New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications †
Abstract
1. Introduction
2. Development of Antibody-Based Biomarker of Exposure to Aedes Bites
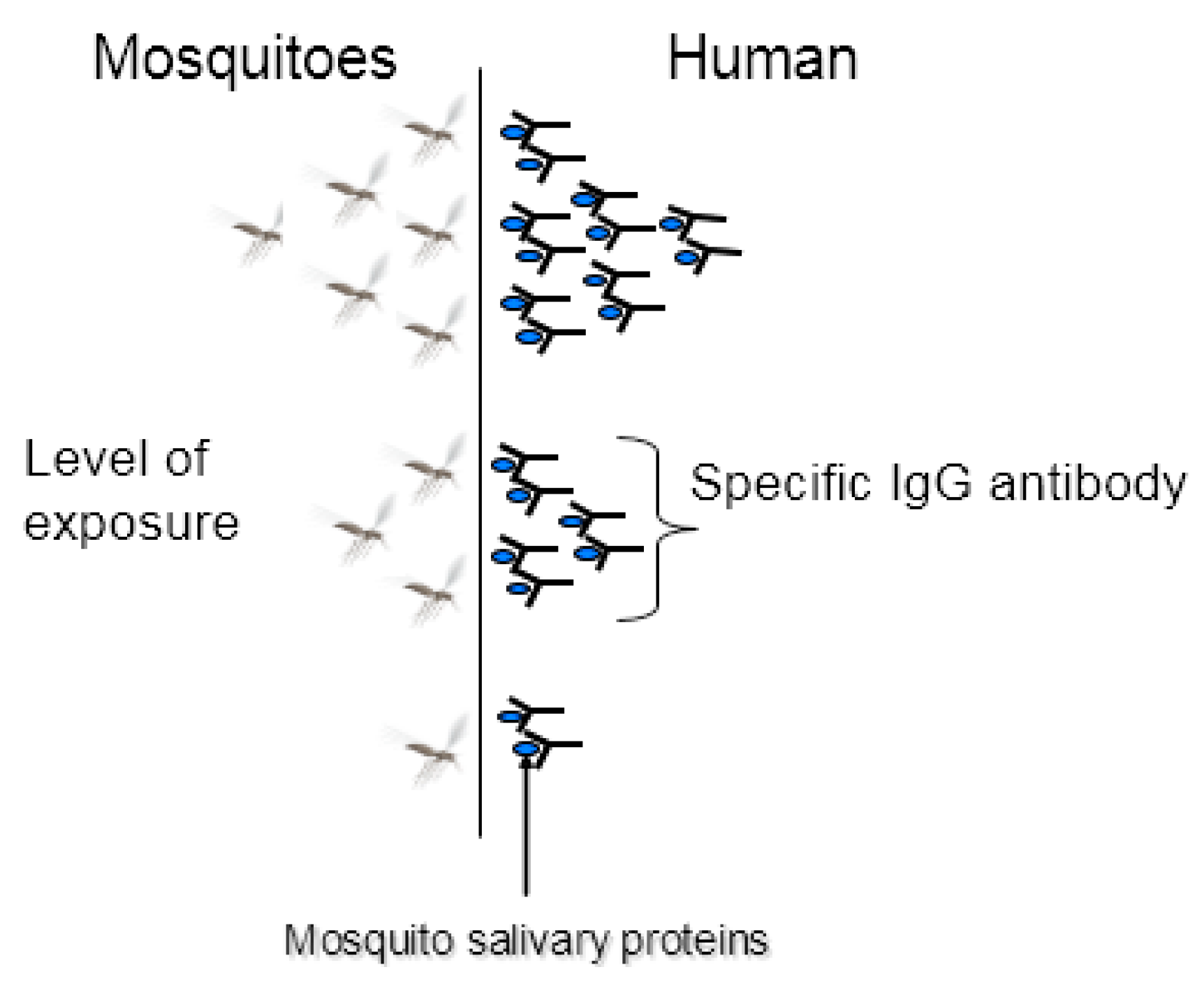
2.1. The Concept
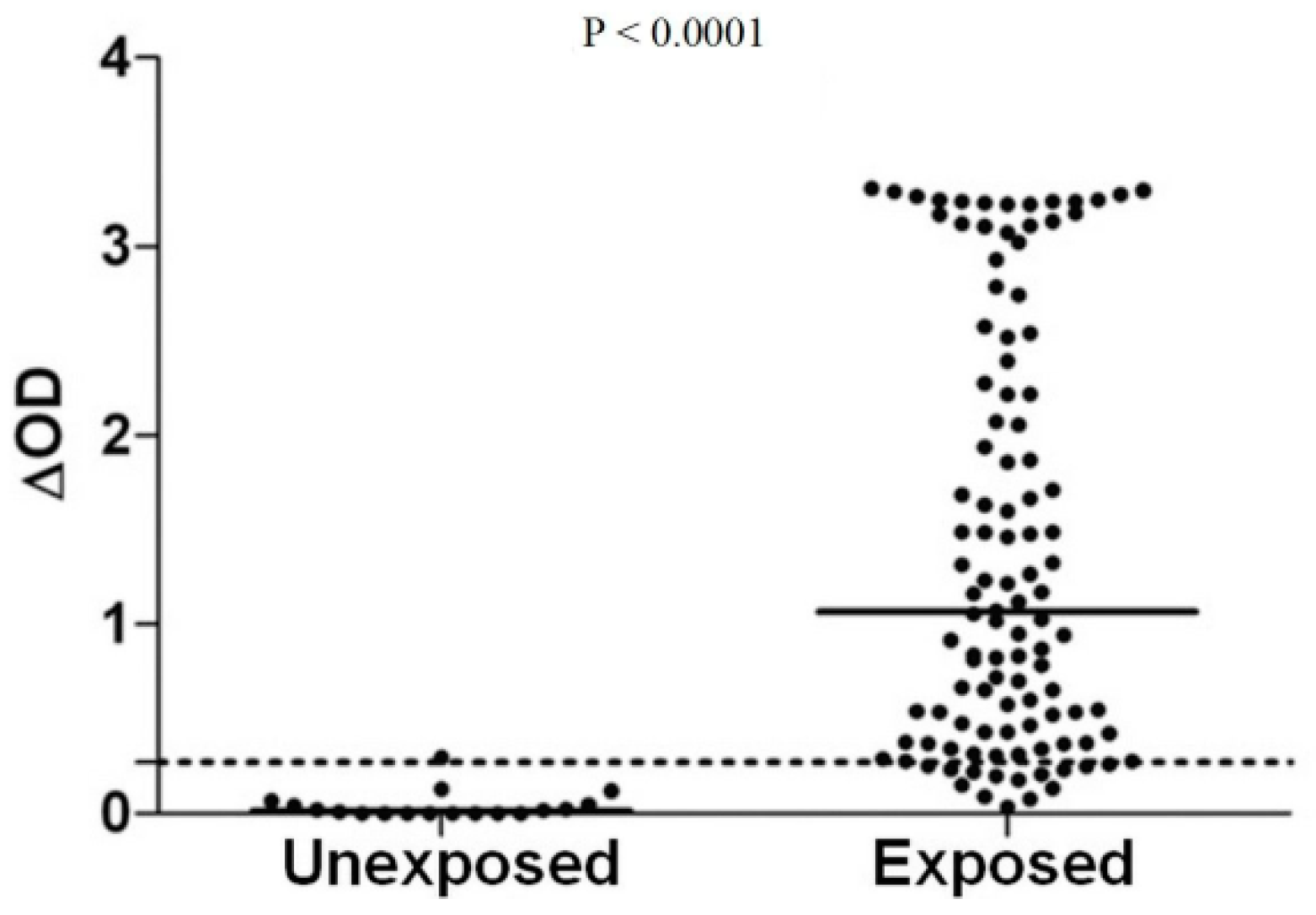
2.2. Validation of the Concept
2.3. From the Whole Saliva to Synthetic Salivary Peptide of Aedes
2.3.1. The Recombinant Protein Approach
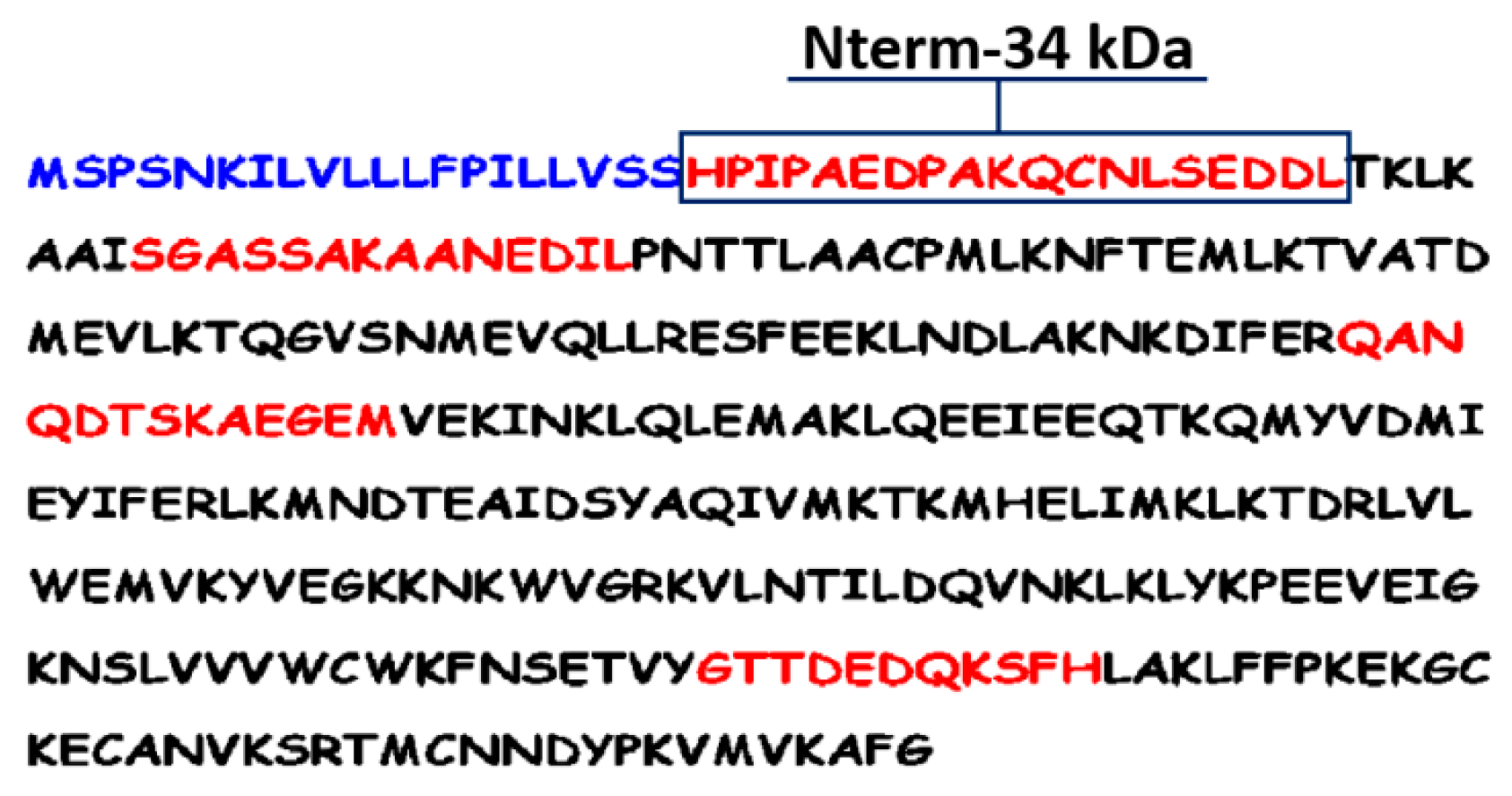
2.3.2. The Peptide Approach
2.3.3. Validation of the Synthetic Aedes Nterm-34 kDa Salivary Peptide
3. Potential Applications of Aedes Salivary Biomarker in the Field
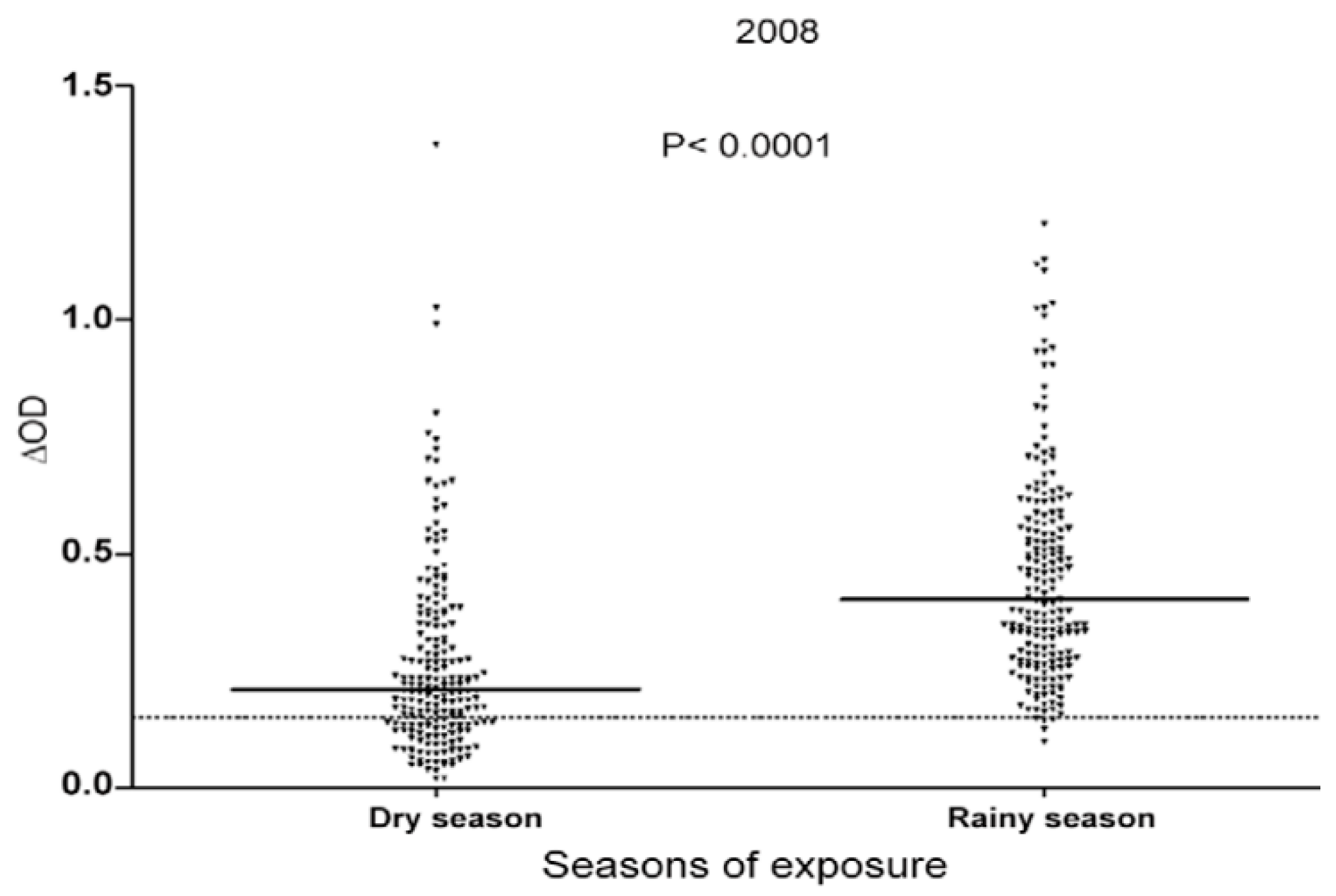
3.1. Evaluation of Human Exposure to Aedes Bites
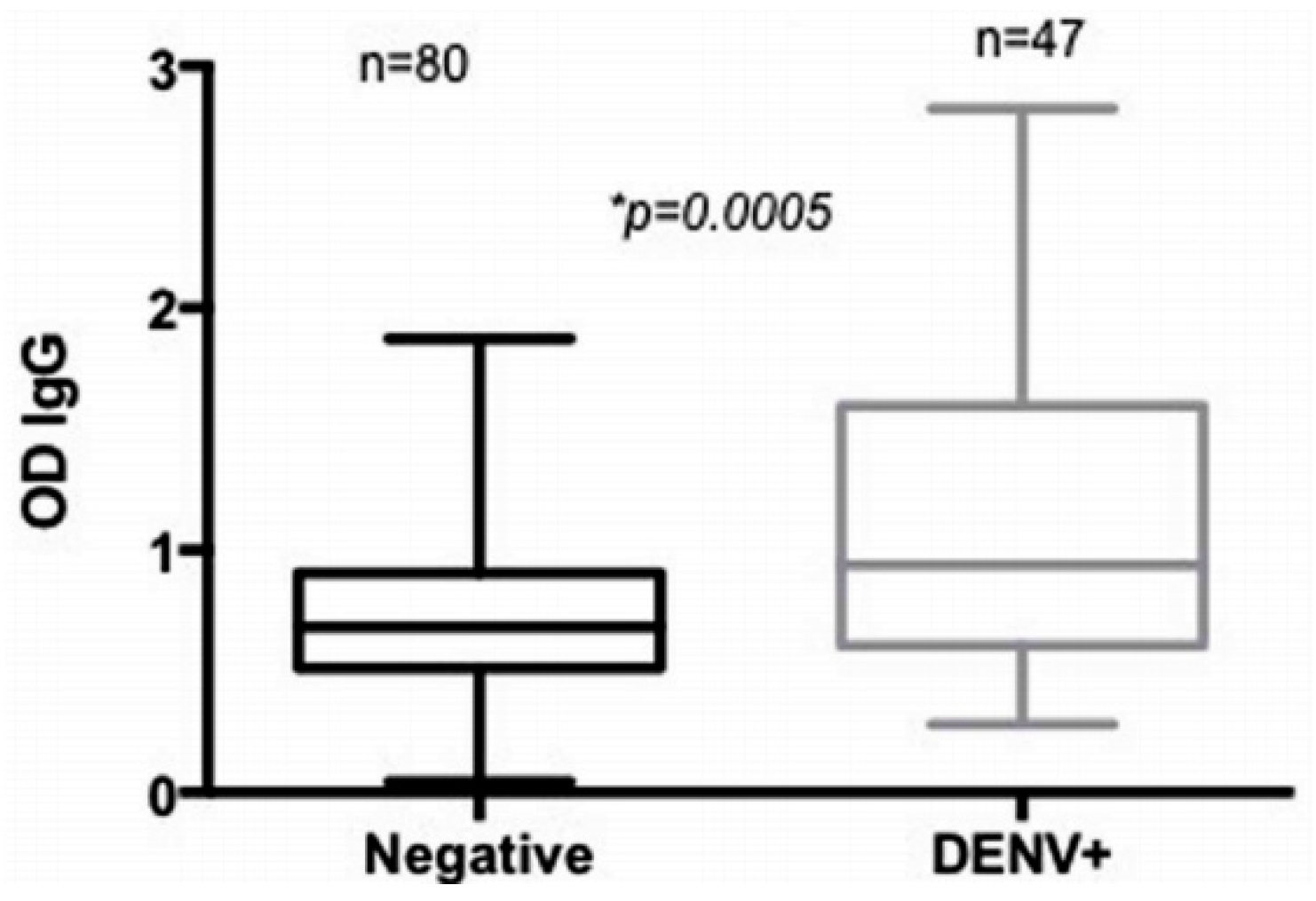
3.2. Evaluation of Arboviral Transmission Risk
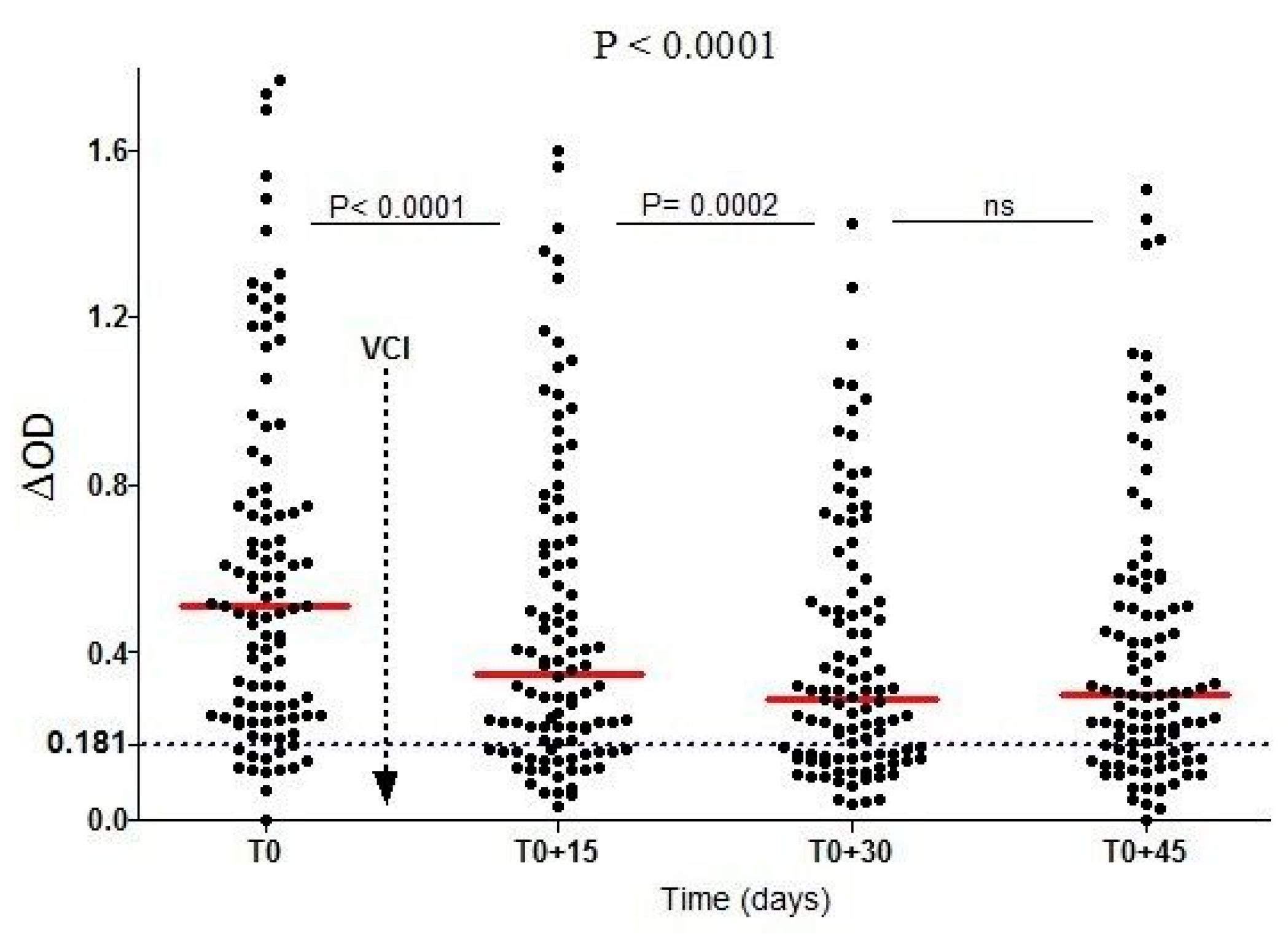
3.3. Evaluation of Vector Control Strategies’ Effectiveness
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gubler, D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002, 33, 330–342. [Google Scholar] [CrossRef]
- World Health Organization (WHO). A Global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014; p. 54. [Google Scholar]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Musso, D. Zika Virus transmission from French Polynesia to Brazil. Emerg. Infect. Dis. 2015, 21, 1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, X.; Zhu, Z.; Huang, L.; Liang, S.; Xu, Y.; Liao, R.; Zhou, L.; Zhang, Y.; Wilder-Smith, A. Early detection of Zika virus infection among travellers from areas of ongoing transmission in China. J. Travel Med. 2016, 23, taw047. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017; p. 53. [Google Scholar]
- Reiter, P.; Fontenille, D.; Paupy, C. Aedes albopictus as an epidemic vector of chikungunya virus: Another emerging problem? Lancet Infect. Dis. 2006, 6, 463–464. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Focks, D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Dibo, M.R.; Chierotti, A.P.; Ferrari, M.S.; Mendonca, A.L.; Chiaravalloti Neto, F. Study of the relationship between Aedes (Stegomyia) aegypti egg and adult densities, dengue fever and climate in Mirassol, state of Sao Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2008, 103, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Francischetti, I.M. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, P.F.; Baird, J.; Mitchell, J.A.; Drakeley, C. Immune interactions between mosquitoes and their hosts. Parasite Immunol. 2006, 28, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Remoue, F.; Cisse, B.; Ba, F.; Sokhna, C.; Herve, J.P.; Boulanger, D.; Simondon, F. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, A.; Diouf, I.; Bakkali, N.; Misse, D.; Pages, F.; Fusai, T.; Rogier, C.; Almeras, L. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasites Vectors 2011, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Remoue, F.; Alix, E.; Cornelie, S.; Sokhna, C.; Cisse, B.; Doucoure, S.; Mouchet, F.; Boulanger, D.; Simondon, F. IgE and IgG4 antibody responses to Aedes saliva in African children. Acta Trop. 2007, 104, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Brummer-Korvenkontio, H.; Palosuo, K.; Miyanij, M.; Ruiz-Maldonado, R.; Love, A.; Francois, G.; Palosuo, T. Frequent occurrence of IgE and IgG4 antibodies against saliva of Aedes communis and Aedes aegypti mosquitoes in children. Int. Arch. Allergy Immunol. 1994, 104, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Brummer-Korvenkontio, H.; Palosuo, K.; Palosuo, T.; Brummer-Korvenkontio, M.; Leinikki, P.; Reunala, T. Detection of mosquito saliva-specific IgE antibodies by capture ELISA. Allergy 1997, 52, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Orlandi-Pradines, E.; Almeras, L.; Denis de Senneville, L.; Barbe, S.; Remoue, F.; Villard, C.; Cornelie, S.; Penhoat, K.; Pascual, A.; Bourgouin, C.; et al. Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007, 9, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Drame, P.M.; Poinsignon, A.; Besnard, P.; Le Mire, J.; Dos-Santos, M.A.; Sow, C.S.; Cornelie, S.; Foumane, V.; Toto, J.C.; Sembene, M.; et al. Human antibody response to Anopheles gambiae saliva: An immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am. J. Trop. Med. Hyg. 2010, 83, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, A.; Pascual, A.; Orlandi-Pradines, E.; Diouf, I.; Remoue, F.; Pages, F.; Fusai, T.; Rogier, C.; Almeras, L. Relationship between exposure to vector bites and antibody responses to mosquito salivary gland extracts. PLoS ONE 2011, 6, e29107. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Mouchet, F.; Cornelie, S.; DeHecq, J.S.; Rutee, A.H.; Roca, Y.; Walter, A.; Herve, J.P.; Misse, D.; Favier, F.; et al. Evaluation of the human IgG antibody response to Aedes albopictus saliva as a new specific biomarker of exposure to vector bites. PLoS Negl. Trop. Dis. 2012, 6, e1487. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Cardenas, J.C.; Cardenas, L.D.; Christofferson, R.C.; Chisenhall, D.M.; Wesson, D.M.; McCracken, M.K.; Carvajal, D.; Mores, C.N. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS ONE 2013, 8, e81211. [Google Scholar] [CrossRef] [PubMed]
- Palosuo, K.; Brummer-Korvenkontio, H.; Mikkola, J.; Sahi, T.; Reunala, T. Seasonal increase in human IgE and IgG4 antisaliva antibodies to Aedes mosquito bites. Int. Arch. Allergy Immunol. 1997, 114, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Waitayakul, A.; Somsri, S.; Sattabongkot, J.; Looareesuwan, S.; Cui, L.; Udomsangpetch, R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop. 2006, 98, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Elanga Ndille, E.; Doucoure, S.; Damien, G.; Mouchet, F.; Drame, P.M.; Cornelie, S.; Noukpo, H.; Yamadjako, S.; Djenontin, A.; Moiroux, N.; et al. First attempt to validate human IgG antibody response to Nterm-34 kDa salivary peptide as biomarker for evaluating exposure to Aedes aegypti bites. PLoS Negl. Trop. Dis. 2012, 6, e1905. [Google Scholar] [CrossRef] [PubMed]
- Ndille, E.E.; Dubot-Peres, A.; Doucoure, S.; Mouchet, F.; Cornelie, S.; Sidavong, B.; Fournet, F.; Remoue, F. Human IgG antibody response to Aedes aegypti Nterm-34 kDa salivary peptide as an indicator to identify areas at high risk for dengue transmission: A retrospective study in urban settings of Vientiane city, Lao PDR. Trop. Med. Int. Health 2014, 19, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Elanga Ndille, E.; Doucoure, S.; Poinsignon, A.; Mouchet, F.; Cornelie, S.; D’Ortenzio, E.; DeHecq, J.S.; Remoue, F. Human IgG antibody response to Aedes Nterm-34 kDa salivary peptide, an epidemiological tool to assess vector control in chikungunya and dengue transmission area. PLoS Negl. Trop. Dis. 2016, 10, e0005109. [Google Scholar] [CrossRef] [PubMed]
- Yobo, M.C.; Sadia-Kacou, A.M.C.; Adja, A.M.; Elanga-Ndille, E.; Sagna, A.B.; Guindo-Coulibaly, N.; Poinsignon, A.; Remoue, F.; Koudou, B.G. Evaluation of human exposure to Aedes bites in rubber and palm cultivations using an immuno-epidemiological biomarker. BioMed Res. Int. 2018. accepted. [Google Scholar]
- Ribeiro, J.M. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 1987, 32, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Brummer-Korvenkontio, H.; Lappalainen, P.; Reunala, T.; Palosuo, T. Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J. Allergy Clin. Immunol. 1994, 93, 551–555. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, W.; James, A.A.; Lam, H.; Sun, D.; Cheng, L.; Simons, F.E. Expression, purification, characterization and clinical relevance of rAed a 1—A 68-kDa recombinant mosquito Aedes aegypti salivary allergen. Int. Immunol. 2001, 13, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xu, W.; Lam, H.; Cheng, L.; James, A.A.; Simons, F.E. A new recombinant mosquito salivary allergen, rAed a 2: Allergenicity, clinical relevance, and cross-reactivity. Allergy 2006, 61, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xu, W.W.; Sham, Y.; Lam, H.; Sun, D.; Cheng, L.; Rasic, N.F.; Guan, Q.; James, A.A.; Simons, F.E. Mosquito salivary allergen Aed a 3: Cloning, comprehensive molecular analysis, and clinical evaluation. Allergy 2016, 71, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Caihe, L.; Beckett, A.N.; Guan, Q.; James, A.A.; Simons, F.E. rAed a 4: A new 67-kDa Aedes aegypti mosquito salivary allergen for the diagnosis of mosquito allergy. Int. Arch. Allergy Immunol. 2016, 170, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.S.; Ribeiro, J.M.; Goldstein, M.D. Anti-tick antibodies: An epidemiologic tool in Lyme disease research. Am. J. Epidemiol. 1990, 132, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Rasic, N.; Liu, Y.; Simons, F.E. Mosquito saliva-specific IgE and IgG antibodies in 1059 blood donors. J. Allergy Clin. Immunol. 2002, 110, 816–817. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Mouchet, F.; Cournil, A.; Le Goff, G.; Cornelie, S.; Roca, Y.; Giraldez, M.G.; Simon, Z.B.; Loayza, R.; Misse, D.; et al. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: A new biomarker of exposure to dengue vector bites. Am. J. Trop. Med. Hyg. 2012, 87, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Mouchet, F.; Cornelie, S.; Drame, P.M.; D’Ortenzio, E.; DeHecq, J.S.; Remoue, F. Human antibody response to Aedes albopictus salivary proteins: A potential biomarker to evaluate the efficacy of vector control in an area of chikungunya and dengue virus transmission. BioMed Res. Int. 2014, 2014, 746509. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Sternberg, J.M.; Johnston, V.; Medrano-Mercado, N.; Anderson, J.M.; Hume, J.C.; Valenzuela, J.G.; Schaub, G.A.; Billingsley, P.F. Antibody responses of domestic animals to salivary antigens of Triatoma infestans as biomarkers for low-level infestation of triatomines. Int. J. Parasitol. 2009, 39, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Medrano-Mercado, N.; Billingsley, P.F.; Schaub, G.A.; Sternberg, J.M. IgM-antibody responses of chickens to salivary antigens of Triatoma infestans as early biomarkers for low-level infestation of triatomines. Int. J. Parasitol. 2010, 40, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Cardenas, J.C.; Giovanni, J.E.; Cardenas, L.; Villamizar, P.; Rolon, J.; Chisenhall, D.M.; Christofferson, R.C.; Carvajal, D.J.; Perez, O.G.; et al. Aedes aegypti anti-salivary gland antibody concentration and dengue virus exposure history in healthy individuals living in an endemic area in Colombia. Biomedica 2015, 35, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Drame, P.M. Salivary biomarkers in the control of mosquito-borne diseases. Insects 2015, 6, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Arca, B.; Lombardo, F.; Francischetti, I.M.; Pham, V.M.; Mestres-Simon, M.; Andersen, J.F.; Ribeiro, J.M. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem. Mol. Biol. 2007, 37, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Arca, B.; Lombardo, F.; Calvo, E.; Phan, V.M.; Chandra, P.K.; Wikel, S.K. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genom. 2007, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Martin-Martin, I.; Arca, B.; Calvo, E. A deep insight into the sialome of male and female Aedes aegypti mosquitoes. PLoS ONE 2016, 11, e0151400. [Google Scholar] [CrossRef] [PubMed]
- Cornelie, S.; Remoue, F.; Doucoure, S.; Ndiaye, T.; Sauvage, F.X.; Boulanger, D.; Simondon, F. An insight into immunogenic salivary proteins of Anopheles gambiae in African children. Malar. J. 2007, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Cornelie, S.; Patramool, S.; Mouchet, F.; Demettre, E.; Seveno, M.; Dehecq, J.S.; Rutee, H.; Herve, J.P.; Favier, F.; et al. First screening of Aedes albopictus immunogenic salivary proteins. Insect Mol. Biol. 2013, 22, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Helling, S.; Collin, N.; Teixeira, C.R.; Medrano-Mercado, N.; Hume, J.C.; Assumpcao, T.C.; Marcus, K.; Stephan, C.; Meyer, H.E.; et al. Immunogenic salivary proteins of Triatoma infestans: Development of a recombinant antigen for the detection of low-level infestation of triatomines. PLoS Negl. Trop. Dis. 2009, 3, e532. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.; Andrade, B.B.; Aquino, D.; Entringer, P.; Miranda, J.C.; Alcantara, R.; Ruiz, D.; Soto, M.; Teixeira, C.R.; Valenzuela, J.G.; et al. Using recombinant proteins from Lutzomyia longipalpis saliva to estimate human vector exposure in visceral leishmaniasis endemic areas. PLoS Negl. Trop. Dis. 2010, 4, e649. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Gomes, R.; Collin, N.; Reynoso, D.; Jochim, R.; Oliveira, F.; Seitz, A.; Elnaiem, D.E.; Caldas, A.; de Souza, A.P.; et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl. Trop. Dis. 2010, 4, e638. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Ronca, R.; Fiorentino, G.; Verra, F.; Mangano, V.; Poinsignon, A.; Sirima, S.B.; Nebie, I.; Lombardo, F.; Remoue, F.; et al. Humoral response to the Anopheles gambiae salivary protein gSG6: A serological indicator of exposure to Afrotropical malaria vectors. PLoS ONE 2011, 6, e17980. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Ronca, R.; Fiorentino, G.; Mangano, V.D.; Sirima, S.B.; Nebie, I.; Petrarca, V.; Modiano, D.; Arca, B. Wide cross-reactivity between Anopheles gambiae and Anopheles funestus SG6 salivary proteins supports exploitation of gSG6 as a marker of human exposure to major malaria vectors in tropical Africa. Malar. J. 2011, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.M.; Bakli, M.; Fontaine, A.; Bakkali, N.; Vu Hai, V.; Audebert, S.; Boublik, Y.; Pages, F.; Remoue, F.; Rogier, C.; et al. Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar. J. 2012, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; (MIVEGEC-IRD, Montpellier, France). IgG antibody response against Aedes aegypti saliva in a Bolivian urban population: Towards a new biomarker of exposure to dengue vector bites. Personal communication, 2009. [Google Scholar]
- Poinsignon, A.; Cornelie, S.; Mestres-Simon, M.; Lanfrancotti, A.; Rossignol, M.; Boulanger, D.; Cisse, B.; Sokhna, C.; Arca, B.; Simondon, F.; et al. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS ONE 2008, 3, e2472. [Google Scholar] [CrossRef] [PubMed]
- Poinsignon, A.; Samb, B.; Doucoure, S.; Drame, P.M.; Sarr, J.B.; Sow, C.; Cornelie, S.; Maiga, S.; Thiam, C.; Rogerie, F.; et al. First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Trop. Med. Int. Health 2010, 15, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Drame, P.M.; Weitzel, T.; Rosas, R.; Gripping, C.; Cardenas, J.C.; Alvares, M.; Wesson, D.M.; Poinsignon, A.; Remoue, F.; et al. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: A pilot study. Parasites Vectors 2015, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Ya-Umphan, P.; Cerqueira, D.; Parker, D.M.; Cottrell, G.; Poinsignon, A.; Remoue, F.; Brengues, C.; Chareonviriyaphap, T.; Nosten, F.; Corbel, V. Use of an Anopheles salivary biomarker to assess malaria transmission risk along the Thailand-Myanmar Border. J. Infect. Dis. 2017, 215, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Sagna, A.B.; Kassie, D.; Couvray, A.; Hermann, E.; Riveau, G.; Salem, G.; Fournet, F.; Remoue, F. Spatial risk of human exposure to Anopheles and Aedes mosquito bites in African urban settings using antibody-based biomarkers. Unpublished data.


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagna, A.B.; Yobo, M.C.; Elanga Ndille, E.; Remoue, F. New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications. Trop. Med. Infect. Dis. 2018, 3, 80. https://doi.org/10.3390/tropicalmed3030080
Sagna AB, Yobo MC, Elanga Ndille E, Remoue F. New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications. Tropical Medicine and Infectious Disease. 2018; 3(3):80. https://doi.org/10.3390/tropicalmed3030080
Chicago/Turabian StyleSagna, André B., Mabo C. Yobo, Emmanuel Elanga Ndille, and Franck Remoue. 2018. "New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications" Tropical Medicine and Infectious Disease 3, no. 3: 80. https://doi.org/10.3390/tropicalmed3030080
APA StyleSagna, A. B., Yobo, M. C., Elanga Ndille, E., & Remoue, F. (2018). New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications. Tropical Medicine and Infectious Disease, 3(3), 80. https://doi.org/10.3390/tropicalmed3030080




