Control Strategies for Scabies
Abstract
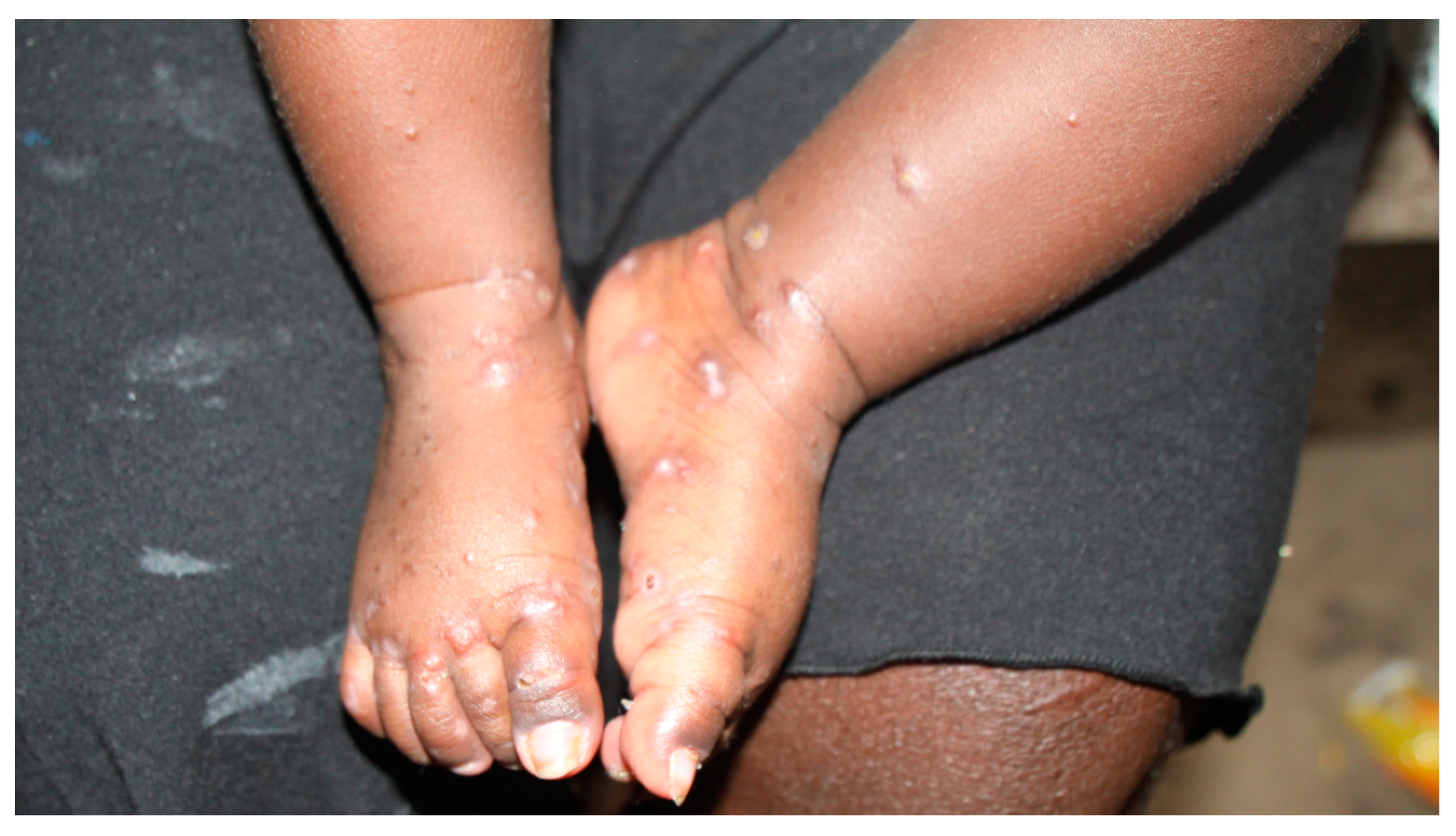
:1. Introduction
2. Burden of Disease
3. The Need for Surveillance and Control
4. Community Control
5. Outstanding Issues
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hay, R.J.; Steer, A.C.; Engelman, D.; Walton, S. Scabies in the developing world—Its prevalence, complications, and management. Clin. Microbiol. Infect. 2012, 18, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chosidow, O. Clinical practices. Scabies. New Engl. J. Med. 2006, 354, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; McGloughlin, S.; Tong, S.Y.; Walton, S.F.; Currie, B.J. A novel clinical grading scale to guide the management of crusted scabies. PLoS Negl. Trop. Dis. 2013, 7, e2387. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F.; Beroukas, D.; Roberts-Thomson, P.; Currie, B.J. New insights into disease pathogenesis in crusted (Norwegian) scabies: The skin immune response in crusted scabies. Br. J. Dermatol. 2008, 158, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Worth, C.; Heukelbach, J.; Fengler, G.; Walter, B.; Lisenfeld, O.; Feldmeier, H. Impaired quality of life in adults and children with scabies from an impoverished community in Brazil. Int. J. Dermatol. 2012, 51, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hofstraat, K.; van Brakel, W.H. Social stigma towards neglected tropical diseases: A systematic review. Int. Health 2016, 8 (Suppl. 1), i53–i70. [Google Scholar] [CrossRef]
- Olsen, J.R.; Gallacher, J.; Finlay, A.Y.; Piguet, V.; Francis, N.A. Quality of life impact of childhood skin conditions measured using the Children’s Dermatology Life Quality Index (CDLQI): A meta-analysis. Br. J. Dermatol. 2016, 174, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Steer, A.C.; Jenney, A.W.; Kado, J.; Batzloff, M.R.; La Vincente, S.; Waqatakirewa, L.; Mulholland, E.K.; Carapetis, J.R. High burden of impetigo and scabies in a tropical country. PLoS Negl. Trop. Dis. 2009, 3, e467. [Google Scholar] [CrossRef] [PubMed]
- Swe, P.M.; Zakrzewski, M.; Kelly, A.; Krause, L.; Fischer, K. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLoS Negl. Trop. Dis. 2014, 8, e2897. [Google Scholar] [CrossRef] [PubMed]
- Swe, P.M.; Fischer, K. A scabies mite serpin interferes with complement-mediated neutrophil functions and promotes staphylococcal growth. PLoS Negl. Trop. Dis. 2014, 8, e2928. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Steer, A.C.; Chosidow, O.; Currie, B.J. Scabies: A suitable case for a global control initiative. Curr. Opin. Infect. Dis. 2013, 26, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Smeesters, P.R.; Steer, A.C. Streptococcal skin infection and rheumatic heart disease. Curr. Opin. Infect. Dis. 2012, 25, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Thornley, S.; Marshall, R.; Jarrett, P.; Sundborn, G.; Reynolds, E.; Schofield, G. Scabies is strongly associated with acute rheumatic fever in a cohort study of Auckland children. J. Paediatr. Child Health 2018, 54, 625–632. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Karimkhani, C.; Colombara, D.V.; Drucker, A.M.; Norton, S.A.; Hay, R.; Engelman, D.; Steer, A.; Whitfeld, M.; Naghavi, M.; Dellavalle, R.P. The global burden of scabies: A cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1247–1254. [Google Scholar] [CrossRef]
- Romani, L.; Steer, A.C.; Whitfeld, M.J.; Kaldor, J.M. Prevalence of scabies and impetigo worldwide: A systematic review. Lancet Infect. Dis. 2015, 15, 960–967. [Google Scholar] [CrossRef]
- Mason, D.S.; Marks, M.; Sokana, O.; Solomon, A.W.; Mabey, D.C.; Romani, L.; Kaldor, J.; Steer, A.C.; Engelman, D. The prevalence of scabies and impetigo in the solomon islands: A population-based survey. PLoS Negl. Trop. Dis. 2016, 10, e0004803. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, D.K.; Anderson, A.; Cleland, G.; Bowen, A.C. Are scabies and impetigo ‘normalised’? A cross-sectional comparative study of hospitalised children in northern Australia assessing clinical recognition and treatment of skin infections. PLoS Negl. Trop. Dis. 2017, 11, e0005726. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Engelman, D.; Gholam, K.; Fuller, L.C.; Steer, A.C. Systematic review of the diagnosis of scabies in therapeutic trials. Clin. Exp. Dermatol. 2017, 42, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L.; Whitfeld, M.J.; Koroivueta, J.; Kama, M.; Wand, H.; Tikoduadua, L.; Tuicakau, M.; Koroi, A.; Ritova, R.; Andrews, R.; et al. The epidemiology of scabies and impetigo in relation to demographic and residential characteristics: Baseline findings from the Skin Health Intervention Fiji Trial. Am. J. Trop. Med. Hyg. 2017, 97, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Koroivueta, J.; Steer, A.C.; Kama, M.; Kaldor, J.M.; Wand, H.; Hamid, M.; Whitfeld, M.J. Scabies and impetigo prevalence and risk factors in Fiji: A national survey. PLoS Negl. Trop. Dis. 2015, 9, e0003452. [Google Scholar] [CrossRef] [PubMed]
- Aung, P.T.Z.; Cuningham, W.; Hwang, K.; Andrews, R.M.; Carapetis, J.R.; Kearns, T.; Clucas, D.; McVernon, J.; Simpson, J.A.; Tong, S.Y.C.; et al. Scabies and risk of skin sores in remote Australian Aboriginal communities: A self-controlled case series study. PLoS Negl. Trop. Dis. 2018, 12, e0006668. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the Tenth Meeting of the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. 2018. Available online: http://www.who.int/neglected_diseases/NTD_STAG_report_2017.pdf?ua=1 (accessed on 17 March 2018).
- World Health Organization. 9th NTD-STAG Global Working Group Meeting on Monitoring and Evaluation of Neglected Tropical Diseases. 2018. Available online: http://www.who.int/neglected_diseases/events/STAG_Working_Group_on_Monitoring_Evaluation/en/ (accessed on 12 August 2018).
- Engelman, D.; Kiang, K.; Chosidow, O.; McCarthy, J.; Fuller, C.; Lammie, P.; Hay, R.; Steer, A. Members of the International Alliance for The Control of Scabies. Toward the global control of human scabies: Introducing the International Alliance for the Control of Scabies. PLoS Negl. Trop. Dis. 2013, 7, e2167. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.; Fuller, L.C.; Steer, A.C. Consensus criteria for the diagnosis of scabies: A Delphi study of international experts. PLoS Negl. Trop. Dis. 2018, 12, e0006549. [Google Scholar] [CrossRef] [PubMed]
- Taplin, D.; Porcelain, S.L.; Meinking, T.L.; Athey, R.L.; Chen, J.A.; Castillero, P.M.; Sanchez, R. Community control of scabies: A model based on use of permethrin cream. Lancet 1991, 337, 1016–1018. [Google Scholar] [CrossRef]
- Taplin, D.; Arrue, C.; Walker, J.; Roth, W.; Rivera, A. Eradication of scabies with a single treatment schedule. J. Am. Acad. Dermatol. 1983, 9, 546–550. [Google Scholar] [CrossRef]
- Lawrence, G.; Leafasia, J.; Sheridan, J.; Hills, S.; Wate, J.; Wate, C.; Montgomery, J.; Pandeya, N.; Purdie, D. Control of scabies, skin sores and haematuria in children in the Solomon Islands: Another role for ivermectin. Bull. World Health Organ. 2005, 83, 34–42. [Google Scholar] [PubMed]
- Leppard, B.; Naburi, A.E. The use of ivermectin in controlling an outbreak of scabies in a prison. Br. J. Dermatol. 2000, 143, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Pacque, M.; Munoz, B.; Greene, B.M.; White, A.T.; Dukuly, Z.; Taylor, H.R. Safety of and compliance with community-based ivermectin therapy. Lancet 1990, 335, 1377–1380. [Google Scholar] [CrossRef]
- Pacque, M.C.; Dukuly, Z.; Greene, B.M.; Munoz, B.; Keyvan-Larijani, E.; Williams, P.N.; Taylor, H.R. Community-based treatment of onchocerciasis with ivermectin: Acceptability and early adverse reactions. Bull. World Health Organ. 1989, 67, 721–730. [Google Scholar] [PubMed]
- Marks, M.; Taotao-Wini, B.; Satorara, L.; Engelman, D.; Nasi, T.; Mabey, D.C.; Steer, A.C. Long term control of scabies fifteen years after an intensive treatment programme. PLoS Negl. Trop. Dis. 2015, 9, e0004246. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.M.; Kearns, T.; Connors, C.; Parker, C.; Carville, K.; Currie, B.J.; Carapetis, J.R. A regional initiative to reduce skin infections amongst aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Negl. Trop. Dis. 2009, 3, e554. [Google Scholar] [CrossRef] [PubMed]
- La Vincente, S.; Kearns, T.; Connors, C.; Cameron, S.; Carapetis, J.; Andrews, R. Community management of endemic scabies in remote aboriginal communities of northern Australia: Low treatment uptake and high ongoing acquisition. PLoS Negl. Trop. Dis. 2009, 3, e444. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Marks, M.; Sokana, O.; Nasi, T.; Kamoriki, B.; Wand, H.; Whitfeld, M.; Engelman, D.; Solomon, A.W.; Steer, A.C.; et al. Feasibility and safety of mass drug co-administration with azithromycin and ivermectin for the control of neglected tropical diseases: A single-arm intervention trial. Lancet Glob. Health 2018, in press. [Google Scholar]
- Marks, M.; Toloka, H.; Baker, C.; Kositz, C.; Asugeni, J.; Puiahi, E.; Asugeni, R.; Azzopardi, K.; Diau, J.; Kaldor, J.M.; et al. Randomised trial of community treatment with azithromycin and ivermectin mass drug administration for control of scabies and impetigo. Clin. Infect. Dis. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.; Martin, D.L.; Hay, R.J.; Chosidow, O.; McCarthy, J.S.; Fuller, L.C.; Steer, A.C. Opportunities to investigate the effects of ivermectin mass drug administration on scabies. Parasit Vectors 2013, 6, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, K.A.; Deb, R.M.; Stanton, M.C.; Molyneux, D.H. Soil transmitted helminths and scabies in Zanzibar, Tanzania following mass drug administration for lymphatic filariasis—A rapid assessment methodology to assess impact. Parasit Vectors 2012, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Wiegand, R.; Goodhew, B.; Lammie, P.; Mkocha, H.; Kasubi, M. Impact of ivermectin mass drug administration for lymphatic filariasis on scabies in eight villages in Kongwa District, Tanzania. Am. J. Trop. Med. Hyg. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Cassell, J.A.; Middleton, J.; Nalabanda, A.; Lanza, S.; Head, M.G.; Bostock, J.; Hewitt, K.; Jones, C.I.; Darley, C.; Karir, S.; et al. Scabies outbreaks in ten care homes for elderly people: A prospective study of clinical features, epidemiology, and treatment outcomes. Lancet Infect. Dis. 2018, 18, 894–902. [Google Scholar] [CrossRef]
- Agrawal, S.; Puthia, A.; Kotwal, A.; Tilak, R.; Kunte, R.; Kushwaha, A.S. Mass scabies management in an orphanage of rural community: An experience. Med. J. Armed Forces India 2012, 68, 403–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beeres, D.T.; Ravensbergen, S.J.; Heidema, A.; Cornish, D.; Vonk, M.; Wijnholds, L.D.; Hendriks, J.J.H.; Kleinnijenhuis, J.; Omansen, T.F.; Stienstra, Y. Efficacy of ivermectin mass-drug administration to control scabies in asylum seekers in the Netherlands: A retrospective cohort study between January 2014–March 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006401. [Google Scholar] [CrossRef] [PubMed]
- White, L.C.; Lanza, S.; Middleton, J.; Hewitt, K.; Freire-Moran, L.; Edge, C.; Nicholls, M.; Rajan-Iyer, J.; Cassell, J.A. The management of scabies outbreaks in residential care facilities for the elderly in England: A review of current health protection guidelines. Epidemiol. Infect. 2016, 144, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, K.A.; Nalabanda, A.; Cassell, J.A. Scabies outbreaks in residential care homes: Factors associated with late recognition, burden and impact. A mixed methods study in England. Epidemiol. Infect. 2015, 143, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Mounsey, K.E.; Murray, H.C.; King, M.; Oprescu, F. Retrospective analysis of institutional scabies outbreaks from 1984 to 2013: Lessons learned and moving forward. Epidemiol. Infect. 2016, 144, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- WHO Africa. Ethiopia-Scabies Outbreak Response in Amhara Regional State. Available online: https://www.afro.who.int/news/ethiopia-scabies-outbreak-response-amhara-regional-state (accessed on 6 July 2018).
- Currie, B.J.; McCarthy, J.S. Permethrin and ivermectin for scabies. New Engl. J. Med. 2010, 362, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Stolk, W.A.; Walker, M.; Coffeng, L.E.; Basanez, M.G.; de Vlas, S.J. Required duration of mass ivermectin treatment for onchocerciasis elimination in Africa: A comparative modelling analysis. Parasit Vectors 2015, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, M.A.; Stolk, W.A.; Smith, M.E.; Subramanian, S.; Singh, B.K.; Weil, G.J.; Michael, E.; Hollingsworth, T.D. Effectiveness of a triple-drug regimen for global elimination of lymphatic filariasis: A modelling study. Lancet Infect. Dis. 2017, 17, 451–458. [Google Scholar] [CrossRef]
- Lydeamore, M.J.; Campbell, P.T.; Regan, D.G.; Tong, S.Y.C.; Andrews, R.M.; Steer, A.C.; Romani, L.; Kaldor, J.M.; McVernon, J.; McCaw, J.M. A biological model of scabies infection dynamics and treatment informs mass drug administration strategies to increase the likelihood of elimination. Math. Biosci. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.L.; Steer, A.C.; Cranswick, N.; Gwee, A. Is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg? Arch. Dis. Child 2018, 103, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Gyapong, J.O.; Chinbuah, M.A.; Gyapong, M. Inadvertent exposure of pregnant women to ivermectin and albendazole during mass drug administration for lymphatic filariasis. Trop. Med. Int. Health 2003, 8, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, S.F.; Currie, B.J. Problems in diagnosing scabies, a global disease in human and animal populations. Am. Soc. Rev. 2007, 20, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Boeras, D.I.; Nkengasong, J. Re-imagining the future of diagnosis of neglected tropical diseases. Comput. Struct. Biotechnol. J. 2017, 15, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Marks, M.; Bertran, L.; Kollie, K.; Argaw, D.; Fahal, A.; Fitzpatrick, C.; Fuller, C.; Garcia-Izquierdo, B.; Hay, R.; et al. Integrated control and management of neglected tropical diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005136. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recognizing Neglected Tropical Diseases through Changes on the Skin: A Training Guide for Front-Line Health Workers. 2018. Available online: http://apps.who.int/iris/bitstream/handle/10665/272723/9789241513531-eng.pdf (accessed on 12 August 2018).
- Estrada, R.; Chavez-Lopez, G.; Estrada-Chavez, G.; Paredes-Solis, S. Specialized dermatological care for marginalized populations and education at the primary care level: Is community dermatology a feasible proposal? Int. J. Dermatol. 2012, 51, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.; Estrada, R.; Grossmann, H. Managing skin disease in resource-poor environments-the role of community-oriented training and control programs. Int. J. Dermatol. 2011, 50, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Bagayoko, C.; Dicko, A.; Cissé, L.; Berthé, S.; Traoré, B.; Fofana, Y.; Niang, M.; Traoré, S.; Karabinta, Y.; et al. A teledermatology pilot programme for the management of skin diseases in primary health care centres: Experiences from a resource-limited country (Mali, West Africa). Trop. Med. Infect. Dis. 2018, 3, 88. [Google Scholar] [CrossRef]
- Currie, B.J.; Harumal, P.; McKinnon, M.; Walton, S.F. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin. Infect. Dis. 2004, 39, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Mounsey, K.E.; Holt, D.C.; McCarthy, J.; Currie, B.J.; Walton, S.F. Scabies: Molecular perspectives and therapeutic implications in the face of emerging drug resistance. Future Microbiol. 2008, 3, 57–66. [Google Scholar] [CrossRef] [PubMed]
- McNair, C.M. Ectoparasites of medical and veterinary importance: Drug resistance and the need for alternative control methods. J. Pharm Pharmacol. 2015, 67, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Abbas, O.; Kibbi, A.G.; Kurban, M. Scabies in the age of increasing drug resistance. PLoS Negl. Trop. Dis. 2017, 11, e0005920. [Google Scholar] [CrossRef] [PubMed]
- Opoku, N.O.; Bakajika, D.K.; Kanza, E.M.; Howard, H.; Mambandu, G.L.; Nyathirombo, A.; Nigo, M.M.; Kasonia, K.; Masembe, S.L.; Mumbere, M.; et al. Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: A randomised, controlled, double-blind phase 3 trial. Lancet 2018, in press. [Google Scholar] [CrossRef]
- Cotreau, M.M.; Warren, S.; Ryan, J.L.; Fleckenstein, L.; Vanapalli, S.R.; Brown, K.R.; Rock, D.; Chen, C.Y.; Schwertschlag, U.S. The antiparasitic moxidectin: Safety, tolerability, and pharmacokinetics in humans. J. Clin. Pharmacol. 2003, 43, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Mounsey, K.E.; Bernigaud, C.; Chosidow, O.; McCarthy, J.S. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl. Trop. Dis. 2016, 10, e0004389. [Google Scholar] [CrossRef] [PubMed]
- Bernigaud, C.; Fang, F.; Fischer, K.; Lespine, A.; Aho, L.; Dreau, D.; Kelly, A.; Sutra, J.F.; Moreau, F.; Lilin, T.; et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: Efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl. Trop. Dis. 2016, 10, e0005030. [Google Scholar] [CrossRef] [PubMed]
- Medicines Development for Global Health. Moxidectin Program. 2018. Available online: https://www.medicinesdevelopment.com/development-programs.htm (accessed on 3 August 2018).
- Thomas, J.; Christenson, J.K.; Walker, E.; Baby, K.E.; Peterson, G.M. Scabies - An ancient itch that is still rampant today. J. Clin. Pharm. Ther. 2017, 42, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.; Fuller, L.C.; Solomon, A.W.; McCarthy, J.S.; Hay, R.J.; Lammie, P.J.; Steer, A.C. Opportunities for integrated control of neglected tropical diseases that affect the skin. Trends Parasitol. 2016, 32, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Hay, R. Skin NTDs: An opportunity for integrated care. Trans. Royal Soc. Trop. Med. Hyg. 2016, 110, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Yotsu, R.R.; Kouadio, K.; Vagamon, B.; N’Guessan, K.; Akpa, A.J.; Yao, A.; Ake, J.; Abbet, R.; Tchamba Agbor Agbor, B.; Bedimo, R.; et al. Skin disease prevalence study in schoolchildren in rural Cote d’Ivoire: Implications for integration of neglected skin diseases (skin NTDs). PLoS Negl. Trop. Dis. 2018, 12, e0006489. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, E.K.; Sanuku, N.; Baea, M.; Satofan, S.; Maki, E.; Lombore, B.; Schmidt, M.S.; Siba, P.M.; Weil, G.J.; Kazura, J.W.; et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin. Infect. Dis. 2016, 62, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.U.; King, C.L.; Jacobson, J.A.; Weil, G.J. Potential value of triple drug therapy with ivermectin, diethylcarbamazine, and albendazole (IDA) to accelerate elimination of lymphatic filariasis and onchocerciasis in Africa. PLoS Negl. Trop. Dis. 2017, 11, e0005163. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.; Engels, D. Leaving no one behind: A neglected tropical disease indicator and tracers for the Sustainable Development Goals. Int. Health 2016, 8, i15–i18. [Google Scholar] [CrossRef] [PubMed]
| Level | Criteria |
|---|---|
| A. Confirmed scabies | At least one of the following: A1: Mites, eggs or faeces on light microscopy of skin samples A2: Mites, eggs or faeces visualized on individual using high-powered imaging device A3: Mite visualized on individual using dermoscopy |
| B. Clinical scabies | At least one of the following: B1: Scabies burrows B2: Typical lesions affecting male genitalia B3 Typical lesions in a typical distribution and two history features |
| C. Suspected scabies | One of the following: C1: Typical lesions in a typical distribution and one history feature C2: Atypical lesions or atypical distribution and two history features |
| History features | H1: Itching H2: Close contact with an individual who has itching or typical lesions in a typical distribution |
| Notes: | Diagnosis can be made at one of the three levels (A, B, or C) A diagnosis of clinical or suspected scabies should only be made if differential diagnoses are considered less likely than scabies. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelman, D.; Steer, A.C. Control Strategies for Scabies. Trop. Med. Infect. Dis. 2018, 3, 98. https://doi.org/10.3390/tropicalmed3030098
Engelman D, Steer AC. Control Strategies for Scabies. Tropical Medicine and Infectious Disease. 2018; 3(3):98. https://doi.org/10.3390/tropicalmed3030098
Chicago/Turabian StyleEngelman, Daniel, and Andrew C. Steer. 2018. "Control Strategies for Scabies" Tropical Medicine and Infectious Disease 3, no. 3: 98. https://doi.org/10.3390/tropicalmed3030098
APA StyleEngelman, D., & Steer, A. C. (2018). Control Strategies for Scabies. Tropical Medicine and Infectious Disease, 3(3), 98. https://doi.org/10.3390/tropicalmed3030098





