New Imaging Parameters to Predict Sudden Cardiac Death in Chagas Disease
Abstract
1. Introduction
2. ECG/Holter Monitoring/Exercise Test
2.1. Eletrocardiogram (ECG)
2.2. 24 h Holter
2.3. Exercise Test
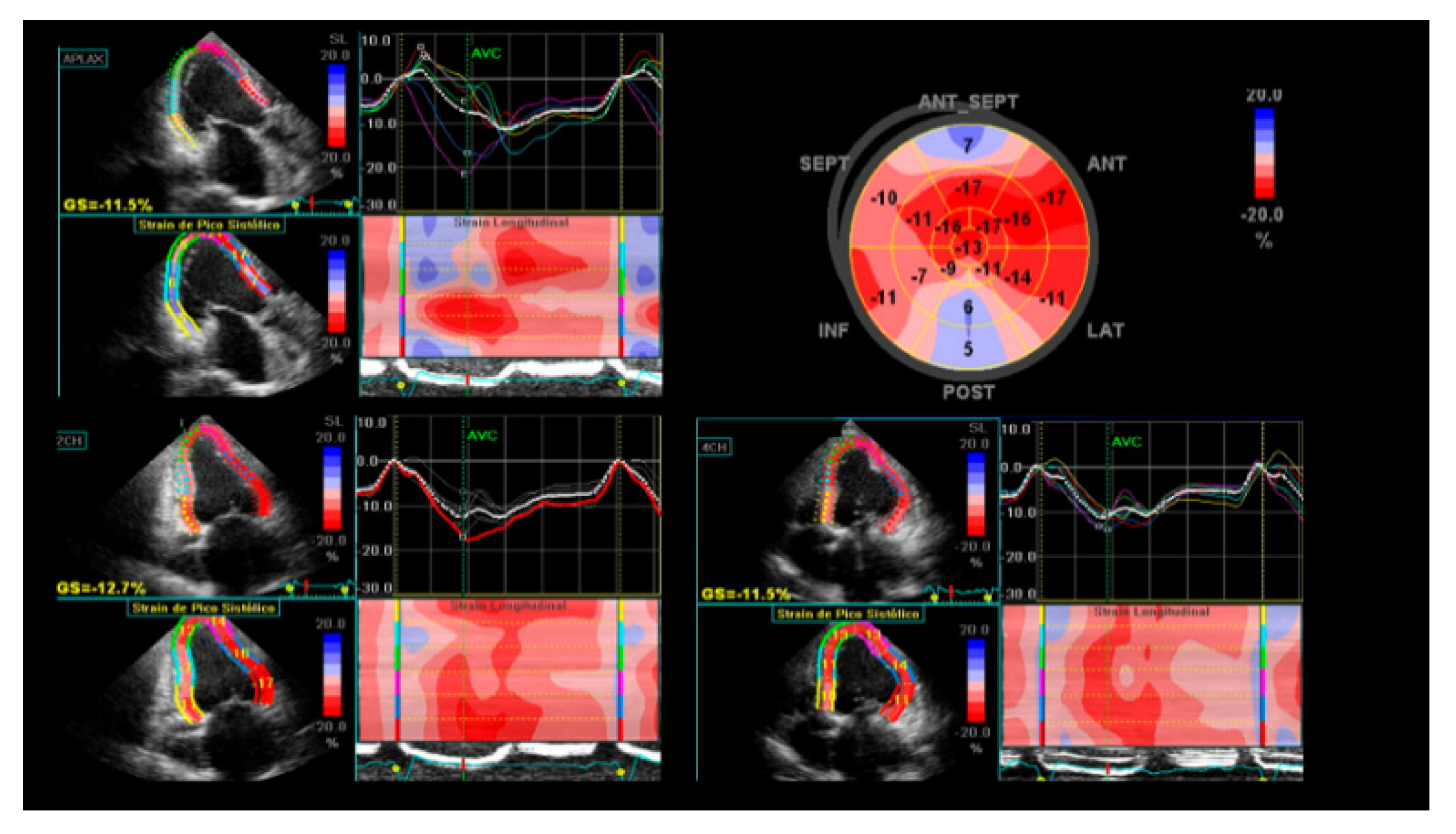
3. Echocardiography
4. Computed Tomography
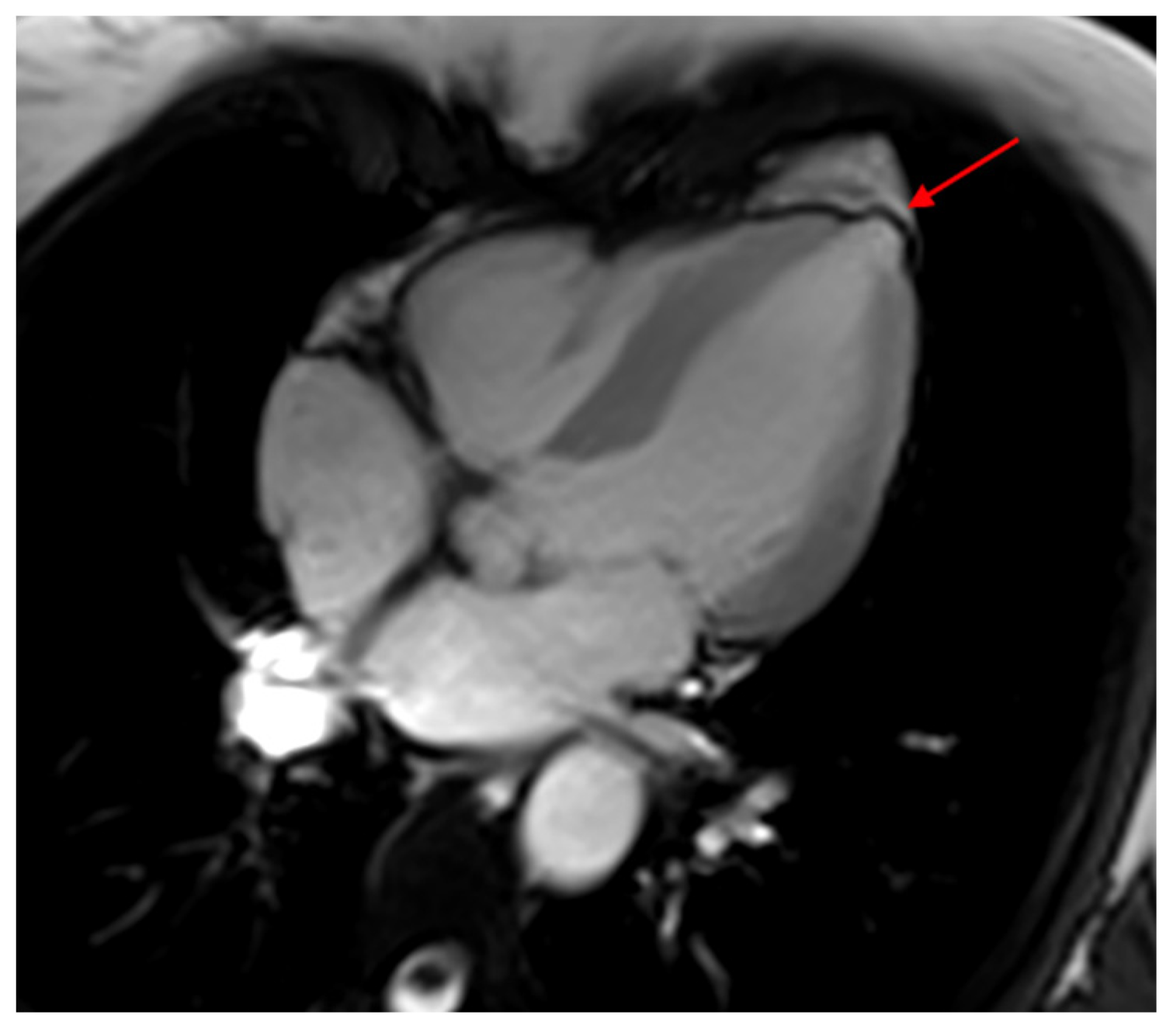
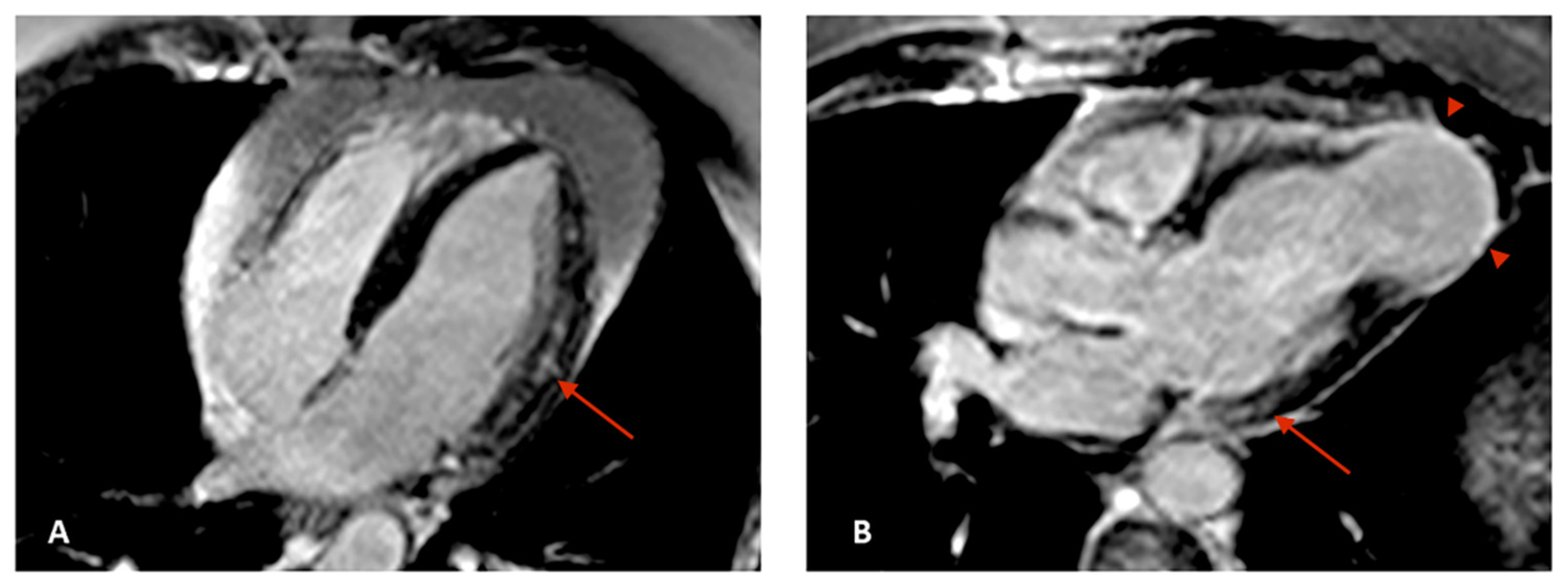
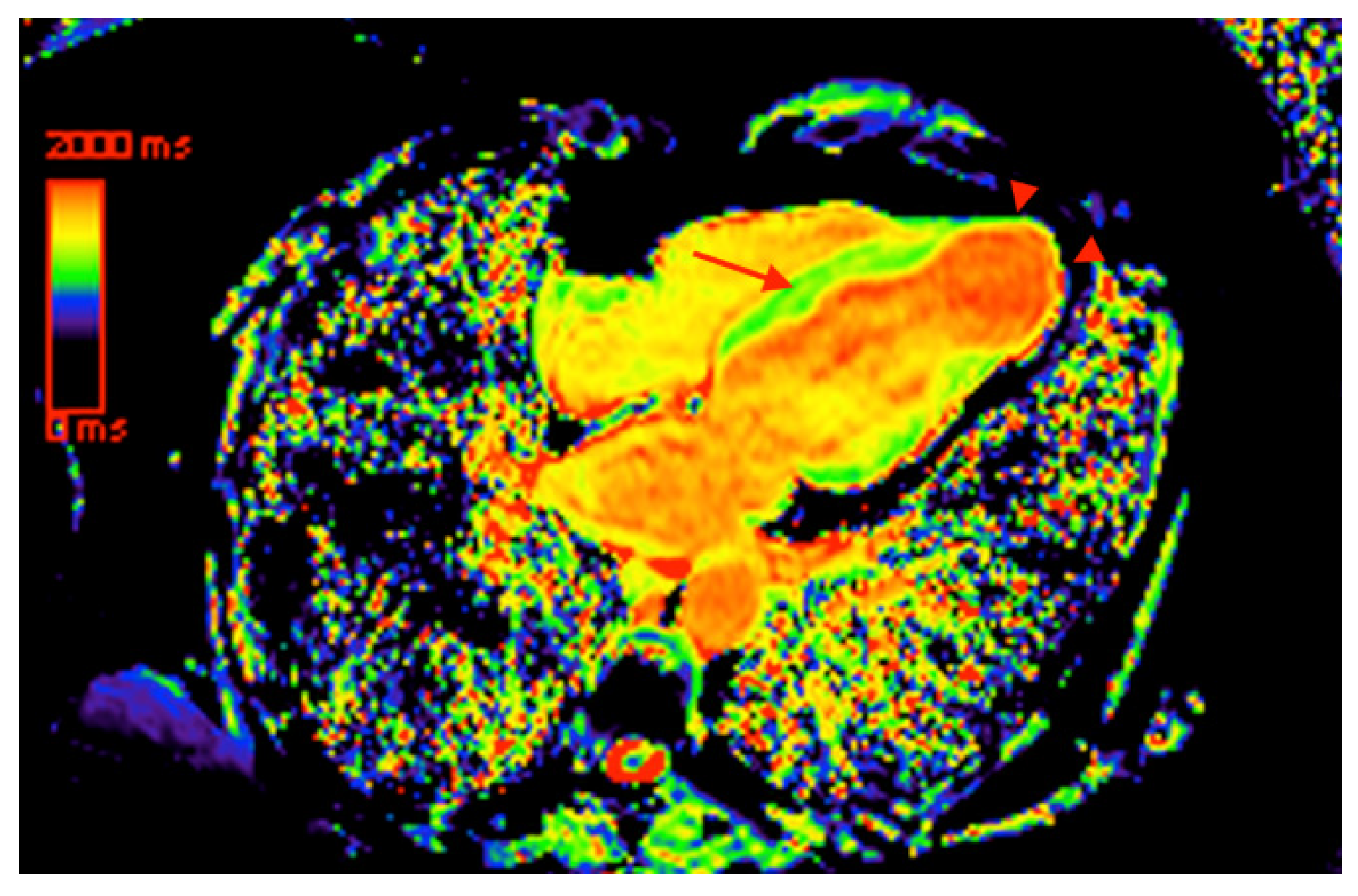
5. Cardiovascular Magnetic Resonance
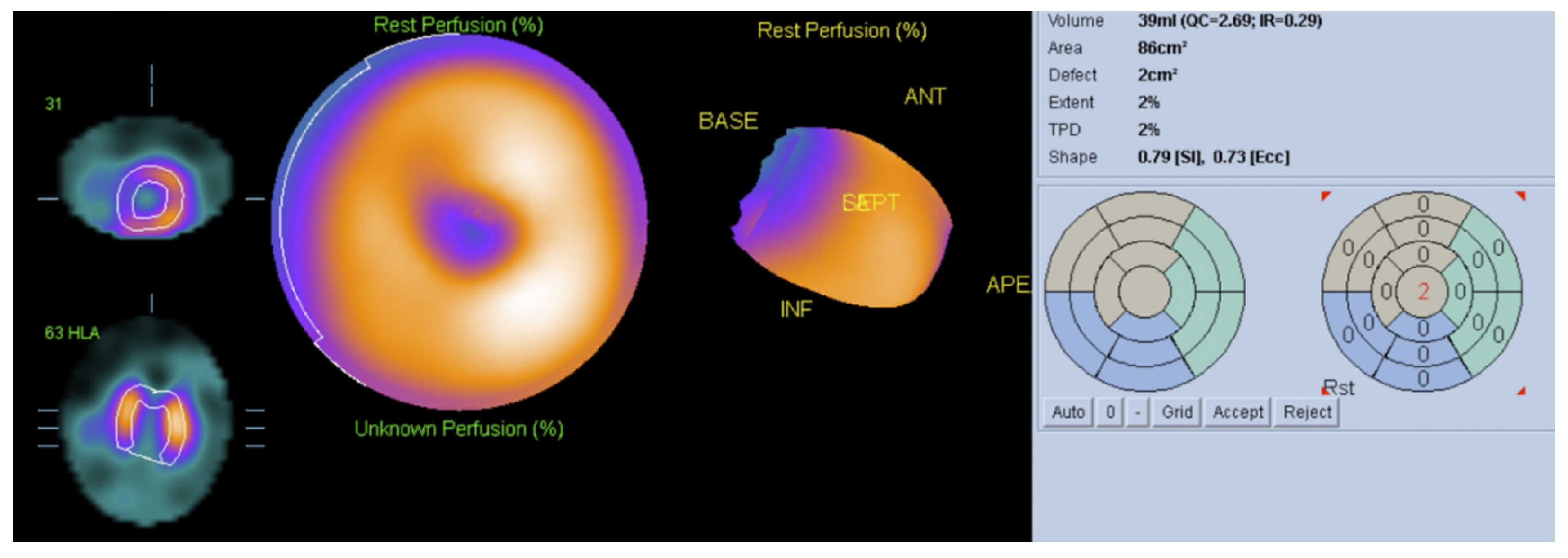
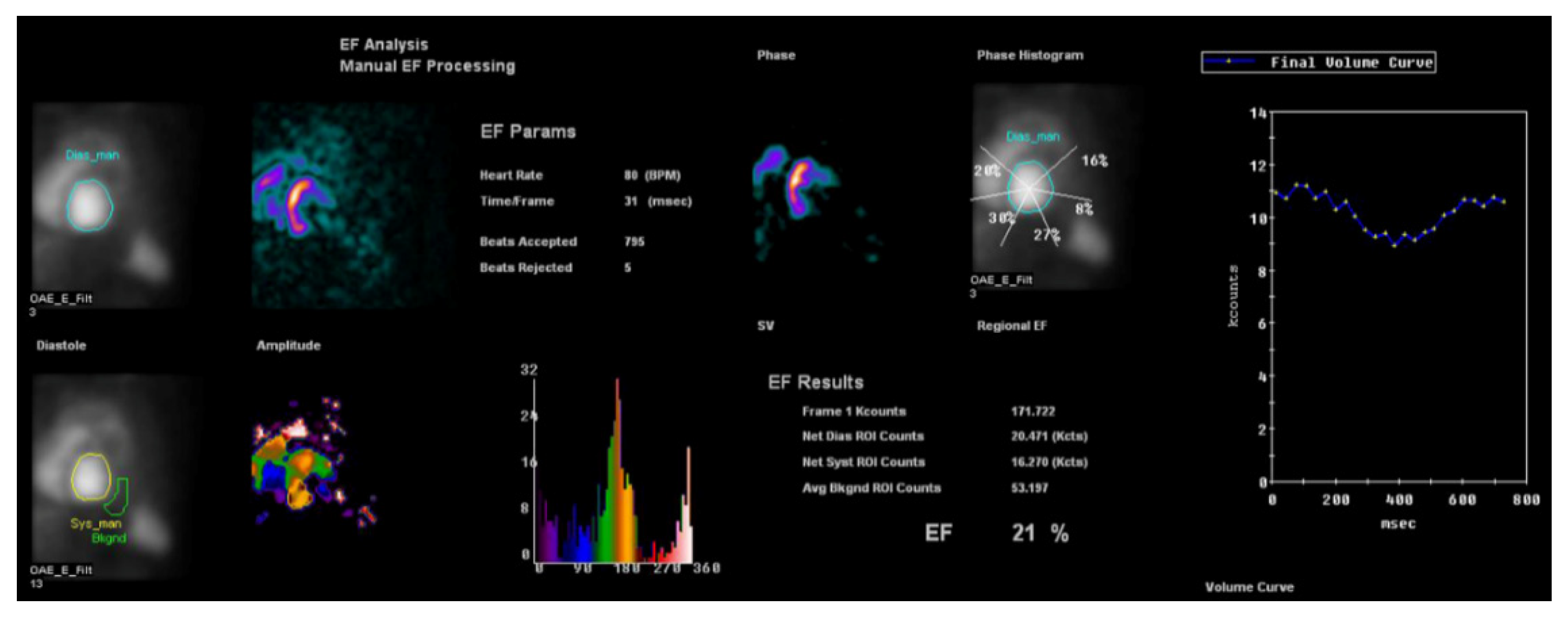
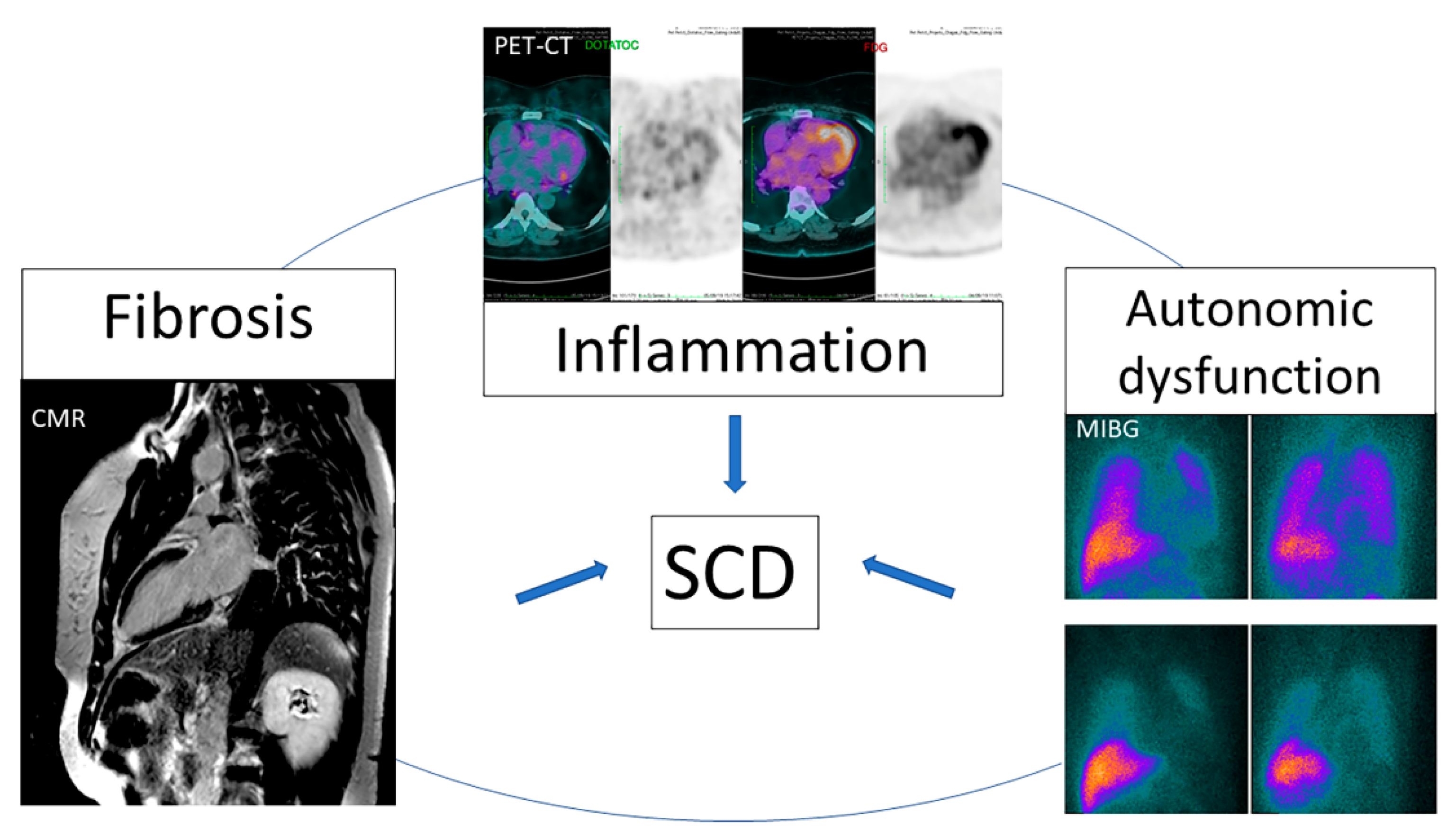
6. Nuclear Medicine
7. Conclusions
Funding
Conflicts of Interest
References
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverria, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Filho, H.A.; de Sousa, A.S.; de Holanda, M.T.; Haffner, P.M.A.; Atié, J.; do Brasil, P.E.A.A.; Hasslocher-Moreno, A.; Xavier, S.S. Valor prognóstico independente da taquicardia ventricular não-sustentada na fase crônica da Doença de Chagas. Rev. SOCERJ 2007, 20, 395–405. [Google Scholar]
- Andrade, J.; Marin-Neto, J.; Paola, A.; Vilas-Boas, F.; Oliveira, G.; Bacal, F.; Bocchi, E.; Almeida, D.; Fragata, F.A.; Moreira, M.C. I Latin American guidelines for the diagnosis and treatment of Chagas cardiomyopathy. Arq. Bras. Cardiol. 2011, 97, 1. [Google Scholar] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Little, W.C.; Xavier, S.S.; Rassi, S.G.; Rassi, A.G.; Rassi, G.G.; Hasslocher-Moreno, A.; Sousa, A.S.; Scanavacca, M.I. Development and validation of a risk score for predicting death in Chagas’ heart disease. N. Engl. J. Med. 2006, 355, 799–808. [Google Scholar] [CrossRef]
- Acquatella, H.; Asch, F.M.; Barbosa, M.M.; Barros, M.; Bern, C.; Cavalcante, J.L.; Echeverria Correa, L.E.; Lima, J.; Marcus, R.; Marin-Neto, J.A.; et al. Recommendations for Multimodality Cardiac Imaging in Patients with Chagas Disease: A Report from the American Society of Echocardiography in Collaboration with the InterAmerican Association of Echocardiography (ECOSIAC) and the Cardiovascular Imaging Department of the Brazilian Society of Cardiology (DIC-SBC). J. Am. Soc. Echocardiogr. 2018, 31, 3–25. [Google Scholar]
- Dias, J.C.; Ramos, A.N., Jr.; Gontijo, E.D.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.; Almeida, E.A.; Oliveira, W., Jr.; et al. Brazilian Consensus on Chagas Disease, 2015. Epidemiol. Serv. Saude 2016, 25, 7–86. [Google Scholar] [CrossRef]
- Xavier, S.; Sousa, A.; Hasslocher-Moreno, A. Application of the new classification of cardiac insufficiency (ACC/AHA) in chronic Chagas cardiopathy: A critical analysis of the survival curves. Rev. SOCERJ 2005, 18, 227–232. [Google Scholar]
- Shen, L.; Ramires, F.; Martinez, F.; Bodanese, L.C.; Echeverría, L.E.; Gómez, E.A.; Abraham, W.T.; Dickstein, K.; Køber, L.; Packer, M. Contemporary characteristics and outcomes in chagasic heart failure compared with other nonischemic and ischemic cardiomyopathy. Circ. Heart Fail. 2017, 10, e004361. [Google Scholar] [CrossRef]
- De Souza, A.C.J.; Salles, G.; Hasslocher-Moreno, A.M.; de Sousa, A.S.; do Brasil, P.E.A.A.; Saraiva, R.M.; Xavier, S.S. Development of a risk score to predict sudden death in patients with Chaga’s heart disease. Int. J. Cardiol. 2015, 187, 700–704. [Google Scholar] [CrossRef]
- Sternick, E.B.; Martinelli, M.; Sampaio, R.C.; Gerken, L.M.; Teixeira, R.A.; Scarpelli, R.; Scanavacca, M.; Nishioka, S.D.O.; Sosa, E. Sudden cardiac death in patients with chagas heart disease and preserved left ventricular function. J. Cardiovasc. Electrophysiol. 2006, 17, 113–116. [Google Scholar] [CrossRef]
- Moll-Bernardes, R.J.; Saraiva, R.M.; Sarmento de Oliveira, R.; Tavares Pinheiro, M.V.; Camargo, G.C.; Xavier de Brito, A.S.; Altino de Almeida, S.; Siqueira, F.P.; de Souza Nogueira Sardinha Mendes, F.; Barbosa, R.M. Case Report: Malignant Ventricular Arrhythmias Mimicking Acute Coronary Syndrome in Chagas Disease. Am. J. Trop. Med. Hyg. 2020, 102, 797–799. [Google Scholar] [CrossRef]
- Bestetti, R.B.; Dalbo, C.M.; Arruda, C.A.; Correia Filho, D.; Freitas, O.C. Predictors of sudden cardiac death for patients with Chagas’ disease: A hospital-derived cohort study. Cardiology 1996, 87, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Bestetti, R.B.; Cardinalli-Neto, A. Sudden cardiac death in Chagas’ heart disease in the contemporary era. Int. J. Cardiol. 2008, 131, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A., Jr.; Queiroz, C.; Carzola, F.; Dourado, J.; Carvalho, W. Variabilidade da freqüência cardíaca em pacientes com doença de Chagas. J. Card. Arrhythm. 2000, 13, 139–142. [Google Scholar]
- Nunes, M.C.P.; Badano, L.P.; Marin-Neto, J.A.; Edvardsen, T.; Fernandez-Golfin, C.; Bucciarelli-Ducci, C.; Popescu, B.A.; Underwood, R.; Habib, G.; Zamorano, J.L.; et al. Multimodality imaging evaluation of Chagas disease: An expert consensus of Brazilian Cardiovascular Imaging Department (DIC) and the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2018, 19, 459–460n. [Google Scholar] [CrossRef] [PubMed]
- Acquatella, H.; Schiller, N.B.; Puigbó, J.; Giordano, H.; Suárez, J.; Casal, H.; Arreaza, N.; Valecillos, R.; Hirschhaut, E. M-mode and two-dimensional echocardiography in chronic Chages’ heart disease. A clinical and pathologic study. Circulation 1980, 62, 787–799. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Barbosa, M.M.; Rocha, M.O.C.; Vidigal, D.F.; de Carvalho Bicalho Carneiro, R.; Araújo, R.D.; Palma, M.C.; de Barros, M.V.L.; Nunes, M.C.P. Early detection of left ventricular contractility abnormalities by two-dimensional speckle tracking strain in Chagas’ disease. Echocardiography 2014, 31, 623–630. [Google Scholar] [CrossRef]
- García-Álvarez, A.; Sitges, M.; Regueiro, A.; Poyatos, S.; Pinazo, M.J.; Posada, E.; Bijnens, B.; Heras, M.; Gascon, J.; Sanz, G. Myocardial deformation analysis in Chagas heart disease with the use of speckle tracking echocardiography. J. Card. Fail. 2011, 17, 1028–1034. [Google Scholar] [CrossRef]
- Gomes, V.A.; Alves, G.F.; Hadlich, M.; Azevedo, C.F.; Pereira, I.M.; Santos, C.R.; Brasil, P.E.; Sangenis, L.H.; Cunha, A.B.; Xavier, S.S.; et al. Analysis of Regional Left Ventricular Strain in Patients with Chagas Disease and Normal Left Ventricular Systolic Function. J. Am. Soc. Echocardiogr. 2016, 29, 679–688. [Google Scholar] [CrossRef]
- Silva Júnior, O.D.; Maeda, P.M.; Borges, M.C.C.; Melo, C.S.D.; Correia, D. One-year cardiac morphological and functional evolution following permanent pacemaker implantation in right ventricular septal position in chagasic patients. Rev. Soc. Bras. Med. Trop. 2012, 45, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.D.O.; Magalhães, L.P.D.; Santana, O.O.; Silva, L.B.D.; Simões, M.; Azevedo, D.O.D. Prevalence and prognostic value of ventricular dyssynchrony in Chagas cardiomyopathy. Arq. Bras. Cardiol. 2011, 96, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; McKenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Halley, C.M.; Carrigan, T.P.; Zysek, V.; Popovic, Z.B.; Setser, R.; Schoenhagen, P.; Starling, R.C.; Flamm, S.D.; Desai, M.Y. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: A delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc. Imaging 2009, 2, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.H.; Powell, A.J.; Geva, T. Myocardial Fibrosis in Congenital Heart Disease. Circ. J. 2016, 80, 1300–1307. [Google Scholar] [CrossRef]
- Senra, T.; Ianni, B.M.; Costa, A.C.; Mady, C.; Martinelli-Filho, M.; Kalil-Filho, R.; Rochitte, C.E. Long-term prognostic value of myocardial fibrosis in patients with chagas cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 2577–2587. [Google Scholar] [CrossRef]
- Regueiro, A.; García-Álvarez, A.; Sitges, M.; Ortiz-Pérez, J.T.; De Caralt, M.T.; Pinazo, M.J.; Posada, E.; Heras, M.; Gascón, J.; Sanz, G. Myocardial involvement in Chagas disease: Insights from cardiac magnetic resonance. Int. J. Cardiol. 2013, 165, 107–112. [Google Scholar] [CrossRef]
- Mello, R.P.D.; Szarf, G.; Schvartzman, P.R.; Nakano, E.M.; Espinosa, M.M.; Szejnfeld, D.; Fernandes, V.; Lima, J.A.; Cirenza, C.; De Paola, A.A. Delayed enhancement cardiac magnetic resonance imaging can identify the risk for ventricular tachycardia in chronic Chagas’ heart disease. Arq. Bras. Cardiol. 2012, 98, 421–430. [Google Scholar] [CrossRef]
- Volpe, G.J.; Moreira, H.T.; Trad, H.S.; Wu, K.C.; Braggion-Santos, M.F.; Santos, M.K.; Maciel, B.C.; Pazin-Filho, A.; Marin-Neto, J.A.; Lima, J.A. Left ventricular scar and prognosis in chronic chagas cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 2567–2576. [Google Scholar] [CrossRef]
- Torreão, J.A.; Ianni, B.M.; Mady, C.; Naia, E.; Rassi, C.H.; Nomura, C.; Parga, J.R.; Avila, L.F.; Ramires, J.A.; Kalil-Filho, R. Myocardial tissue characterization in Chagas’ heart disease by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2015, 17, 97. [Google Scholar] [CrossRef]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A. T1 mapping by CMR imaging: From histological validation to clinical implication. JACC Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.V.; Pintya, A.O.; Bromberg-Marin, G.; Sarabanda, Á.V.; Antloga, C.M.; Pazin-Filho, A.; Maciel, B.C.; Marin-Neto, J.A. Relation of regional sympathetic denervation and myocardial perfusion disturbance to wall motion impairment in Chagas’ cardiomyopathy. Am. J. Cardiol. 2000, 86, 975–981. [Google Scholar] [CrossRef]
- Peix, A.; Garcia, R.; Sanchez, J.; Cabrera, L.O.; Padron, K.; Vedia, O.; Choque, H.V.; Fraga, J.; Bandera, J.; Hernandez-Canero, A. Myocardial perfusion imaging and cardiac involvement in the indeterminate phase of Chagas disease. Arq. Bras. Cardiol. 2013, 100, 114–117. [Google Scholar] [CrossRef]
- Miranda, C.H.; Figueiredo, A.B.; Maciel, B.C.; Marin-Neto, J.A.; Simoes, M.V. Sustained ventricular tachycardia is associated with regional myocardial sympathetic denervation assessed with 123I-metaiodobenzylguanidine in chronic Chagas cardiomyopathy. J. Nucl. Med. 2011, 52, 504–510. [Google Scholar] [CrossRef]
- Landesmann, M.C.P.; da Fonseca, L.M.B.; Pereira, B.D.B.; do Nascimento, E.M.; Rosado-de-Castro, P.H.; de Souza, S.A.L.; de SL Lima, R.; Pedrosa, R.C. Iodine-123 metaiodobenzylguanidine cardiac imaging as a method to detect early sympathetic neuronal dysfunction in chagasic patients with normal or borderline electrocardiogram and preserved ventricular function. Clin. Nucl. Med. 2011, 36, 757–761. [Google Scholar] [CrossRef]
- Gadioli, L.P.; Miranda, C.H.; Pintya, A.O.; de Figueiredo, A.B.; Schmidt, A.; Maciel, B.C.; Marin-Neto, J.A.; Simoes, M.V. The severity of ventricular arrhythmia correlates with the extent of myocardial sympathetic denervation, but not with myocardial fibrosis extent in chronic Chagas cardiomyopathy: Chagas disease, denervation and arrhythmia. J. Nucl. Cardiol. 2018, 25, 75–83. [Google Scholar] [CrossRef]
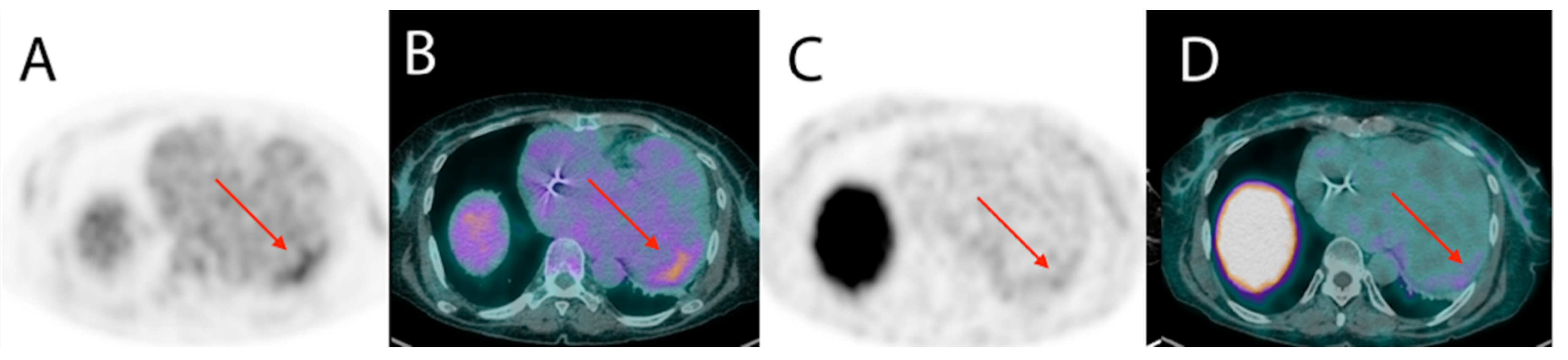
- James, O.G.; Christensen, J.D.; Wong, T.Z.; Borges-Neto, S.; Koweek, L.M. Utility of FDG PET/CT in inflammatory cardiovascular disease. Radiographics 2011, 31, 1271–1286. [Google Scholar] [CrossRef]
- Blankstein, R.; Osborne, M.; Naya, M.; Waller, A.; Kim, C.K.; Murthy, V.L.; Kazemian, P.; Kwong, R.Y.; Tokuda, M.; Skali, H.; et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J. Am. Coll. Cardiol. 2014, 63, 329–336. [Google Scholar] [CrossRef]
- Garg, G.; Cohen, S.; Neches, R.; Travin, M.I. Cardiac (18)F-FDG uptake in chagas disease. J. Nucl. Cardiol. 2016, 23, 321–325. [Google Scholar] [CrossRef]
- Shapiro, H.; Meymandi, S.; Shivkumar, K.; Bradfield, J.S. Cardiac inflammation and ventricular tachycardia in Chagas disease. HeartRhythm Case Rep. 2017, 3, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Moll-Bernardes, R.J.; de Oliveira, R.S.; de Brito, A.S.X.; de Almeida, S.A.; Rosado-de-Castro, P.H.; de Sousa, A.S. Can PET/CT be useful in predicting ventricular arrhythmias in Chagas Disease? J. Nucl. Cardiol. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gormsen, L.C.; Haraldsen, A.; Kramer, S.; Dias, A.H.; Kim, W.Y.; Borghammer, P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016, 6, 52. [Google Scholar] [CrossRef] [PubMed]

| Stage | ECG | Echocardiogram | Heart Failure |
|---|---|---|---|
| A | abnormal | Normal | Absent |
| B1 | abnormal | abnormal, LVEF ≥45% | Absent |
| B2 | abnormal | abnormal, LVEF <45% | Absent |
| C | abnormal | Abnormal | Reversible |
| D | abnormal | Abnormal | Refractory |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moll-Bernardes, R.J.; Rosado-de-Castro, P.H.; Camargo, G.C.; Mendes, F.S.N.S.; Brito, A.S.X.; Sousa, A.S. New Imaging Parameters to Predict Sudden Cardiac Death in Chagas Disease. Trop. Med. Infect. Dis. 2020, 5, 74. https://doi.org/10.3390/tropicalmed5020074
Moll-Bernardes RJ, Rosado-de-Castro PH, Camargo GC, Mendes FSNS, Brito ASX, Sousa AS. New Imaging Parameters to Predict Sudden Cardiac Death in Chagas Disease. Tropical Medicine and Infectious Disease. 2020; 5(2):74. https://doi.org/10.3390/tropicalmed5020074
Chicago/Turabian StyleMoll-Bernardes, Renata J., Paulo Henrique Rosado-de-Castro, Gabriel Cordeiro Camargo, Fernanda Souza Nogueira Sardinha Mendes, Adriana S. X. Brito, and Andréa Silvestre Sousa. 2020. "New Imaging Parameters to Predict Sudden Cardiac Death in Chagas Disease" Tropical Medicine and Infectious Disease 5, no. 2: 74. https://doi.org/10.3390/tropicalmed5020074
APA StyleMoll-Bernardes, R. J., Rosado-de-Castro, P. H., Camargo, G. C., Mendes, F. S. N. S., Brito, A. S. X., & Sousa, A. S. (2020). New Imaging Parameters to Predict Sudden Cardiac Death in Chagas Disease. Tropical Medicine and Infectious Disease, 5(2), 74. https://doi.org/10.3390/tropicalmed5020074






