An Evaluation of Programmatic Community-Based Chest X-ray Screening for Tuberculosis in Ho Chi Minh City, Vietnam
Abstract
1. Introduction
2. Materials and Methods
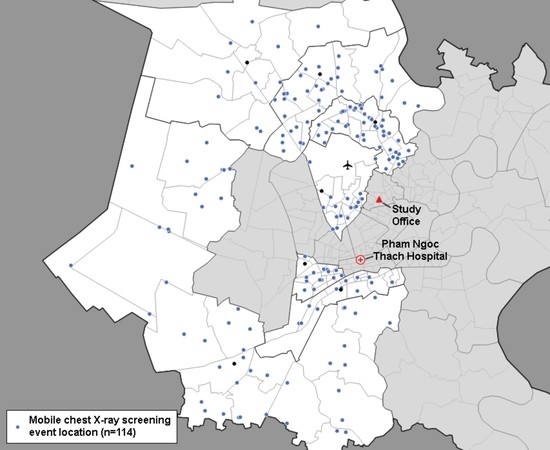
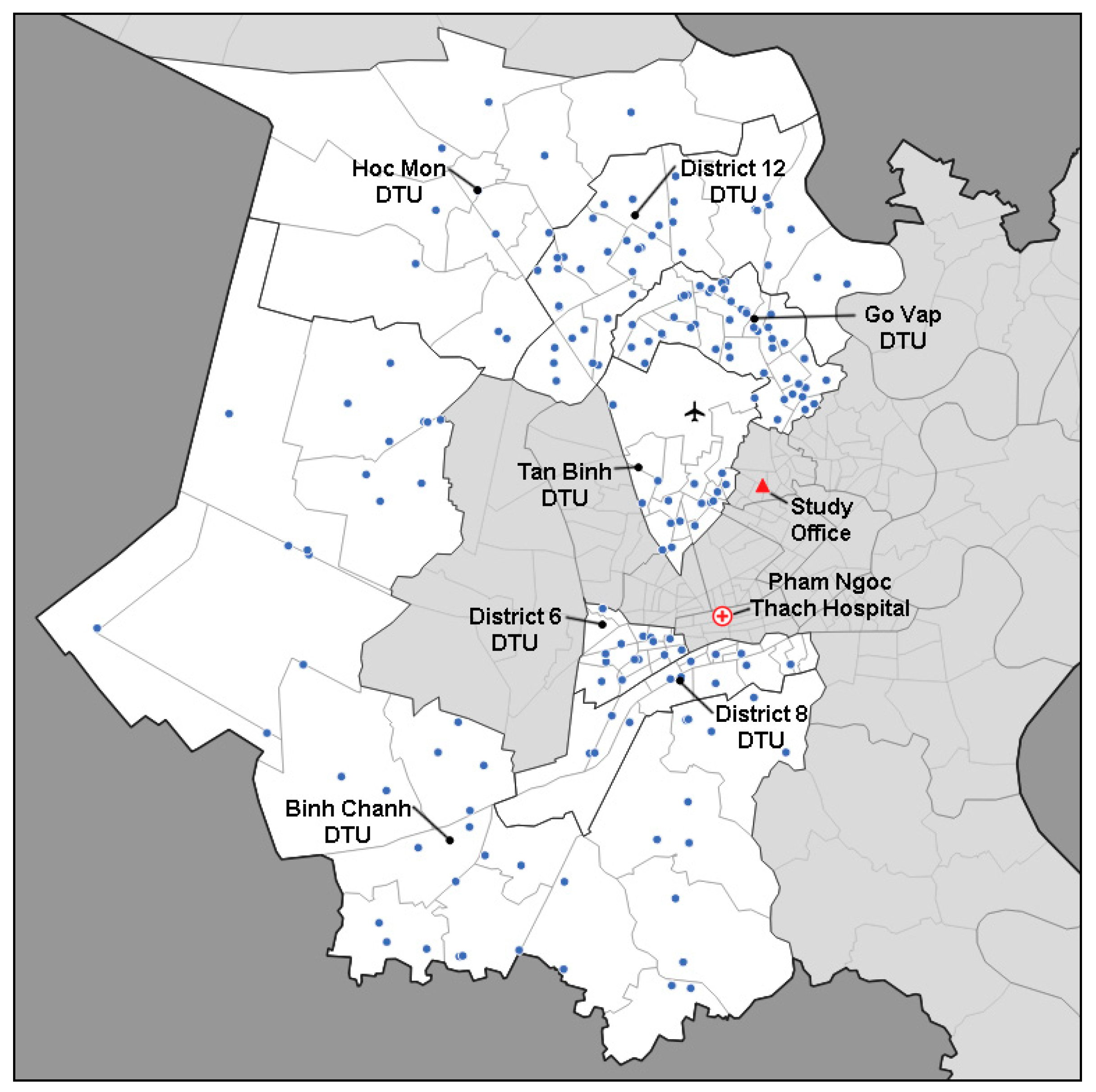
2.1. Study Setting
2.2. Participant Mobilization
2.3. Screening and Diagnosis of TB
2.4. Statistical Analyses
2.5. Ethical Considerations
3. Results
3.1. Yields of TB
3.2. Marginal Yield Analysis and Relative Diagnostic Costing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020.
- Nguyen, H.V.; Tiemersma, E.W.; Nguyen, H.B.; Cobelens, F.G.J.; Finlay, A.; Glaziou, P.; Dao, C.H.; Mirtskhulava, V.; Nguyen, H.V.; Pham, H.T.T.; et al. The second national tuberculosis prevalence survey in Vietnam. PLoS ONE 2020, 15, e0232142. [Google Scholar] [CrossRef]
- Hoa, N.B.; Sy, D.N.; Nhung, N.V.; Tiemersma, E.W.; Borgdorff, M.W.; Cobelens, F.G. National survey of tuberculosis prevalence in Viet Nam. Bull. World Health Organ. 2010, 88, 273–280. [Google Scholar] [CrossRef]
- Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018.
- The End TB Strategy; World Health Organization: Geneva, Switzerland, 2015.
- Onozaki, I.; Law, I.; Sismanidis, C.; Zignol, M.; Glaziou, P.; Floyd, K. National tuberculosis prevalence surveys in Asia, 1990–2012: An overview of results and lessons learned. Trop. Med. Int. Health 2015, 20, 1128–1145. [Google Scholar] [CrossRef]
- Frascella, B.; Richards, A.S.; Sossen, B.; Emery, J.C.; Odone, A.; Law, I.; Onozaki, I.; Esmail, H.; Houben, R.M.G.J. Subclinical tuberculosis disease—A review and analysis of prevalence surveys to inform definitions, burden, associations and screening methodology. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Chest Radiography in Tuberculosis Detection: Summary of Current WHO Recommendations and Guidance on Programmatic Approaches; World Health Organization: Geneva, Switzerland, 2016.
- Biermann, O.; Lönnroth, K.; Caws, M.; Viney, K. Factors influencing active tuberculosis case-finding policy development and implementation: A scoping review. BMJ Open 2019, 9, e031284. [Google Scholar] [CrossRef]
- Guidelines on TB Diagnosis, Treatment and Prevention (1314/QĐ-BYT); Viet Nam Ministry of Health: Ha Noi, Viet Nam, 2020.
- 2019 Final Report: Activities of the National TB Program; National TB Program: Ha Noi, Viet Nam, 2020.
- Morishita, F.; Garfin, A.M.C.G.; Lew, W.; Oh, K.H.; Yadav, R.-P.; Reston, J.C.; Infante, L.L.; Acala, M.R.C.; Palanca, D.L.; Kim, H.J.; et al. Bringing state-of-the-art diagnostics to vulnerable populations: The use of a mobile screening unit in active case finding for tuberculosis in Palawan, the Philippines. PLoS ONE 2017, 12, e0171310. [Google Scholar] [CrossRef]
- Camelique, O.; Scholtissen, S.; Dousset, J.-P.; Bonnet, M.; Bastard, M.; Hewison, C. Mobile community-based active case-finding for tuberculosis among older populations in rural Cambodia. Int. J. Tuberc. Lung Dis. 2019, 23, 1107–1114. [Google Scholar] [CrossRef]
- Madhani, F.; Maniar, R.A.; Burfat, A.; Ahmed, M.; Farooq, S.; Sabir, A.; Domki, A.K.; Page-Shipp, L.; Khowaja, S.; Safdar, N.; et al. Automated chest radiography and mass systematic screening for tuberculosis. Int. J. Tuberc. Lung Dis. 2020, 24, 665–673. [Google Scholar] [CrossRef]
- Muyoyeta, M.; Maduskar, P.; Moyo, M.; Kasese, N.; Milimo, D.; Spooner, R.; Kapata, N.; Hogeweg, L.; van Ginneken, B.; Ayles, H. The Sensitivity and Specificity of Using a Computer Aided Diagnosis Program for Automatically Scoring Chest X-Rays of Presumptive TB Patients Compared with Xpert MTB/RIF in Lusaka Zambia. PLoS ONE 2014, 9, e93757. [Google Scholar] [CrossRef]
- Ananthakrishnan, R.; Thiagesan, R.; Auguesteen, S.; Karunakaran, N.; Jayabal, L.; Stevens, R.; Codlin, A.; Creswell, J. The impact of chest radiography and Xpert MTB/RIF testing among household contacts in Chennai, India. PLoS ONE 2020, 15, e0241203. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Zishiri, V.; Page-Shipp, L.; Makgopa, S.; Churchyard, G.J.; Dowdy, D.; Charalambous, S.; Hoffmann, C.J. Symptom and digital chest X-ray TB screening in South African prisons: Yield and cost-effectiveness. Int. J. Tuberc. Lung Dis. 2020, 24, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, D.M.; Kuhleis, D.C.; Bartholomay, P.; Barreira, D.; Oliveira, C.L.P.; de Jesus, R.S.; Possa, L.A.; Jarczewski, C.A.; Nemeth, L.T.; de Araujo, N.D.; et al. Prevalence and screening of active tuberculosis in a prison in the South of Brazil. Int. J. Tuberc. Lung Dis. 2018, 22, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Vo, L.N.Q.; Forse, R.J.; Codlin, A.J.; Vu, T.N.; Le, G.T.; Do, G.C.; Van Truong, V.; Dang, H.M.; Nguyen, L.H.; Nguyen, H.B.; et al. A comparative impact evaluation of two human resource models for community-based active tuberculosis case finding in Ho Chi Minh City, Viet Nam. BMC Public Health 2020, 20, 934. [Google Scholar] [CrossRef] [PubMed]
- Vo, L.N.Q.; Forse, R.J.; Codlin, A.J.; Nguyen, N.T.; Vu, T.N.; Le, G.T.; Do, G.C.; Truong, V.V.; Dang, H.M.; Nguyen, L.H.; et al. Evaluating the yield of systematic screening for tuberculosis among three priority groups in Ho Chi Minh City, Viet Nam. Infect. Dis. Poverty 2020, 20, 1–12. [Google Scholar]
- Tuberculosis Prevalence Surveys: A Handbook; World Health Organization: Geneva, Switzerland, 2011.
- MacPherson, P.; Houben, R.M.G.J.; Glynn, J.R.; Corbett, E.L.; Kranzer, K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: A systematic review and meta-analysis. Bull. World Health Organ. 2014, 92, 126–138. [Google Scholar] [CrossRef]
- Steingart, K.R.; Schiller, I.; Horne, D.J.; Pai, M.; Boehme, C.C.; Dendukuri, N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2014, CD009593. [Google Scholar] [CrossRef]
- Toman’s Tuberculosis: Case Detection, Treatment and Monitoring: Questions and Answers, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004.
- Minh, H.V.; Mai, V.Q.; Nhung, N.V.; Hoi, L.V.; Giang, K.B.; Chung, L.H.; Kien, V.D.; Duyen, N.T.; Ngoc, N.B.; Anh, T.T.; et al. Costs of providing tuberculosis diagnosis and treatment services in Viet Nam. Int. J. Tuberc. Lung Dis. 2017, 21, 1035–1040. [Google Scholar] [CrossRef]
- Global Drug Facility (GDF) Diagnostics Catalog; Stop TB Partnership: Geneva, Switzerland, 2020.
- External Quality Assessment for AFB Smear Microscopy; Association of Public Health Laboratories (U.S.): Silver Spring, MD, USA; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA; International Union against Tuberculosis and Lung Disease: Paris, France; World Health Organization: Geneva, Switzerland, 2002.
- Systematic Screening for Active Tuberculosis: An Operational Guide; World Health Organization: Geneva, Switzerland, 2015.
- The Global Plan to End TB 2016–2020; Stop TB Partnership: Geneva, Switzerland, 2015.
- Marks, G.B.; Nguyen, N.V.; Nguyen, P.T.B.; Nguyen, T.-A.; Nguyen, H.B.; Tran, K.H.; Nguyen, S.V.; Luu, K.B.; Tran, D.T.T.; Vo, Q.T.N.; et al. Community-wide Screening for Tuberculosis in a High-Prevalence Setting. N. Engl. J. Med. 2019, 381, 1347–1357. [Google Scholar] [CrossRef]
- Behr, M.A.; Warren, S.A.; Salamon, H.; Hopewell, P.C.; Ponce de Leon, A.; Daley, C.L.; Small, P.M. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999, 353, 444–449. [Google Scholar] [CrossRef]
- Xie, Y.L.; Cronin, W.A.; Proschan, M.; Oatis, R.; Cohn, S.; Curry, S.R.; Golub, J.E.; Barry, C.E.; Dorman, S.E. Transmission of Mycobacterium tuberculosis From Patients Who Are Nucleic Acid Amplification Test Negative. Clin. Infect. Dis. 2018, 67, 1653–1659. [Google Scholar] [CrossRef]
- Patterson, B.; Wood, R. Is cough really necessary for TB transmission? Tuberculosis 2019, 117, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cadena, A.M.; Fortune, S.M.; Flynn, J.L. Heterogeneity in tuberculosis. Nat. Rev. Immunol. 2017, 17, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Loveday, M.; Ramjee, A.; Osburn, G.; Master, I.; Kabera, G.; Brust, J.C.M.; Padayatchi, N.; Warren, R.; Theron, G. Drug-resistant tuberculosis in patients with minimal symptoms: Favourable outcomes in the absence of treatment. Int. J. Tuberc. Lung Dis. 2017, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Huong, N.T.; Vree, M.; Duong, B.D.; Khanh, V.T.; Loan, V.T.; Co, N.V.; Borgdorff, M.W.; Cobelens, F.G. Delays in the diagnosis and treatment of tuberculosis patients in Vietnam: A cross-sectional study. BMC Public Health 2007, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Long, N.H.; Diwan, V.K.; Winkvist, A. Gender and tuberculosis control: Perspectives on health seeking behaviour among men and women in Vietnam. Health Policy 2000, 52, 33–51. [Google Scholar] [CrossRef]
- Khan, M.S.; Dar, O.; Sismanidis, C.; Shah, K.; Godfrey-Faussett, P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: A pragmatic randomised controlled trial. Lancet 2007, 369, 1955–1960. [Google Scholar] [CrossRef]
- Qin, Z.Z.; Ahmed, S.; Sarker, M.S.; Paul, K.; Adel, A.S.S.; Naheyan, T.; Banu, S.; Creswell, J. Can artificial intelligence (AI) be used to accurately detect tuberculosis (TB) from chest x-ray? A multiplatform evaluation of five AI products used for TB screening in a high TB-burden setting. arXiv 2020, arXiv:200605509. [Google Scholar]
- Qin, Z.Z.; Sander, M.S.; Rai, B.; Titahong, C.N.; Sudrungrot, S.; Laah, S.N.; Adhikari, L.M.; Carter, E.J.; Puri, L.; Codlin, A.J.; et al. Using artificial intelligence to read chest radiographs for tuberculosis detection: A multi-site evaluation of the diagnostic accuracy of three deep learning systems. Sci. Rep. 2019, 9, 15000. [Google Scholar] [CrossRef]
- Chry, M.; Smelyanskaya, M.; Ky, M.; Codlin, A.J.; Cazabon, D.; Tan Eang, M.; Creswell, J. Can the High Sensitivity of Xpert MTB/RIF Ultra Be Harnessed to Save Cartridge Costs? Results from a Pooled Sputum Evaluation in Cambodia. Trop. Med. Infect. Dis. 2020, 5, 27. [Google Scholar] [CrossRef]
- Sander, M.; Laah, S.; Titahong, C.; Lele, C.; Kinge, T.; de Jong, B.; Abena, J.-L.; Codlin, A.; Creswell, J. Systematic screening for tuberculosis among hospital outpatients in Cameroon: The role of screening and testing algorithms to improve case detection. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 15, 100095. [Google Scholar] [CrossRef]
- Systematic Screening for Active Tuberculosis: Principles and Recommendations; World Health Organization: Geneva, Switzerland, 2013.
| All Participants | Cough ≥ 2 Weeks | Cough < 2 Weeks and No Cough | |
|---|---|---|---|
| All Participants | |||
| Screened by chest X-ray (CXR) | 34,529 | 9430 | 25,099 |
| CXR abnormal | 4722 (13.7%) | 1515 (16.1%) | 3207 (12.8%) |
| Tested using the Xpert MTB/RIF assay (Xpert) | 2670 (56.5%) | 974 (64.3%) | 1696 (52.9%) |
| Xpert(+) TB detected | 256 (9.6%) | 128 (13.1%) | 128 (7.5%) |
| Xpert(+) TB prevalence rate | 741 | 1357 | 510 |
| Aged 15–54 Years | |||
| Screened by CXR | 12,896 | 3311 | 9585 |
| CXR abnormal | 1063 (8.2%) | 337 (10.2%) | 726 (7.6%) |
| Tested by Xpert | 664 (62.5%) | 253 (75.1%) | 411 (56.6%) |
| Xpert(+) TB detected | 95 (14.3%) | 52 (20.6%) | 43 (10.5%) |
| Xpert(+) TB prevalence rate | 737 | 1571 | 449 |
| Aged ≥ 55 Years | |||
| Screened by CXR | 21,633 | 6119 | 15,514 |
| CXR abnormal | 3659 (16.9%) | 1178 (19.3%) | 2481 (16.0%) |
| Tested by Xpert | 2006 (54.8%) | 721 (61.2%) | 1285 (51.8%) |
| Xpert(+) TB detected | 161 (8.0%) | 76 (10.5%) | 85 (6.6%) |
| Xpert(+) TB prevalence rate | 744 | 1242 | 548 |
| Males | |||
| Screened by CXR | 14,609 | 4409 | 10,200 |
| CXR abnormal | 2980 (20.4%) | 1023 (23.2%) | 1957 (19.2%) |
| Tested by Xpert | 1950 (65.4%) | 724 (70.8%) | 1226 (62.6%) |
| Xpert(+) TB detected | 216 (11.1%) | 104 (14.4%) | 112 (9.1%) |
| Xpert(+) TB prevalence rate | 1479 | 2359 | 1098 |
| Females | |||
| Screened by CXR | 19,920 | 5021 | 14,899 |
| CXR abnormal | 1742 (8.7%) | 492 (9.8%) | 1250 (8.4%) |
| Tested by Xpert | 720 (41.3%) | 250 (50.8%) | 470 (37.6%) |
| Xpert(+) TB detected | 40 (5.6%) | 24 (9.6%) | 16 (3.4%) |
| Xpert(+) TB prevalence rate | 201 | 478 | 107 |
| CXR Screens | AFB Tests | Xpert Tests | Estimated TB Yield | Marginal TB Yield | Total Diagnostic Costs | Cost per Bac(+) Case Detected | |
|---|---|---|---|---|---|---|---|
| Cough ≥ 2 weeks followed by acid-fast bacilli smear microscopy (AFB) | 0 | 9430 | 0 | 65 | - | 9915 | 153.12 |
| Any cough followed by AFB | 0 | 15,101 | 0 | 91 | +40.6% | 10,941 | 120.16 |
| Cough ≥ 2 weeks followed by Xpert MTB/RIF assay (Xpert) | 0 | 0 | 9430 | 128 | +97.7% | 113,070 | 883.36 |
| Chest X-ray (CXR) abnormal followed by AFB | 34,529 | 4722 | 0 | 130 | +100.0% | 57,620 | 444.92 |
| Any cough followed by Xpert | 0 | 0 | 15,101 | 180 | +178.0% | 176,131 | 978.51 |
| CXR abnormal followed by Xpert | 34,529 | 0 | 4722 | 256 * | +295.3% | 109,274 | 426.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.H.; Codlin, A.J.; Vo, L.N.Q.; Dao, T.; Tran, D.; Forse, R.J.; Vu, T.N.; Le, G.T.; Luu, T.; Do, G.C.; et al. An Evaluation of Programmatic Community-Based Chest X-ray Screening for Tuberculosis in Ho Chi Minh City, Vietnam. Trop. Med. Infect. Dis. 2020, 5, 185. https://doi.org/10.3390/tropicalmed5040185
Nguyen LH, Codlin AJ, Vo LNQ, Dao T, Tran D, Forse RJ, Vu TN, Le GT, Luu T, Do GC, et al. An Evaluation of Programmatic Community-Based Chest X-ray Screening for Tuberculosis in Ho Chi Minh City, Vietnam. Tropical Medicine and Infectious Disease. 2020; 5(4):185. https://doi.org/10.3390/tropicalmed5040185
Chicago/Turabian StyleNguyen, Lan Huu, Andrew J. Codlin, Luan Nguyen Quang Vo, Thang Dao, Duc Tran, Rachel J. Forse, Thanh Nguyen Vu, Giang Truong Le, Tuan Luu, Giang Chau Do, and et al. 2020. "An Evaluation of Programmatic Community-Based Chest X-ray Screening for Tuberculosis in Ho Chi Minh City, Vietnam" Tropical Medicine and Infectious Disease 5, no. 4: 185. https://doi.org/10.3390/tropicalmed5040185
APA StyleNguyen, L. H., Codlin, A. J., Vo, L. N. Q., Dao, T., Tran, D., Forse, R. J., Vu, T. N., Le, G. T., Luu, T., Do, G. C., Truong, V. V., Minh, H. D. T., Nguyen, H. H., Creswell, J., Caws, M., Nguyen, H. B., & Nguyen, N. V. (2020). An Evaluation of Programmatic Community-Based Chest X-ray Screening for Tuberculosis in Ho Chi Minh City, Vietnam. Tropical Medicine and Infectious Disease, 5(4), 185. https://doi.org/10.3390/tropicalmed5040185