Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs
Abstract
1. Introduction
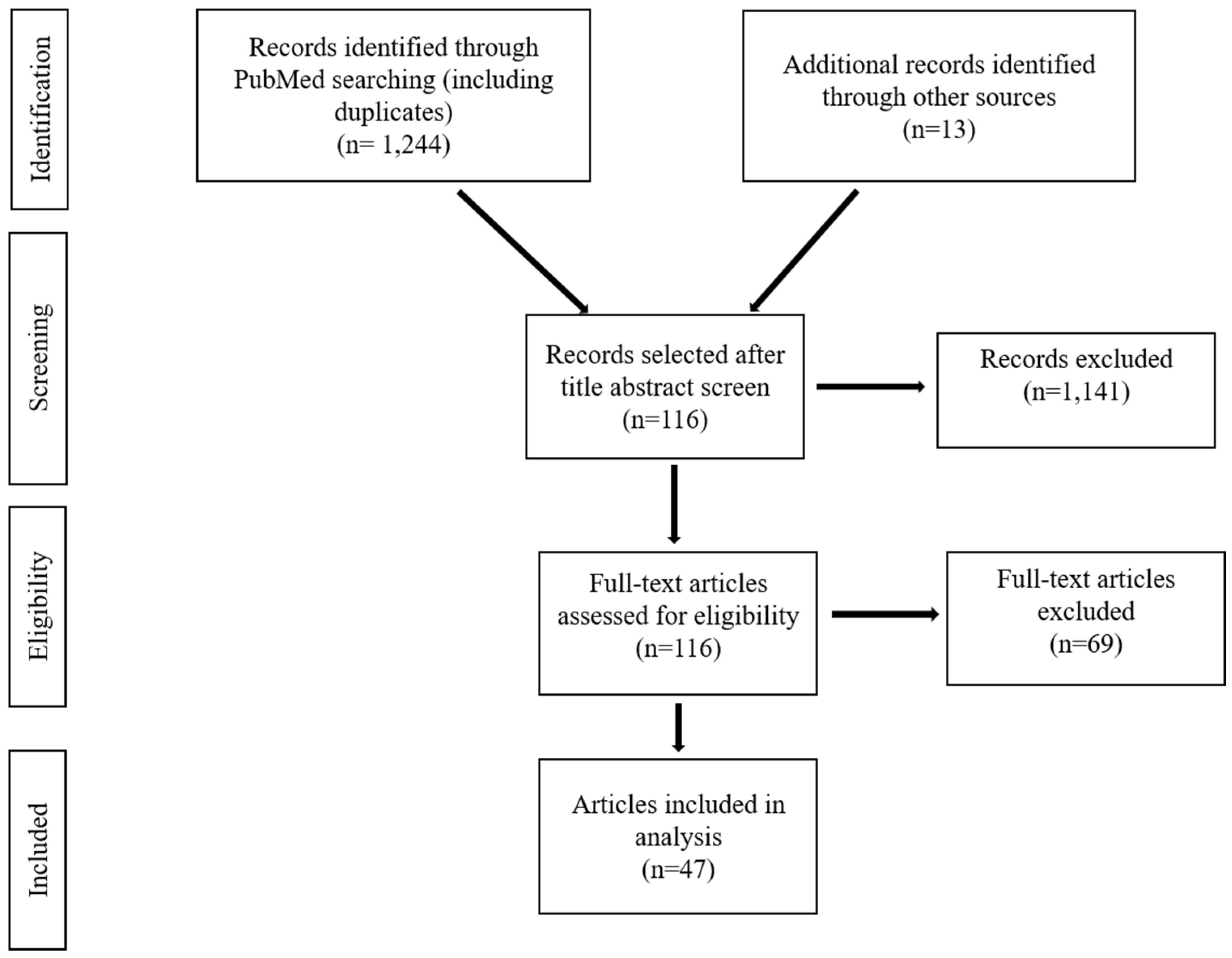
2. Materials and Methods
3. Results
3.1. Current Malaria Diagnostic Options
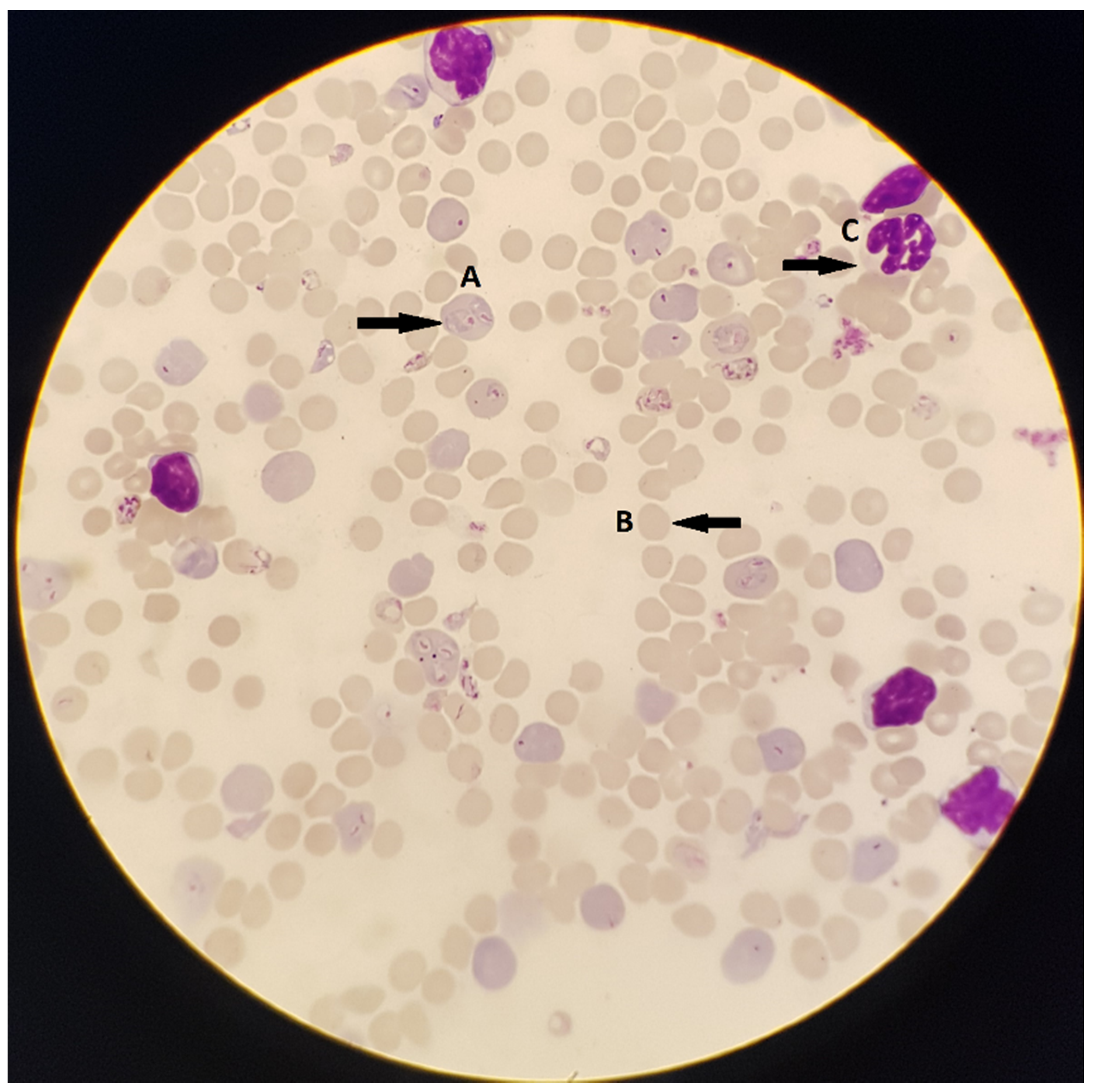
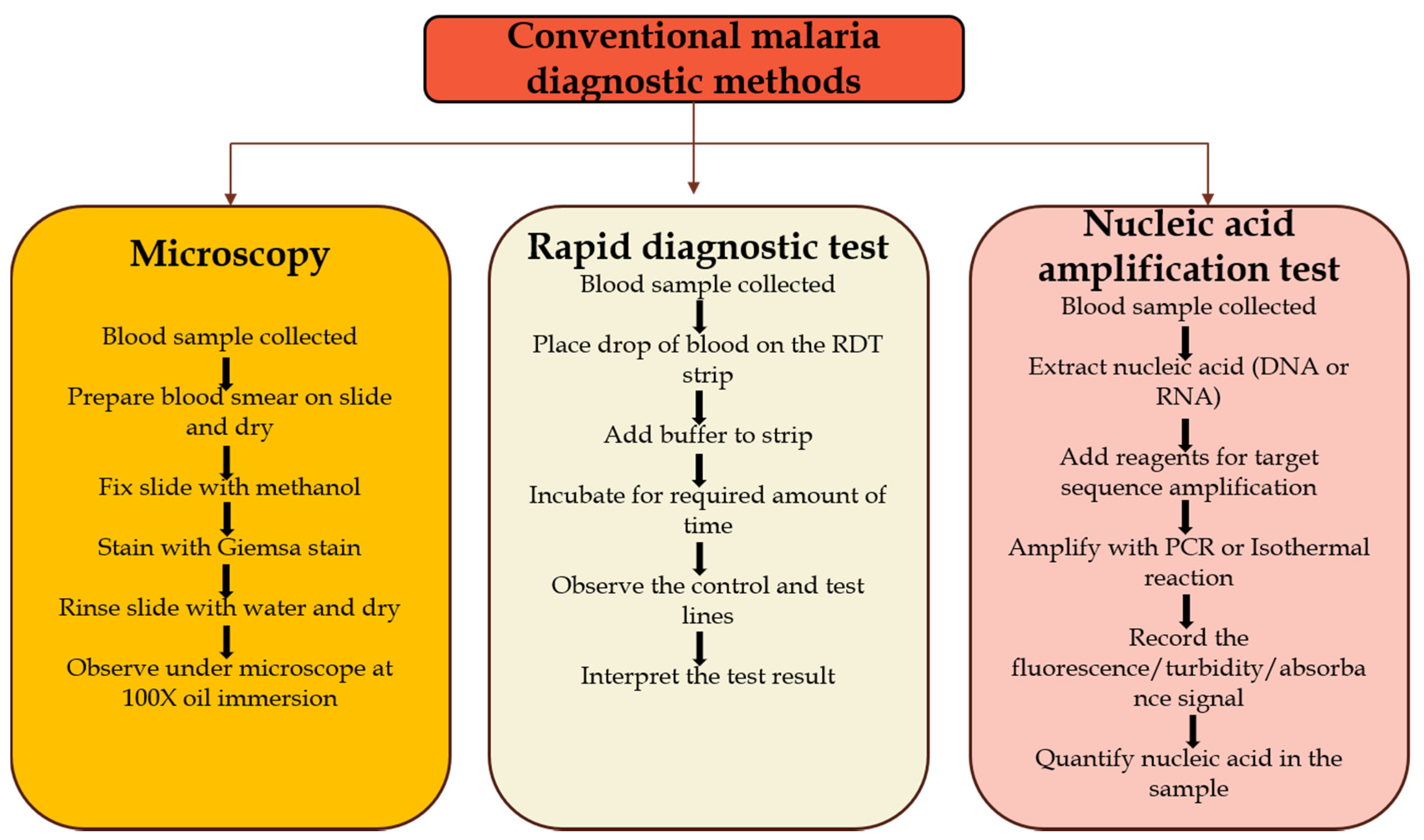
3.1.1. Microscopy
Advantages and Limitations
3.1.2. Rapid Diagnostic Test (RDT)
Advantages and Limitations
3.1.3. Polymerase Chain Reaction (PCR)
Advantages and Limitations
3.2. Novel Malaria Diagnostic Options under Development
3.2.1. Loop-mediated Isothermal Amplification (LAMP)
Advantages and Limitations
3.2.2. Nucleic Acid Sequence-Based Amplification (NASBA)
Advantages and Limitations
3.2.3. Isothermal Thermophilic Helicase-Dependent Amplification (tHDA)
Advantages and Limitations
3.2.4. Saliva-Based Test with Nucleic-Acid Amplification
Advantages and Limitations
3.2.5. Saliva-Based Plasmodium Protein Detection
Advantages and Limitations
3.2.6. Urine-Based Malaria Test
Advantages and Limitations
3.2.7. Transdermal Hemozoin Detection
Advantages and Limitations
3.3. Challenges
3.3.1. Malaria Diagnosis in Resource-limited Settings
3.3.2. Malaria Diagnosis among Symptomatic Carriers
3.3.3. Malaria Diagnosis during Pregnancy
3.3.4. Malaria Diagnosis to Reduce Under-five Mortality
3.3.5. Malaria Diagnosis in Elimination Settings
4. Conclusions and Future Goals
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019. 2019. Available online: https://www.who.int/publications-detail/world-malaria-report-2019 (accessed on 16 January 2020).
- WHO. Countries and Territories Certified Malaria-Free by WHO. 2019. Available online: https://www.who.int/malaria/areas/elimination/malaria-free-countries/en/ (accessed on 18 September 2019).
- Mahmoudi, S.; Keshavarz, H. Efficacy of phase 3 trial of RTS, S/AS01 malaria vaccine: The need for an alternative development plan. Hum. Vaccines Immunother. 2017, 13, 2098–2101. [Google Scholar] [CrossRef]
- Ashley, E.A.; Phyo, A.P.; Woodrow, C.J. Malaria. Lancet 2018, 391, 21–27. [Google Scholar] [CrossRef]
- Singh, B.; Daneshvar, C. Human infections and detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 2013, 26, 165–184. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Treatment of Malaria. Third edition. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf;jsessionid=0701D7687C6100D4636C9D15714D7544?sequence=1 (accessed on 18 September 2019).
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 22–28. [Google Scholar]
- WHO. Global Technical Strategy for Malaria 2016–2030. 2015. Available online: https://www.who.int/malaria/publications/atoz/9789241564991/en/ (accessed on 20 September 2019).
- Feachem, R.G.; Chen, I.; Akbari, O.; Bertozzi-Villa, A.; Bhatt, S.; Binka, F.; Boni, M.F.; Buckee, C.; Dieleman, J.; Dondorp, P.; et al. Malaria Eradication within a generation. Lancet Comm. 2019, 394, 1056–1112. [Google Scholar] [CrossRef]
- Mathison, B.; Pritt, B. Update on Malaria Diagnostics and Test Utilization. J. Clin. Microbiol. 2017, 55, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Cordray, M.S.; Richards-Kortum, R.R. Emerging nucleic acid-based tests for point-of-care detection of malaria. Am. J. Trop. Med. Hyg. 2012, 87, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, N.; Klapperich, C.M.; Cabodi, M. Towards lab-on-a-chip diagnostics for malaria elimination. Lab. Chip 2018, 18, 75–94. [Google Scholar] [CrossRef]
- Pham, N.M.; Karlen, W.; Beck, H.P.; Delamarche, E. Malaria and the ‘last’ parasite: How can technology help? Malar. J. 2018, 17, 260. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. Malaria rapid diagnostic tests. Clin. Infect. Dis. 2012, 54, 1637–1641. [Google Scholar] [CrossRef]
- Amir, A.; Cheong, F.W.; De Silva, J.R.; Lau, Y.L. Diagnostic tools in childhood malaria. Parasites Vectors 2018, 11, 53. [Google Scholar] [CrossRef]
- Mouatcho, J.C.; Goldring, J.P. Malaria rapid diagnostic tests: Challenges and prospects. J. Med. Microbiol. 2013, 62, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Mukkala, A.N.; Kwan, J.; Lau, R.; Harris, D.; Kain, D.; Boggild, A.K. An Update on Malaria Rapid Diagnostic Tests. Curr. Infect. Dis. Rep. 20 2018. [Google Scholar] [CrossRef] [PubMed]
- WHO. Malaria Rapid Diagnostic Test Performance: Summary Results of WHO Product Testing of Malaria RDTs: Round 1–7 (2008–2016). 2017. Available online: https://www.who.int/malaria/publications/atoz/978924151268/en/ (accessed on 11 October 2019).
- Cunningham, J.; Jones, S.; Gatton, M.L.; Barnwell, J.W.; Cheng, Q.; Chiodini, P.L.; Glenn, J.; Incardona, S.; Kosack, C.; Luchavez, J.; et al. A review of the WHO malaria rapid diagnostic test product testing programme (2008–2018): Performance, procurement and policy. Malar. J. 2019, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Abba, K.; Deeks, J.J.; Olliaro, P.; Naing, C.M.; Jackson, S.M.; Takwoingi, Y.; Glenn, J.; Incardona, S.; Kosack, C.; Luchavez, J. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst. Rev. 2011, CD008122. [Google Scholar] [CrossRef] [PubMed]
- Oriero, E.; Jacobs, J.; Van Geertruyden, J.; Nwakanma, D.; D’Alessandro, U. Molecular-based isothermal tests for field diagnosis of malaria and their potential contribution to malaria elimination. J. Antimicrob. Chemother. 2015, 70, 2–13. [Google Scholar] [CrossRef]
- Vasoo, S.; Pritt, B. Molecular Diagnostics and Parasitic Disease. Clin. Lab. Med. 2013, 33, 461–503. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Korevaar, D.; Leeflang, M.; Mens, P. Molecular malaria diagnostics: A systematic review and meta-analysis. Crit. Rev. Clin. Lab. Sci. 2015, 53, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, K.V.; Kumar, S.; Swaraj, S.; Neethirajan, S. Advances in biosensors and optical assays for diagnosis and detection of malaria. Biosens. Bioelectron. 2018, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Abdul-Ghani, R.; Al-Mekhlafi, A.; Karanis, P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: Would it come to clinical reality as a point-of-care test? Acta Trop. 2012, 122, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Polley, S.D.; Mori, Y.; Watson, J.; Perkins, M.D.; González, I.J.; Notomi, T.; Chiodini, P.L.; Sutherland, C.J. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J. Clin. Microbiol. 2010, 48, 2866–2871. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, N.; Gopalakrishnan, A.; Ginocchio, C.; Manji, R.; Bythrow, M.; Lemieux, B.; Kong, H. Detection and species identification of malaria parasites by isothermal tHDA amplification directly from human blood without sample preparation. J. Mol. Diagn. 2013, 15, 634–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mfuh, K.O.; Yunga, S.T.; Esemu, L.F.; Bekindaka, O.N.; Yonga, J.; Djontu, J.C.; Calixt, D.; Mbakop; Taylor, D.W.; Nerurkar, V.R.; et al. Detection of Plasmodium falciparum DNA in saliva samples stored at room temperature: Potential for a non-invasive saliva-based diagnostic test for malaria. Malar. J. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, D.P.; Gupta, R.; Savargaonkar, D.; Singh, O.P.; Nanda, N.; Bhatt, R.M.; Valecha, N. Comparison of three PCR-based assays for the non-invasive diagnosis of malaria: Detection of Plasmodium parasites in blood and saliva. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1631–1639. [Google Scholar] [CrossRef]
- Ongagna-Yhombi, S.Y.; Corstjens, P.; Geva, E.; Abrams, W.R.; Barber, C.A.; Malamud, D.; Mharakurwa, S. Improved assay to detect Plasmodium falciparum using an uninterrupted, semi-nested PCR and quantitative lateral flow analysis. Malar. J. 2013, 12. [Google Scholar] [CrossRef]
- Gbotosho, G.O.; Happi, C.T.; Folarin, O.; Keyamo, O.; Sowunmi, A.; Oduola, A.M. Rapid detection of lactate dehydrogenase and genotyping of Plasmodium falciparum in saliva of children with acute uncomplicated malaria. Am. J. Trop. Med. Hyg. 2010, 83, 496–501. [Google Scholar] [CrossRef]
- Ouattara, A.; Doumbo, S.; Saye, R.; Beavogui, A.H.; Traoré, B.; Djimdé, A.; Niangaly, A.; Kayentao, K.; Diallo, M.; Doumbo, O.K.; et al. Use of a pLDH-based dipstick in the diagnostic and therapeutic follow-up of malaria patients in Mali. Malar. J. 2011, 10. [Google Scholar] [CrossRef]
- Tao, D.; McGill, B.; Hamerly, T.; Kobayashi, T.; Khare, P.; Dziedzic, A.; Leski, T.; Holtz, A.; Shull, B.; Jedlicka, A.E.; et al. A saliva-based rapid test to quantify the infectious subclinical malaria parasite reservoir. Sci. Transl. Med. 2019, 11, eaan4479. [Google Scholar] [CrossRef]
- Oyibo, W.A.; Ezeigwe, N.; Ntadom, G.; Oladosu, O.O.; Rainwater-Loveth, K.; O’Meara, W.; Okpokoro, E.; Brieger, W. Multicenter Pivotal Clinical Trial of Urine Malaria Test for Rapid Diagnosis of Plasmodium falciparum Malaria. J. Clin. Microbiol. 2016, 55, 253–263. [Google Scholar] [CrossRef][Green Version]
- Fydor Biotechnologies. 2019. Available online: https://www.fyodorbio.com/ (accessed on 28 September 2019).
- Lukianova-Hleb, E.Y.; Campbell, K.M.; Constantinou, P.E.; Braam, J.; Olson, J.S.; Ware, R.E.; Sullivan, D.J., Jr.; Lapotko, D.O. Hemozoin-generated vapor nanobubbles for transdermal reagent- and needle-free detection of malaria. Proc. Natl. Acad. Sci. USA 2014, 111, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.; Bezek, S.; Szigeti, R.; Khodarev, A.; Kelley, T.; Hurrell, A.; Berba, M.; Kumar, N.; D’Alessandro, U.; Lapotko, D. Transdermal Diagnosis of Malaria Using Vapor Nanobubbles. Emerg. Infect. Dis. 2015, 21, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Oguonu, T.; Shu, E.; Ezeonwu, B.U.; Lige, B.; Derrick, A.; Umeh, R.E.; Abgo, E. The performance evaluation of a urine malaria test (UMT) kit for the diagnosis of malaria in individuals with fever in south-east Nigeria: Cross-sectional analytical study. Malar. J. 2014, 13. [Google Scholar] [CrossRef]
- Grimberg, B.T.; Grimberg, K.O. Hemozoin detection may provide an inexpensive, sensitive, 1-minute malaria test that could revolutionize malaria screening. Expert Rev. Anti -Infect. Ther. 2016, 14, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Britton, S.; Cheng, Q.; McCarthy, J.S. Novel molecular diagnostic tools for malaria elimination: A review of options from the point of view of high-throughput and applicability in resource limited settings. Malar. J. 2016, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Clarke, S.E.; Gosling, R.; Hamainza, B.; Killeen, G.; Magill, A.; O’Meara, W.; Price, R.N.; Riley, E.M. “Asymptomatic” Malaria: A Chronic and Debilitating Infection That Should Be Treated. PLoS Med. 2016, 13, e1001942. [Google Scholar] [CrossRef]
- Galatas, B.; Bassat, Q.; Mayor, A. Malaria Parasites in the Asymptomatic: Looking for the Hay in the Haystack. Trends Parasitol. 2016, 32, 296–308. [Google Scholar] [CrossRef]
- Bousema, T.; Okell, L.; Felger, I.; Drakeley, C. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nat. Rev. Microbiol 2014, 12, 833–840. [Google Scholar] [CrossRef]
- Aguilar, R.; Machevo, S.; Menéndez, C.; Bardají, A.; Nhabomba, A.; Alonso, P.L.; Mayor, A. Comparison of placental blood microscopy and the ICT HRP2 rapid diagnostic test to detect placental malaria. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 573–575. [Google Scholar] [CrossRef]
- Fried, M.; Muehlenbachs, A.; Duffy, P.E. Diagnosing malaria in pregnancy: An update. Expert Rev. Anti -Infect. Ther. 2012, 10, 1177–1187. [Google Scholar] [CrossRef]
- WHO. Malaria Elimination Certification Process. 2018. Available online: https://www.who.int/malaria/areas/elimination/certification/en/ (accessed on 13 October 2019).
- WHO. The E-2020 Initiative of 21 Malaria-Eliminating Countries: 2019 Progress Report. 2019. Available online: https://www.who.int/malaria/publications/atoz/e-2020-progress-report-2019/en/ (accessed on 13 October 2019).
| Method | Microscopy | RDT | PCR |
|---|---|---|---|
| Target | N/A | pHRP-2, LDH, Aldolase | 18S rRNA |
| Sensitivity | 95% 1 | 85% to 94.8% | 98% to 100% |
| Specificity | 98% 1 | 95.2% to 99% | 88% to 94% |
| Limit of detection | 50–200 parasites per μL of blood | 50–100 parasites per μL of blood | 0.5–5 parasites per μL of blood |
| Advantages | Identification of parasite morphologies, species and stage | Fast and easy to use | Low limit of detection making it easier to detect low parasitemia, High throughput |
| Limitations | Requires trained personnel and microscopes | Mutation in pHRP-2 leading to false negatives, Unable to quantify parasitemia | Needs expensive instrument and is not able to quantify parasitemia |
| Cost per test | $0.12–$0.40 | $0.85 | $7–$8 |
| Time | 60 min | 15–20 min | 2 h |
| References | [11,12,13] | [11,12,13,20] | [11,21] |
| Method | LAMP | NASBA | tHDA | Saliva-Based Test with Nucleic-Acid Amplification | Saliva-based Test with Plasmodium Protein Detection | UMT | Transdermal Hemozoin Detection | |
|---|---|---|---|---|---|---|---|---|
| Target | 18S rRNA, Mitochondrial DNA | 18S mRNA | 18S rRNA | 18S rRNA, P. falciparum dihydrofolate reductase gene | pLDH | PSSP17 | pHRP-2 | Hemozoin |
| Sensitivity | 98.3% to 100% | 97.40–100% | 96.6% | 86.36–95% | 77.9–97.2% | 83% | 79% (overall) 93% (children under 5 years) 1 | Unknown |
| Specificity | 94.3% to 100% | 80.90–94% | 100% | 93–98.46% | 95.4% | Unknown | 89% (overall) 83% (children under 5 years) 1 | Unknown |
| Limit of detection | 1–5 parasites per μL of blood | 0.01–0.1 parasites/μL of blood | 200 parasites per μL of blood | 1–10 parasites per μL of blood | 1000 parasites per μL of blood | 1 to 10 gametocytes per μL of blood | 125 parasites/µL of blood | Able to detect 0.00034% parasitemia |
| Advantages | Low limit of detection, faster reaction time than PCR, no thermocycler needed, high throughput | No thermocycler needed | Uses whole blood directly | Non-invasive sample collection | Non-invasive sample collection | Non-invasive sample collection | Non-invasive, fast and easy to use | Non-invasive, no reagents, fast, very low limit of detection |
| Limitations | Easily susceptible to contamination | Requires highly trained personnel, expensive | Limit of detection is higher than that of other methods | Needs expensive instrument and is not able to quantify parasitemia | Test is unable to quantify parasitemia percentage | Test is unable to quantify parasitemia percentage | Lower sensitivity and specificity than other methods | Needs highly trained personnel |
| Cost per test | <$1–$5.31 | $5 to $20 | Similar to that of LAMP | Similar to that of PCR | Unknown | Unknown | $1.50 | Potentially more expensive than other methods due to the cost of instrument |
| Time | 30–60 min | 1–2 h | 1–2 h | 6 h | 22 min | 3 min to 30 min | 25 min | Seconds |
| References | [21,26,27] | [11,21] | [21,28] | [29,30,31] | [32,33] | [34] | [13,35,36] | [37,38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbanefo, A.; Kumar, N. Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Trop. Med. Infect. Dis. 2020, 5, 102. https://doi.org/10.3390/tropicalmed5020102
Mbanefo A, Kumar N. Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Tropical Medicine and Infectious Disease. 2020; 5(2):102. https://doi.org/10.3390/tropicalmed5020102
Chicago/Turabian StyleMbanefo, Afoma, and Nirbhay Kumar. 2020. "Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs" Tropical Medicine and Infectious Disease 5, no. 2: 102. https://doi.org/10.3390/tropicalmed5020102
APA StyleMbanefo, A., & Kumar, N. (2020). Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Tropical Medicine and Infectious Disease, 5(2), 102. https://doi.org/10.3390/tropicalmed5020102






