoglobinuria | 27 (27.3) | 18 (21.2) | 9 (64.3) | 0.002 |
| Acute renal failure (Cr > 265 µmol/L) | 25 (25.3) | 17 (20.0) | 8 (57.1) | 0.006 |
| Hypoglycaemia (blood sugar < 0.4 g/L) | 13 (13.1) | 11 (12.9) | 2 (14.3) | NS |
| Jaundice | 12 (12.1) | 6 (7.1) | 6 (42.9) | 0.002 |
| Abnormal bleeding | 7 (7.1) | 3 (3.5) | 4 (28.6) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boushab, B.M.; Ould Ahmedou Salem, M.S.; Ould Mohamed Salem Boukhary, A.; Parola, P.; Basco, L. Clinical Features and Mortality Associated with Severe Malaria in Adults in Southern Mauritania. Trop. Med. Infect. Dis. 2021, 6, 1. https://doi.org/10.3390/tropicalmed6010001
Boushab BM, Ould Ahmedou Salem MS, Ould Mohamed Salem Boukhary A, Parola P, Basco L. Clinical Features and Mortality Associated with Severe Malaria in Adults in Southern Mauritania. Tropical Medicine and Infectious Disease. 2021; 6(1):1. https://doi.org/10.3390/tropicalmed6010001
Chicago/Turabian StyleBoushab, Boushab Mohamed, Mohamed Salem Ould Ahmedou Salem, Ali Ould Mohamed Salem Boukhary, Philippe Parola, and Leonardo Basco. 2021. "Clinical Features and Mortality Associated with Severe Malaria in Adults in Southern Mauritania" Tropical Medicine and Infectious Disease 6, no. 1: 1. https://doi.org/10.3390/tropicalmed6010001
APA StyleBoushab, B. M., Ould Ahmedou Salem, M. S., Ould Mohamed Salem Boukhary, A., Parola, P., & Basco, L. (2021). Clinical Features and Mortality Associated with Severe Malaria in Adults in Southern Mauritania. Tropical Medicine and Infectious Disease, 6(1), 1. https://doi.org/10.3390/tropicalmed6010001






