Mycetoma: The Spectrum of Clinical Presentation
Abstract
1. Introduction
2. Age, Gender and Occupation in Mycetoma
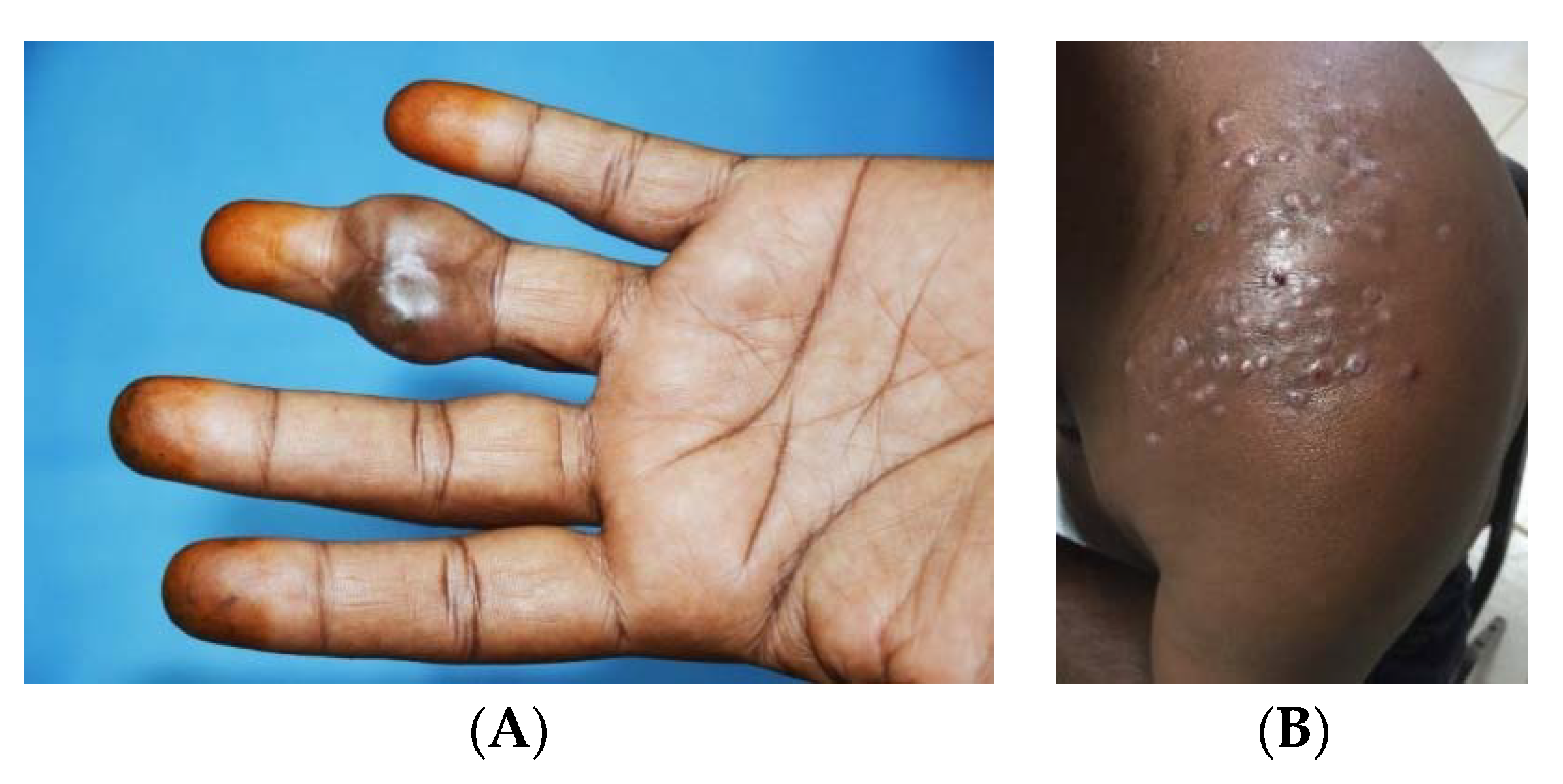
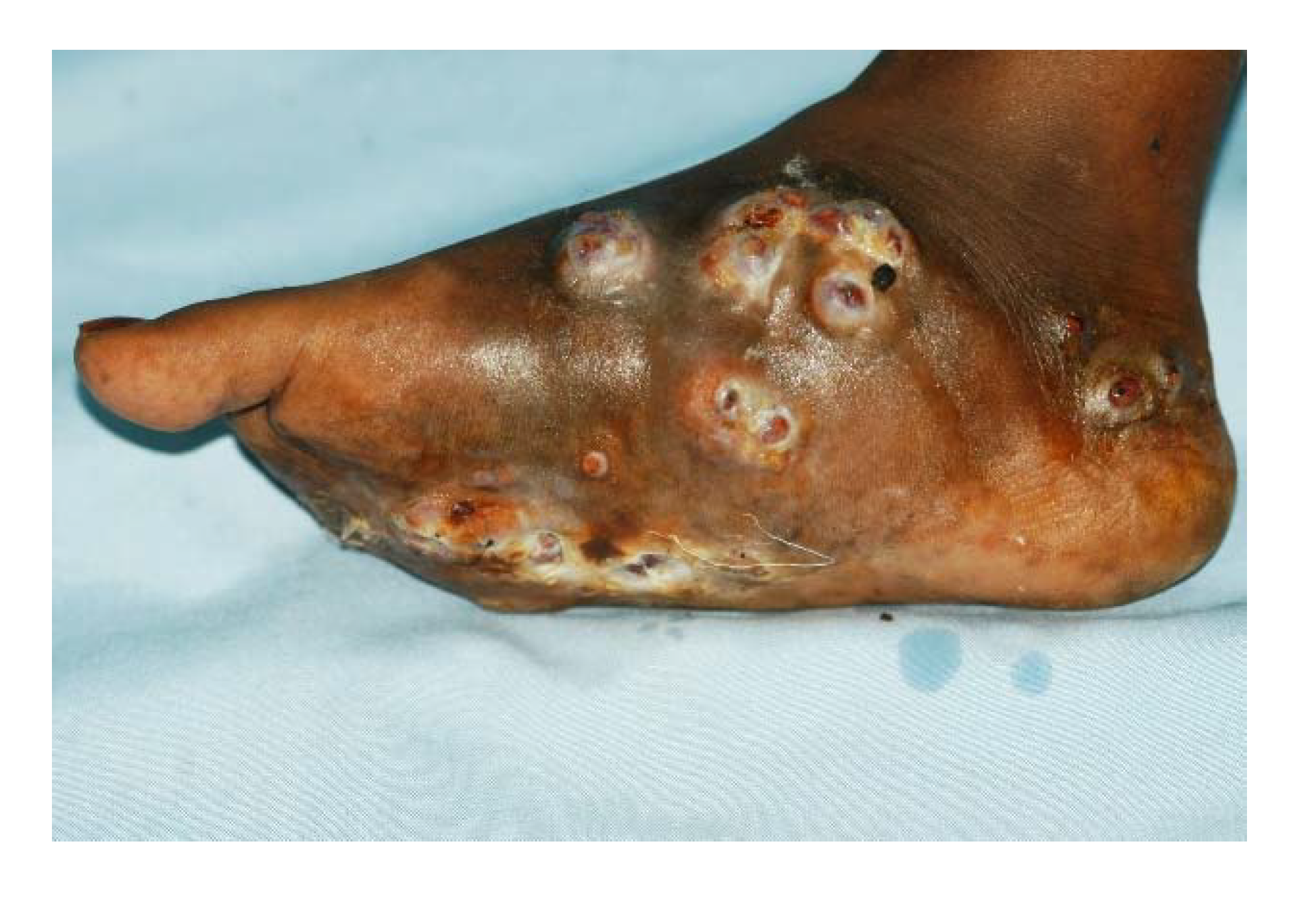
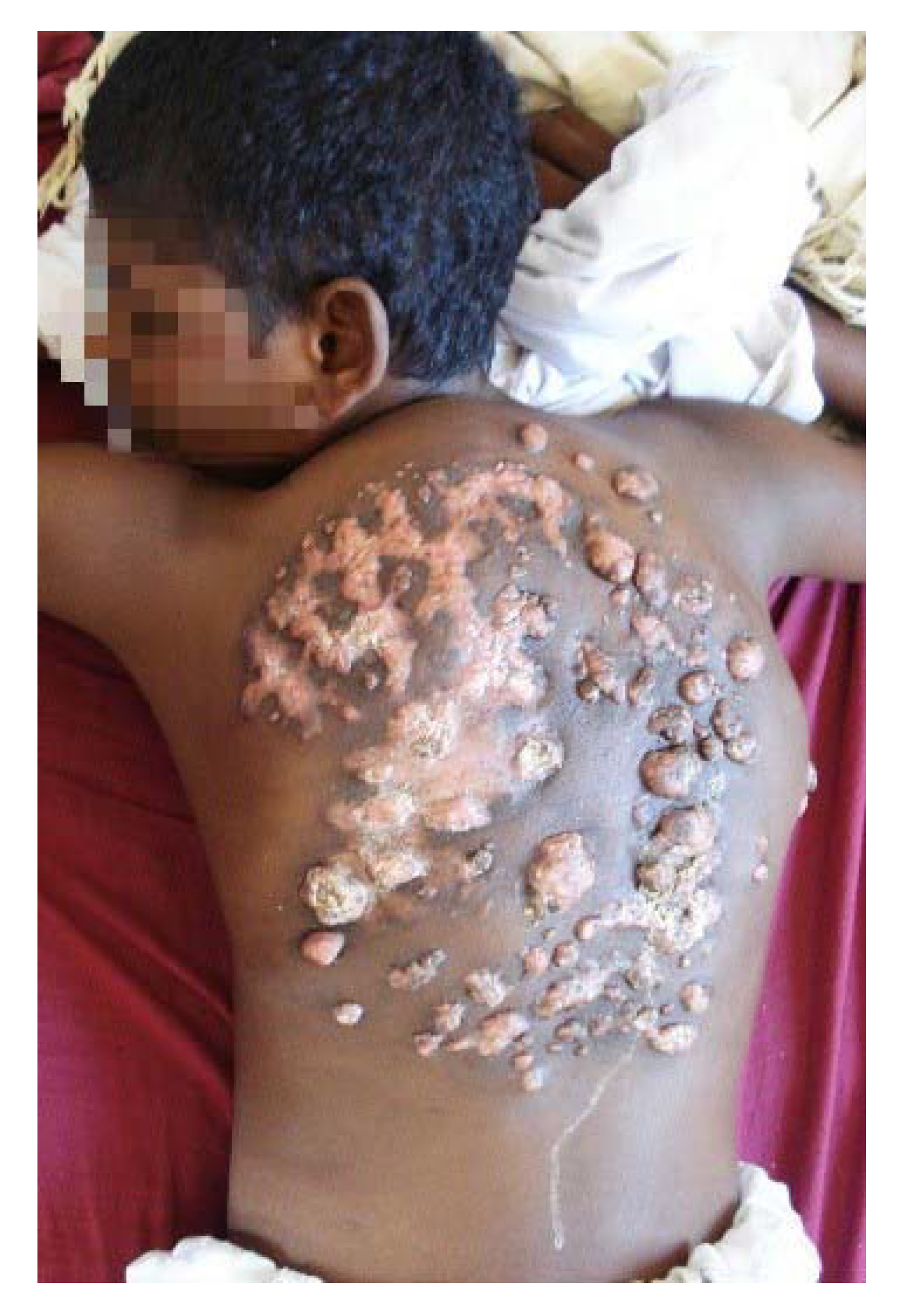
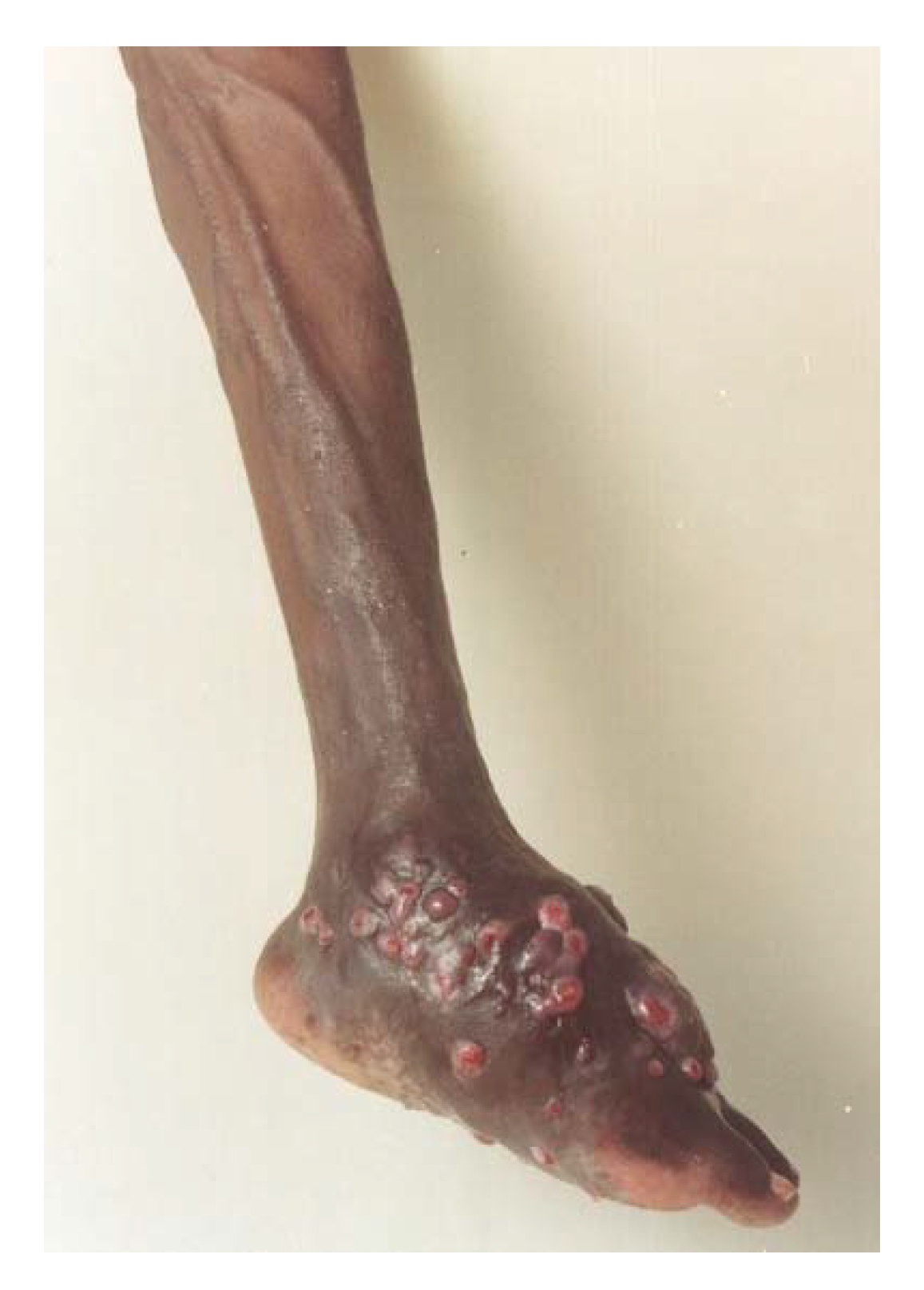
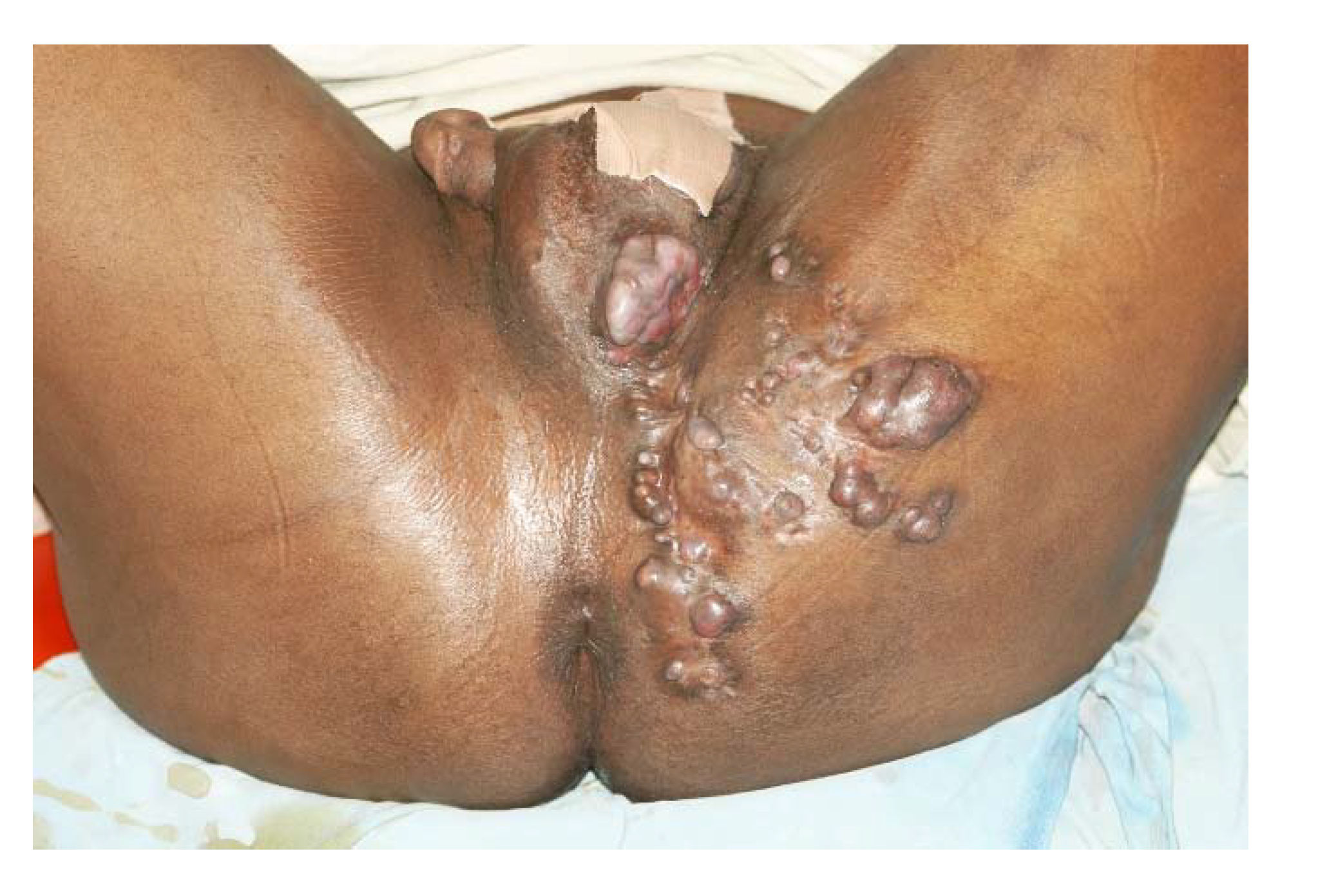
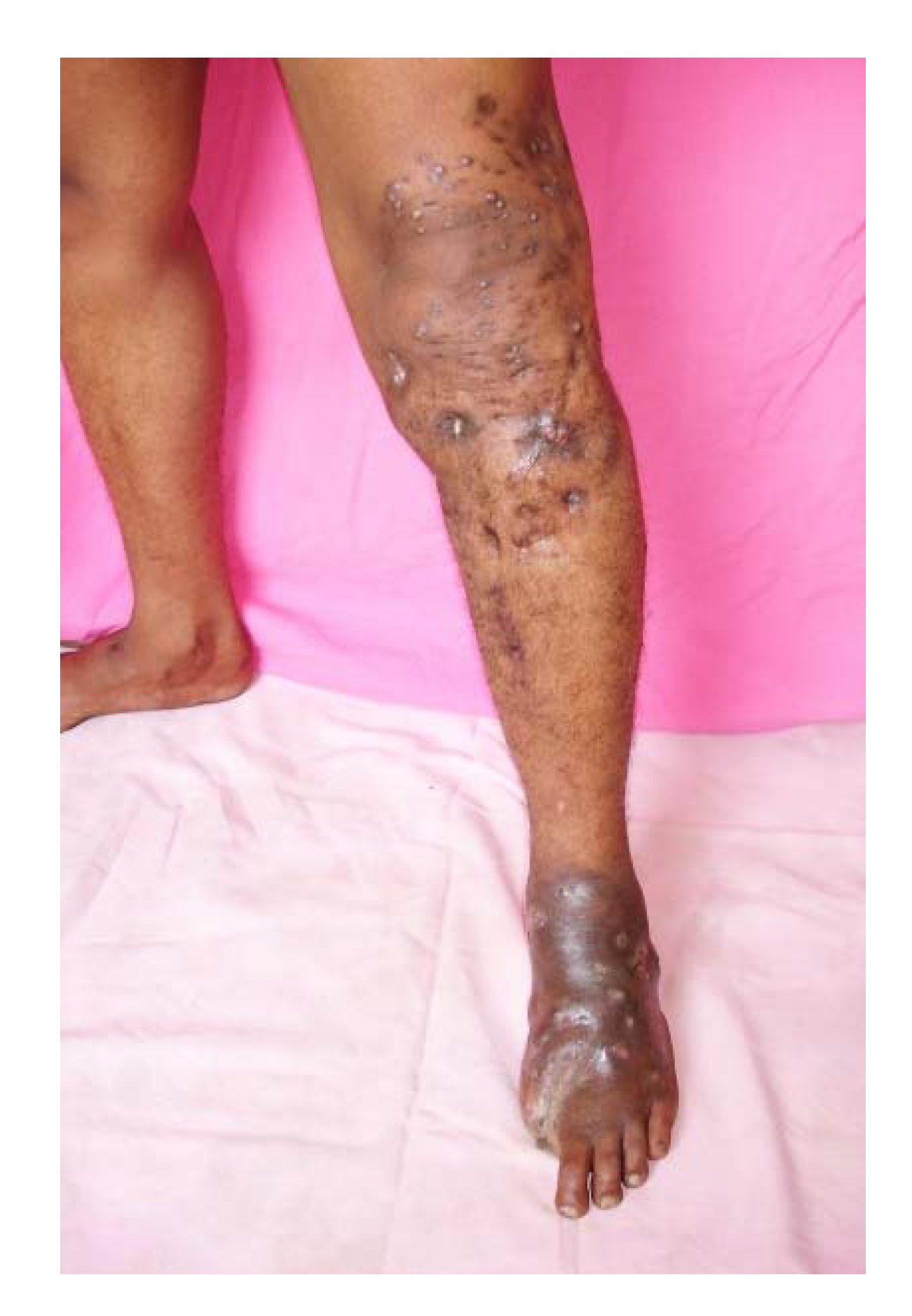
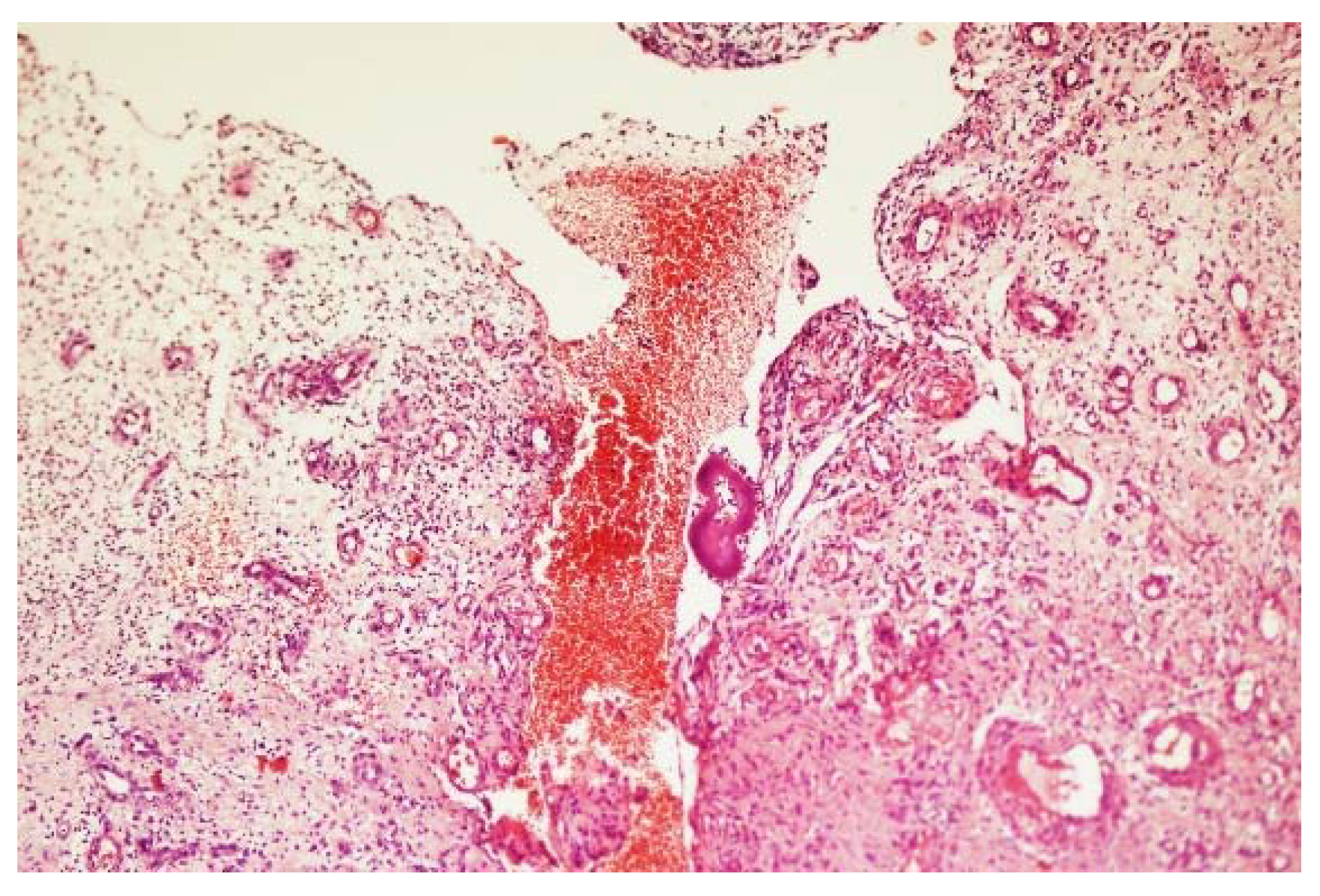
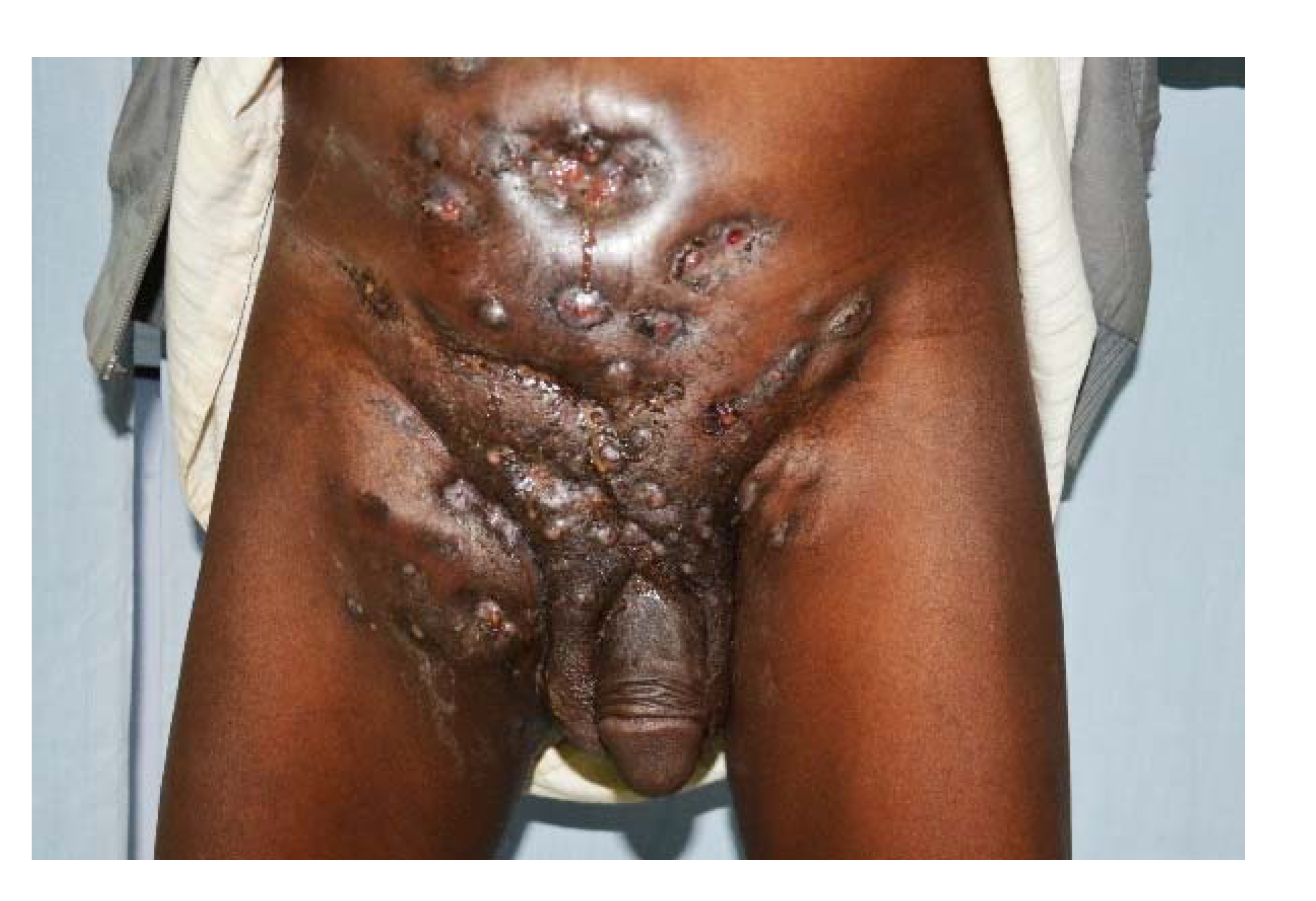
3. The Clinical Presentation
4. The Site of Mycetoma
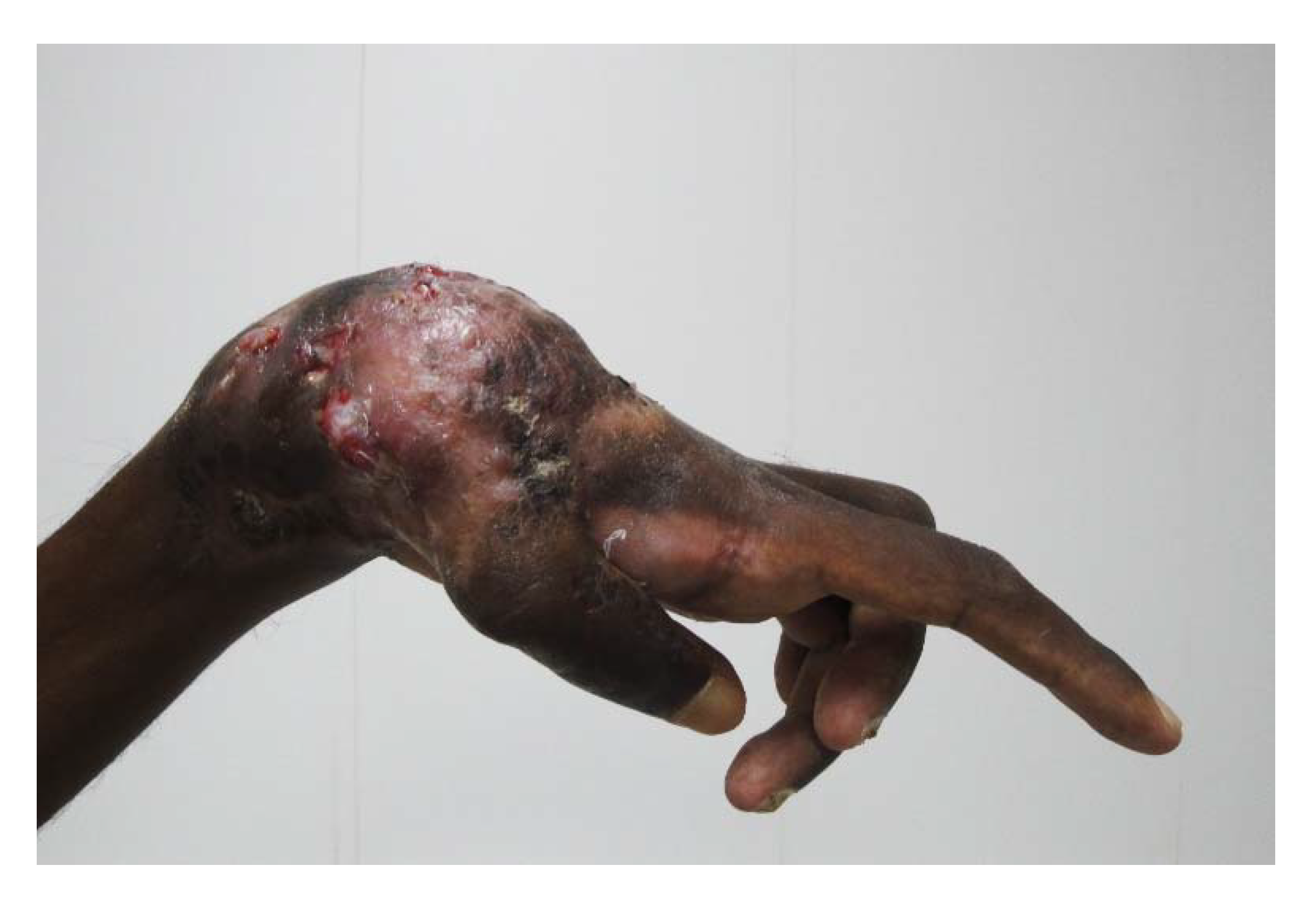
5. Mycetoma Spread
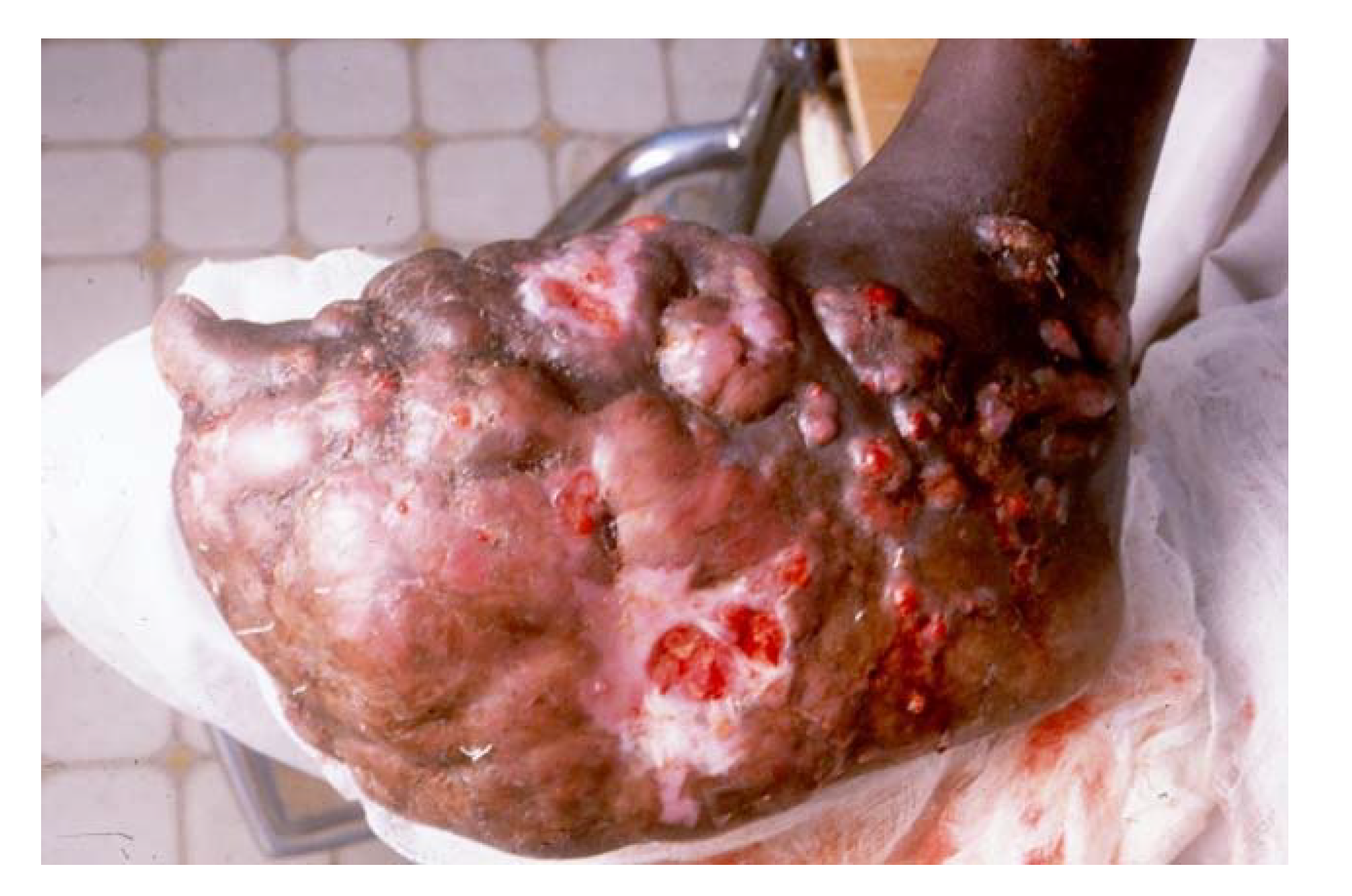
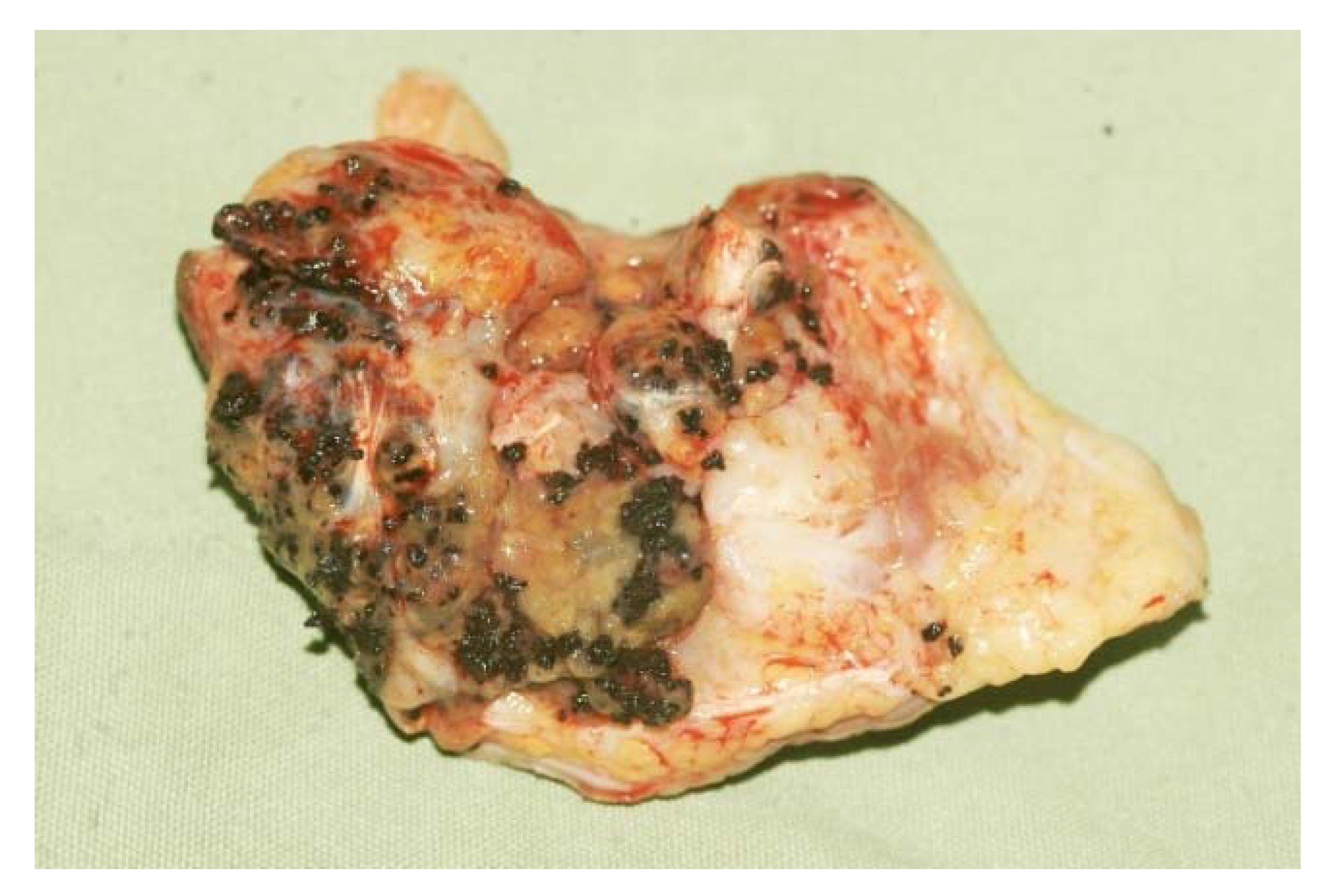
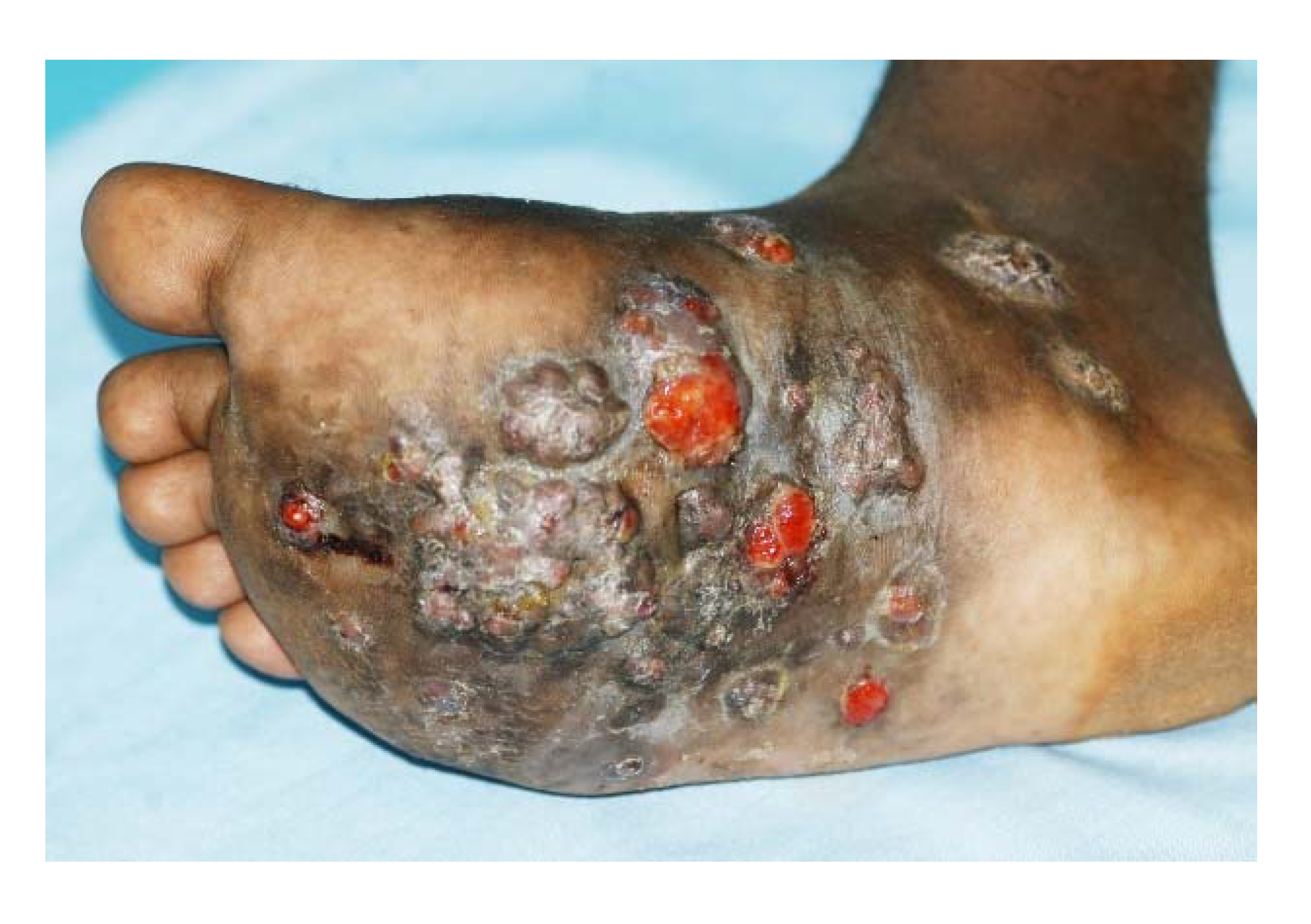
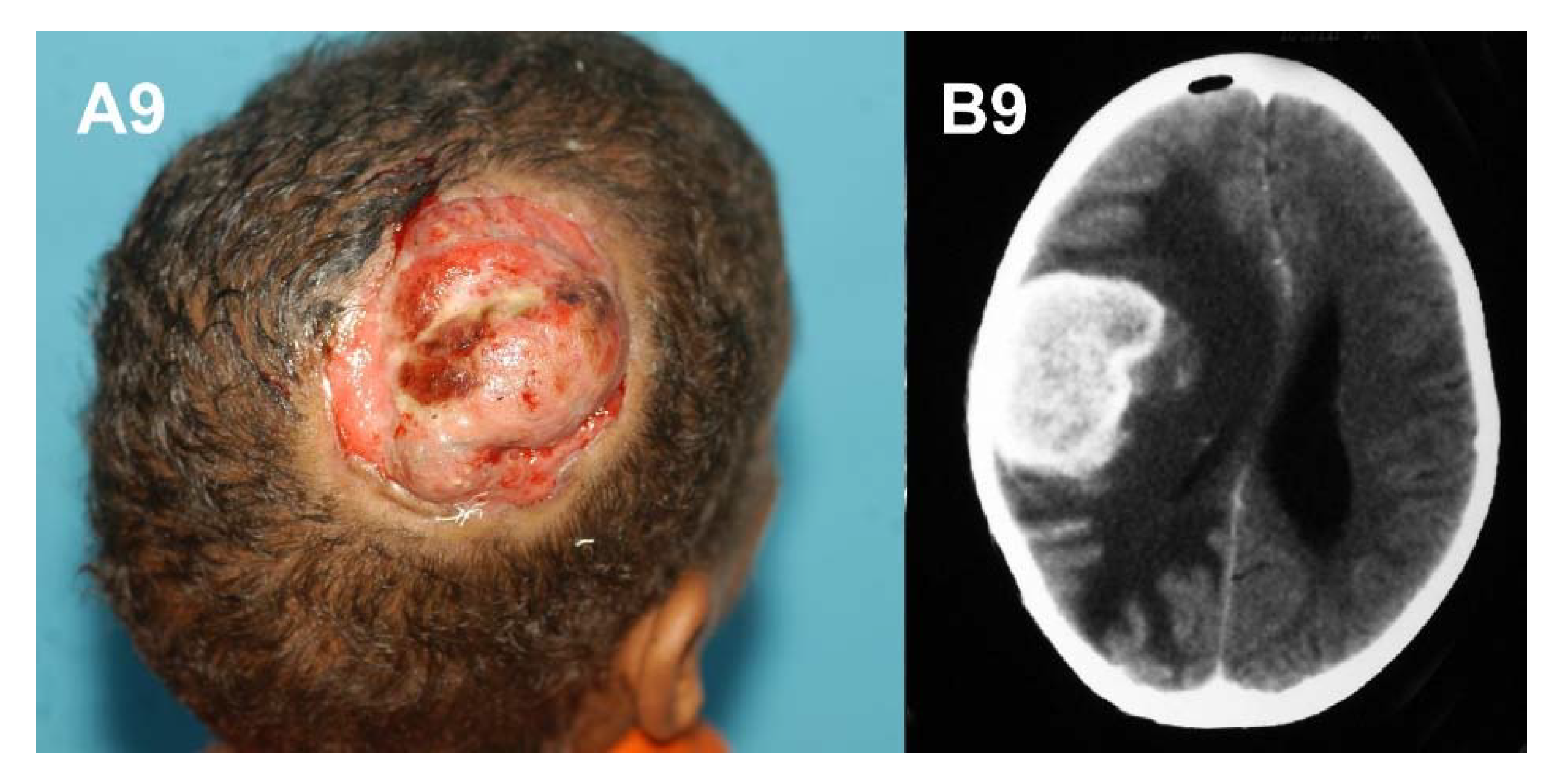
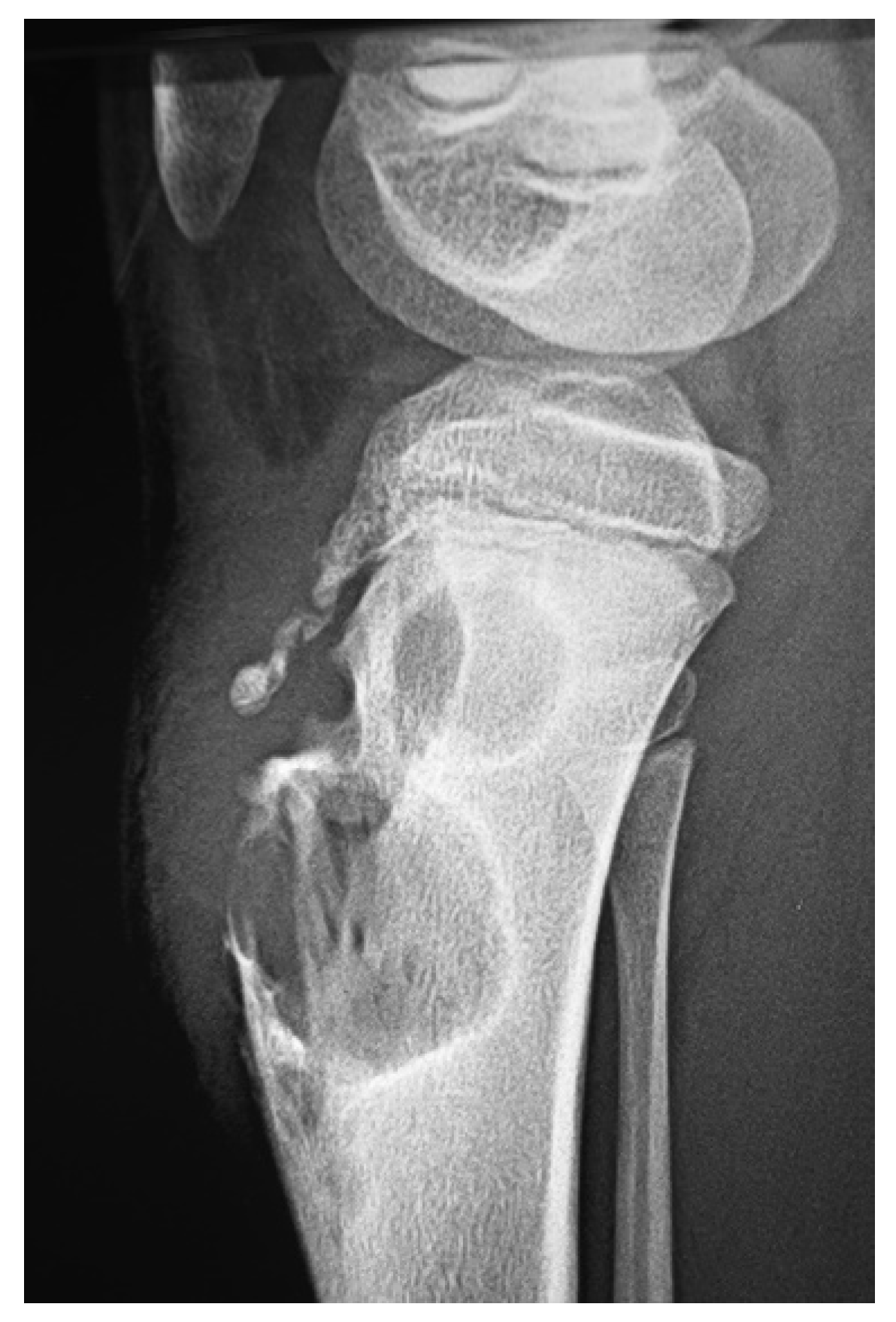
6. Aggressive Mycetoma
7. The Differential Diagnosis
Author Contributions
Funding
Conflicts of Interest
References
- Fahal, A.H. Mycetoma: A thorn in the flesh. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 3–11. [Google Scholar]
- Fahal, A.H.; Hassan, M.A. Mycetoma. Br. J. Surg. 1992, 79, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Tirado-Sánchez, A.; Calderón, L.; Saúl, A.; Araiza, J.; Hernández, M.; González, G.M.; Ponce, R.M. Mycetoma: Experience of 482 cases in a single center in Mexico. PLoS Negl. Trop. Dis. 2014, 8, e3102. [Google Scholar]
- Ahmed, A.O.; van Leeuwen, W.; Fahal, A.; van de Sande, W.; Verbrugh, H.; van Belkum, A. Mycetoma caused by Madurella mycetomatis: A neglected infectious burden. Lancet Infect. Dis. 2004, 4, 566–574. [Google Scholar] [CrossRef]
- Ahmed, A.O.A.; van de Sande, W.W.; Fahal, A.; Bakker-Woudenberg, I.; Verbrugh, H.; van Belkum, A. Management of mycetoma: Major challenge in tropical mycoses with limited international recognition. Curr. Opin. Infect. Dis. 2007, 20, 146–151. [Google Scholar] [PubMed]
- Fahal, A.H.; van de Sandy, W.W. The epidemiology of mycetoma. Curr. Fungal Infect. Rep. 2012, 6, 320–326. [Google Scholar] [CrossRef]
- Fahal, A.H. Mycetoma. In Tropical Infectious Diseases. Principles, Pathogens and Practice, 3rd ed.; Guerrant, R.L., Walker, D.H., Weller, P.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 565–568. [Google Scholar]
- Williams, N.S.; Bulstrode, C.; O’Connell, P.R. Bailey and Love’s Short Practice of Surgery 26E, 26th ed.; Taylor & Francis Ltd.: London, UK, 2013; pp. 84–88. [Google Scholar]
- Fahal, A.H. Mycetoma: Clinico-Pathological Monograph; University of Khartoum Press: Khurtum, Sudan, 2006; pp. 40–49. [Google Scholar]
- Hassan, M.A.; Fahal, A.H. Mycetoma: In Tropical Surgery; Kamil, R., Lumbly, J., Eds.; Westminster Publication Ltd.: London, UK, 2004; pp. 30–45. [Google Scholar]
- Fahal, A.H.; Suliman, S.H.; Gadir, A.F.A.; EL Hag, I.A.; EL Amin, F.I.; Gumaa, S.A.; Mahgoub, E.S. Abdominal wall mycetoma: An unusual presentation. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 78–80. [Google Scholar] [CrossRef]
- Fahal, A.H.; Suliman, S.H. Clinical presentation of mycetoma. Sudan Med. J. 1994, 32, 46–66. [Google Scholar]
- Fahal, A.H.; El Hassan, A.M.; Abdelalla, A.O.; Sheikh, H.E. Cystic mycetoma: An unusual clinical presentation of Madurella mycetomatis. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 66–67. [Google Scholar] [CrossRef]
- Fahal, A.H.; EL Toum, E.A.; EL Hassan, A.M.; Gumaa, S.A.; Mahgoub, E.S. A preliminary study on the ultrastructure of Actinomadura pelletieri and its host tissue reaction. J. Med. Vet. Mycol. 1994, 32, 343–348. [Google Scholar] [CrossRef] [PubMed]
- EL Hassan, A.M.; Fahal, A.H.; EL Hag, I.A.; Khalil, E.A.G. The pathology of mycetoma: Light microscopic and ultrastructural features. Sudan Med. J. 1994, 32, 23–45. [Google Scholar]
- Fahal, A.H.; Hassan, M.A.; Sanhouri, M. Surgical treatment of mycetoma. Sudan Med. J. 1994, 32, 98–104. [Google Scholar]
- Fahal, A.H.; EL Toum, E.A.; EL Hassan, A.M.; Gumaa, S.A.; Mahgoub, E.S. Host tissue reaction to Madurella mycetomatis: New classification. J. Med. Vet. Mycol. 1995, 33, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, W.W.; Fahal, A.H.; Goodfellow, M.; Maghoub, E.S.; Welsh, O.; Zijlstra, E.E. The merits and pitfalls of the currently used diagnostic tools in mycetoma. PLoS Negl. Trop. Dis. 2014, 3, e2918. [Google Scholar] [CrossRef] [PubMed]
- Welsh, O.; Al-Abdely, H.M.; Salinas-Carmona, M.C.; Fahal, A.H. Mycetoma medical therapy. PLoS Negl. Trop. Dis. 2014, 8, e3218. [Google Scholar] [CrossRef] [PubMed]
- Welsh, O.; Vera-Cabrera, L.; Welsh, E.; Salinas, M.C. Actinomycetoma and advances in its treatment. Clin. Dermatol. 2012, 30, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Mhmoud, N.A.; Ahmed, S.A.; Fahal, A.H.; de Hoog, G.S.; Gerrits van den Ende, A.H.; van de Sande, W.W. Pleurostomophora ochracea, a novel agent of human eumycetoma with yellow grains. J. Clin. Microbiol. 2012, 50, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Van Diepeningen, A.D.; Mahgoub, E.S.; Van de Sande, W.W. New species of Madurella, causative agents of black-grain mycetoma. Clin. Microbiol. 2012, 50, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, E.S.; Gumaa, S.A. Ketoconazole in the treatment of eumycetoma due to Madurella mycetomi. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 376–379. [Google Scholar] [CrossRef]
- Fahal, A.H.; Rahman, I.A.; El-Hassan, A.M.; EL Rahman, M.E.; Zijlstra, E.E. The efficacy of itraconazole in the treatment of patients with eumycetoma due to Madurella mycetomatis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, E.S.; Murray, I.G. Mycetoma; Medical Books Ltd.: London, UK, 1973; pp. 2–25. [Google Scholar]
- Abbott, P.H. Mycetoma in the Sudan. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 11–24. [Google Scholar] [CrossRef]
- Lynch, J.B. Mycetoma in the Sudan. Ann. R. Coll. Surg. Engl. 1964, 35, 319–340. [Google Scholar] [PubMed]
- EL Moghraby, I.M. Mycetoma in Gezira. Sudan Med. J. 1971, 9, 77. [Google Scholar]
- Mahgoub, E.S. Mycoses in the Sudan. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 184–188. [Google Scholar] [CrossRef]
- Fahal, A.H.; Mahgoub, E.S.; EL Hassan, A.M.; Abdel-Rahman, M.E.; Alshambaty, Y.; Hashim, A.; Hago, A.; Zijlstra, E.E. A new model for management of mycetoma in the Sudan. PLoS Negl. Trop. Dis. 2014, 8, e3271. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, R.; Méndez-Tovar, L.J.; Lavalle, P.; Welsh, O.; Saúl, A. Epidemiology of mycetoma in Mexico: Study of 2105 cases. Gac. Med. Mex. 1992, 128, 477–481. [Google Scholar] [PubMed]
- López-Martínez, R.; Méndez-Tovar, L.J.; Bonifaz, A.; Arenas, R.; Mayorga, J.; Welsh, O.; Vera-Cabrera, L.; Padilla-Desgarennes, M.C.; Contreras Pérez, C.; Chávez, G.; et al. Update on the epidemiology of mycetoma in Mexico. A Review of 3933 cases. Gac. Med. Mex. 2013, 149, 586–592. [Google Scholar] [PubMed]
- Ahmed, A.O.A.; Adelmann, D.; Fahal, A.H.; Verbrugh, H.A.; Van Belkum, A.; de Hoog, S.A. Environmental occurrence of Madurella mycetomatis, major agent of human eumycetoma in Sudan. J. Clin. Microbiol. 2002, 40, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.O.A.; van Vianen, W.; ten Kate, M.; van de Sande, W.W.J.; Van Belkum, A. A murine model of Madurella mycetomatis eumycetoma. FEMS Immunol. Med. Microbiol. 2003, 37, 29–36. [Google Scholar] [CrossRef]
- Fahal, A.H.; Azziz, K.A.A.; Suliman, S.H.; Galib, H.V.; Mahgoub, E.S. Dual infection with mycetoma and tuberculosis. East Afr. Med. J. 1995, 72, 749–750. [Google Scholar] [PubMed]
- Fahal, A.H.; Arbab, M.A.R.; EL Hassan, A.M. Aggressive clinical presentation of mycetoma due to Actinomadura pelletierii. Khartoum Med. J. 2012, 5, 699–702. [Google Scholar]
- Ibrahim, A.I.; EL Hassan, A.M.; Fahal, A.H.; van de Sande, W.W. A histopathological exploration of the Madurella mycetomatis grain. PLoS ONE 2013, 8, e57774. [Google Scholar] [CrossRef] [PubMed]
- Aounallah, A.; Boussofara, L.; Ben Saïd, Z.; Ghariani, N.; Saidi, W.; Denguezli, M.; Belajouza, C.; Nouira, R. Analysis of 18 Tunisian cases of mycetoma at the Sousse hospital (1974–2010). Bull. Soc. Pathol. Exot. 2013, 106, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Dieng, M.T.; Niang, S.O.; Diop, B.; Ndiaye, B. Actinomycetomas in Senegal: Study of 90 cases. Bull. Soc. Pathol. Exot. 2005, 98, 18–20. [Google Scholar] [PubMed]
- Marc, S.; Meziane, M.; Hamada, S.; Hassam, B.; Benzekri, L. Clinical and epidemiological features of mycetoma in Morocco. Med. Mal. Infect. 2011, 41, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Philippon, M.; Larroque, D.; Ravisse, P. Mycetoma in Mauritania: Species found, epidemiologic characteristics and country distribution. Report of 122 cases. Bull. Soc. Pathol. Exot. 1992, 85, 107–114. [Google Scholar] [PubMed]
- Fahal, A.H.; Sharfy, A.R.A. Vulval mycetoma: A rare cause of bladder outlet obstruction. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 652–653. [Google Scholar] [CrossRef]
- Mohamed, E.W.; Mohamed, E.N.A.; Yousif, B.M.; Fahal, A.H. Tongue actinomycetoma due to Actinomadura madurae: A rare clinical presentation. J. Oral. Maxillofac. Surg. 2012, 70, e622–e624. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.M.; Fahal, A.H. Oral eumycetoma: A rare and unusual presentation. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 115, e23–e25. [Google Scholar] [CrossRef] [PubMed]
- Fahal, A.H.; Yagi, H.I.; EL Hassan, A.M. Mycetoma induced palatal deficiency and pharyngeal plexus dysfunction. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 676–677. [Google Scholar] [CrossRef]
- Fahal, A.H.; Sharfi, A.R.; Sheikh, H.E.; EL Hassan, A.M. Internal fistula formation: An unusual complication of mycetoma. Trans. R. Soc. Trop. Med. Hyg. 1996, 89, 550–552. [Google Scholar] [CrossRef]
- Mohamed, E.W.; Suleiman, H.S.; Fadella, A.I.; Fahal, A.H. Aggressive perineal and pelvic eumycetoma: An unusual and challenging problem to treat. Khartoum Med. J. 2012, 5, 771–774. [Google Scholar]
- Hussein, A.; Ahmed, A.E.M.; Fahal, A.H.; Mahmoud, A.; Sidig, A.; Awad, K. Cervical cord compression secondary to mycetoma infection. Sud. J. Public Health 2007, 2, 112–115. [Google Scholar]
- Zein, H.A.M.; Fahal, A.H.; Mahgoub, E.S.; EL Hassan, T.; Abdel Rahman, M.E. The predictors of cure, amputation & follow-up dropout among mycetoma patients as seen at the Mycetoma Research Centre, University of Khartoum. Trans R. Soc. Trop. Med. Hyg. 2012, 106, 639–644. [Google Scholar] [PubMed]
- Wendy, W.J.; van de Sande, W.W.; Mahgoub, E.S.; Fahal, A.H.; Goodfellow, M.; Welsh, O. The mycetoma knowledge gap: Identification of research priorities. PLoS Negl. Trop. Dis. 2014, 8, e2667. [Google Scholar]
- EL Hassan, A.M.; Mahgoub, E.S. Lymph node involvement in mycetoma. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 165–169. [Google Scholar] [CrossRef]
- Arbab, M.A.; El Hag, I.A.; Abdul Gadir, A.F.; Siddik, H.E.-R. Intraspinal mycetoma: Report of two cases. Am. J. Trop. Med. Hyg. 1997, 56, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.S.A.; Fahal, A.H. Broncho-pleuro-cutaneous fistula and pneumothorax: Rare challenging complications of chest wall eumycetoma. PLoS Negl. Trop. Dis. 2017, 11, e0005737. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Fahal, A.H. Mycetoma pulmonary secondaries from a gluteal eumycetoma: An unusual presentation. PLoS Negl. Trop. Dis. 2016, 10, e0004945. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.S.W.; Seif El Din, N.; Fahal, A.H. Multiple mycetoma lung secondaries from knee eumycetoma: An unusual complication. PLoS Negl. Trop. Dis. 2016, 10, e0004735. [Google Scholar] [CrossRef] [PubMed]
- Scolding, P.S.; Abbas, M.A.; Omer, R.F.; Fahal, A.H. Madurella mycetomatis-induced massive shoulder joint destruction: A management challenge. PLoS Negl. Trop. Dis. 2016, 10, e0004849. [Google Scholar] [CrossRef] [PubMed]
- Fahal, A.; Mahgoub, E.S.; El Hassan, A.M.; Jacoub, A.O.; Hassan, D. Head and neck mycetoma: The Mycetoma Research Centre experience. PLoS Negl. Trop. Dis. 2015, 9, e0003587. [Google Scholar] [CrossRef] [PubMed]
- Fahal, A.; Mahgoub, E.S.; El Hassan, A.M.; Abdel-Rahman, M.E. Mycetoma in the Sudan: An update from the Mycetoma Research Centre, University of Khartoum, Sudan. PLoS Negl. Trop. Dis. 2015, 9, e0003679. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Marks, M.; Bertran, L.; Kollie, K.; Argaw, D.; Fahal, A.H. Integrated control and management of neglected tropical skin diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005136. [Google Scholar] [CrossRef] [PubMed]
- Bakhiet, S.M.; Fahal, A.H.; Musa, A.M.; Mohamed, E.S.W.; Omer, R.F.; Ahmed, E.S. A holistic approach to the mycetoma management. PLoS Negl. Trop. Dis. 2018, 12, e0006391. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahal, A.H.; Suliman, S.H.; Hay, R. Mycetoma: The Spectrum of Clinical Presentation. Trop. Med. Infect. Dis. 2018, 3, 97. https://doi.org/10.3390/tropicalmed3030097
Fahal AH, Suliman SH, Hay R. Mycetoma: The Spectrum of Clinical Presentation. Tropical Medicine and Infectious Disease. 2018; 3(3):97. https://doi.org/10.3390/tropicalmed3030097
Chicago/Turabian StyleFahal, Ahmed Hassan, Suliman Hussein Suliman, and Roderick Hay. 2018. "Mycetoma: The Spectrum of Clinical Presentation" Tropical Medicine and Infectious Disease 3, no. 3: 97. https://doi.org/10.3390/tropicalmed3030097
APA StyleFahal, A. H., Suliman, S. H., & Hay, R. (2018). Mycetoma: The Spectrum of Clinical Presentation. Tropical Medicine and Infectious Disease, 3(3), 97. https://doi.org/10.3390/tropicalmed3030097





