On the Home Front: Specialised Reference Testing for Dengue in the Australasian Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Samples and Diagnostic Reporting
2.3. Testing Protocols
2.3.1. PHV Overview
RT-rtPCR
DEN 1–4 RT-PCR
Virus Culture
Molecular Genotyping
Serology
2.3.2. ESR Overview
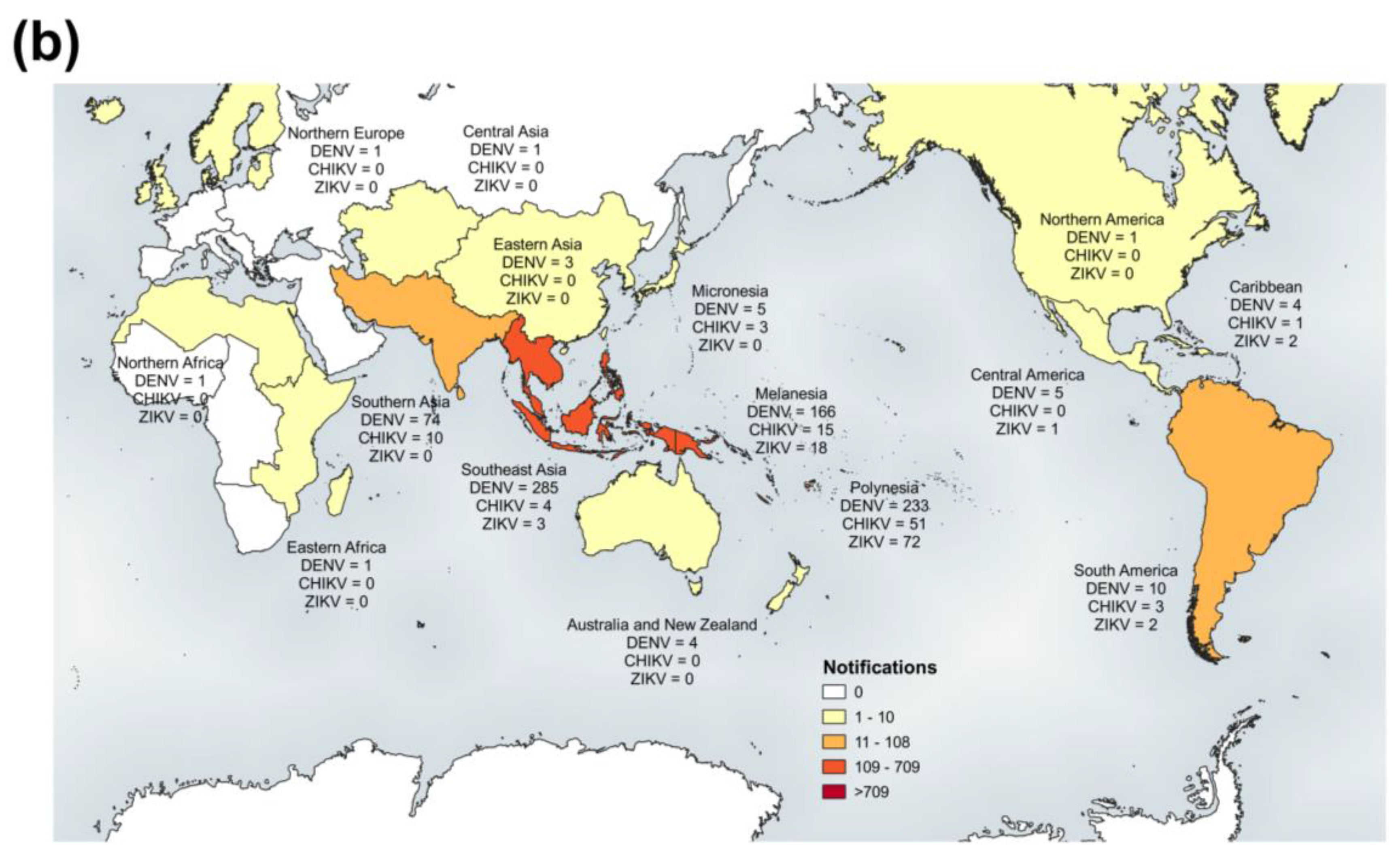
3. Results
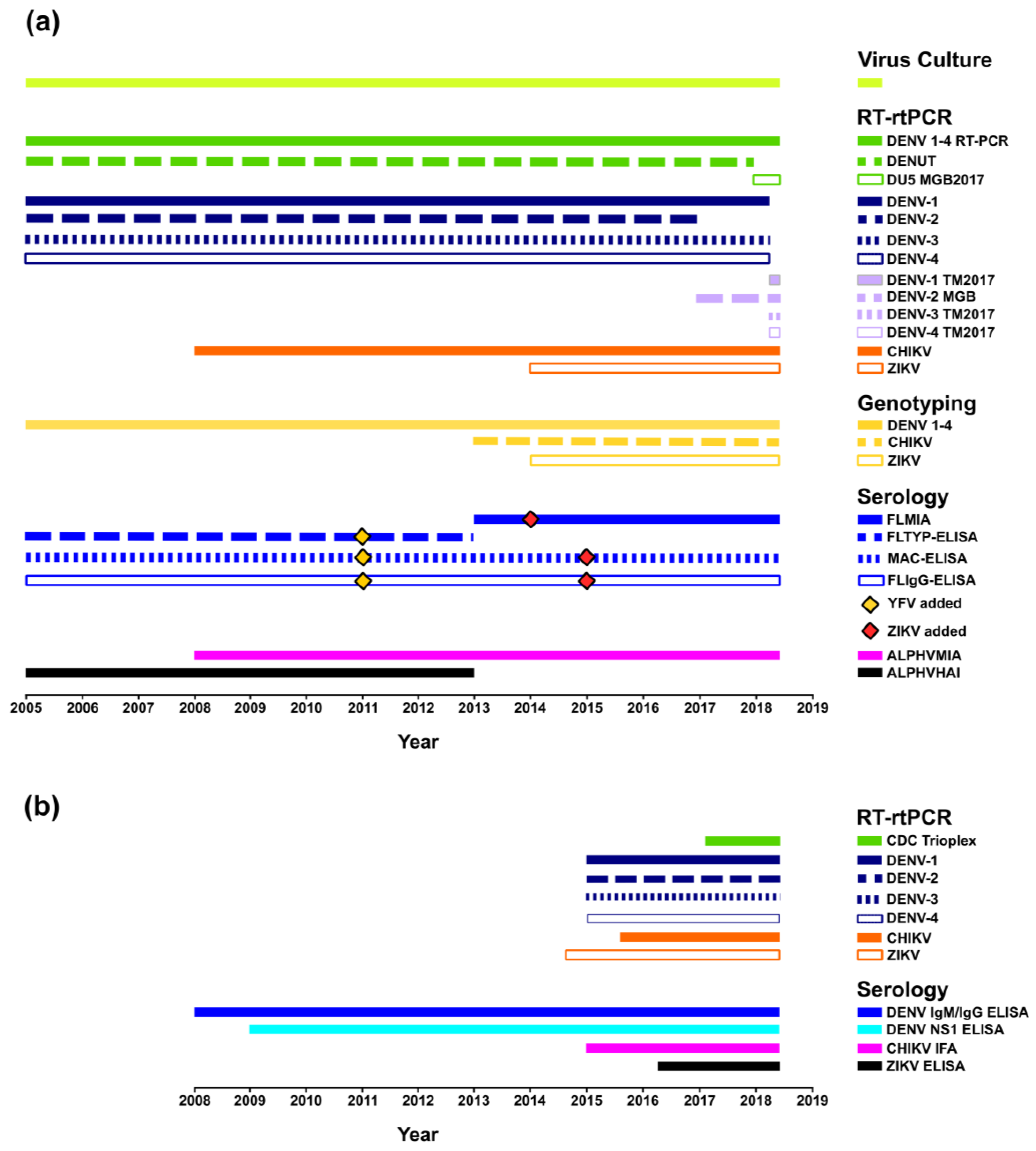
3.1. Multi-Faceted Reference-Based Methods
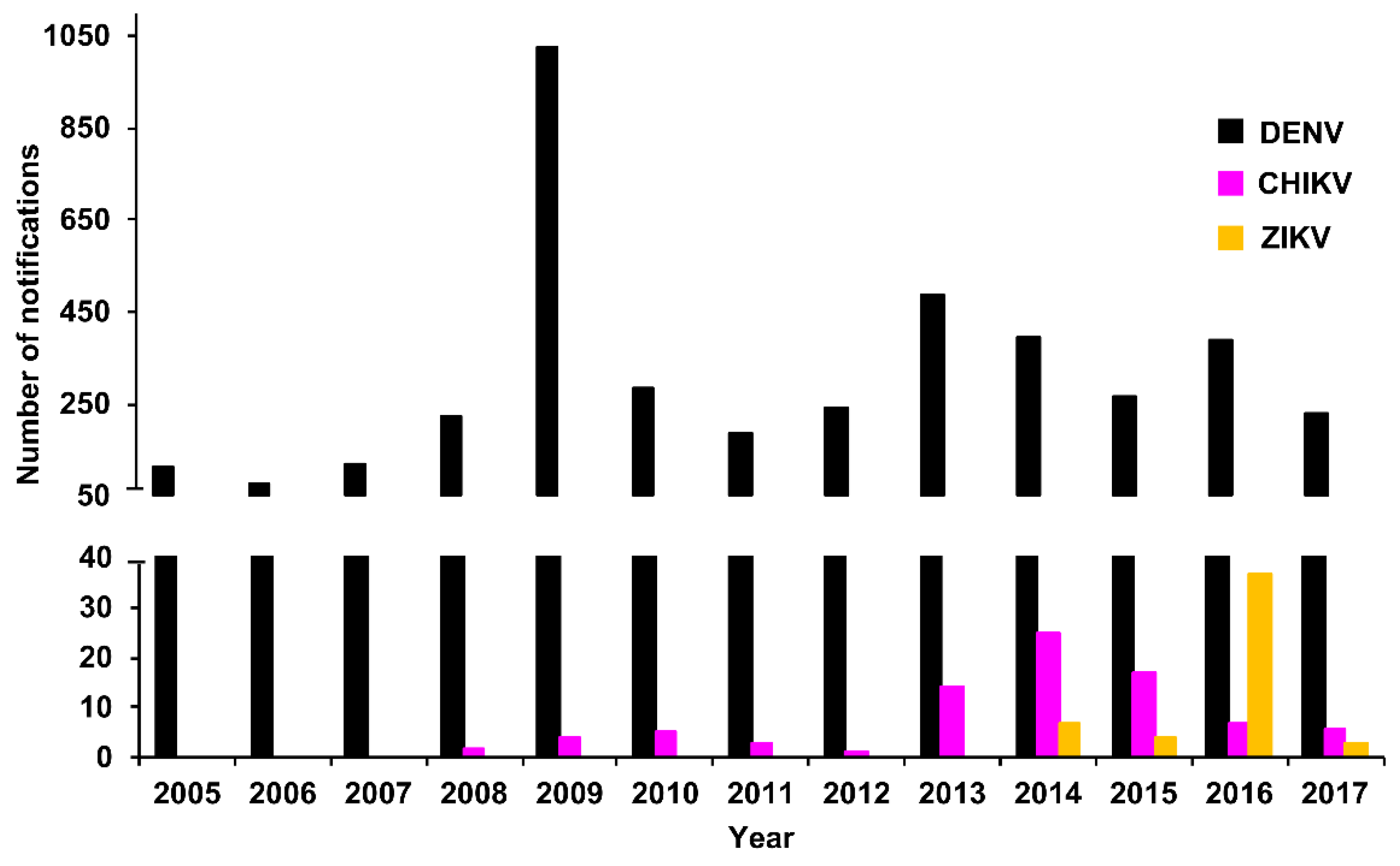
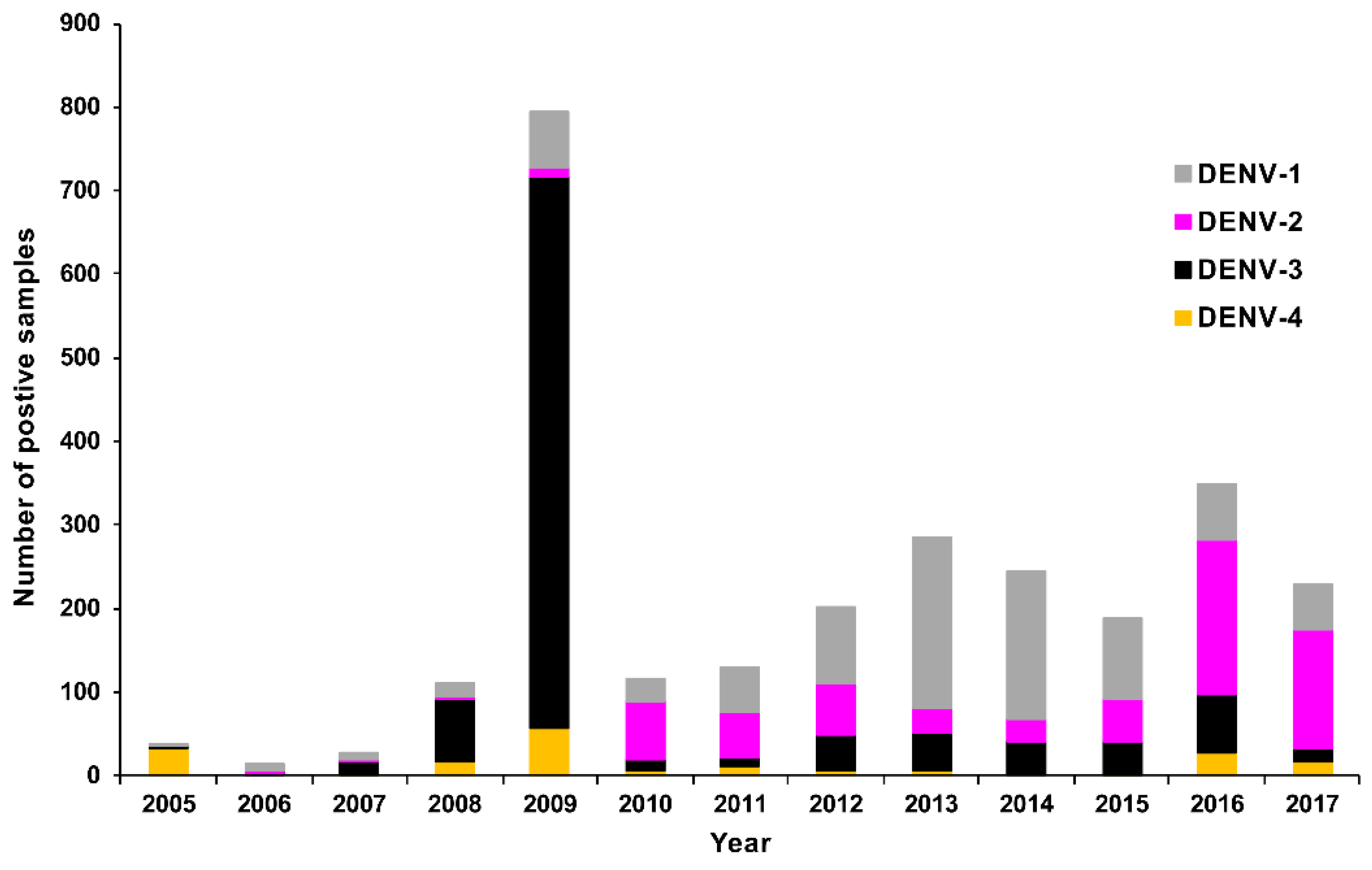
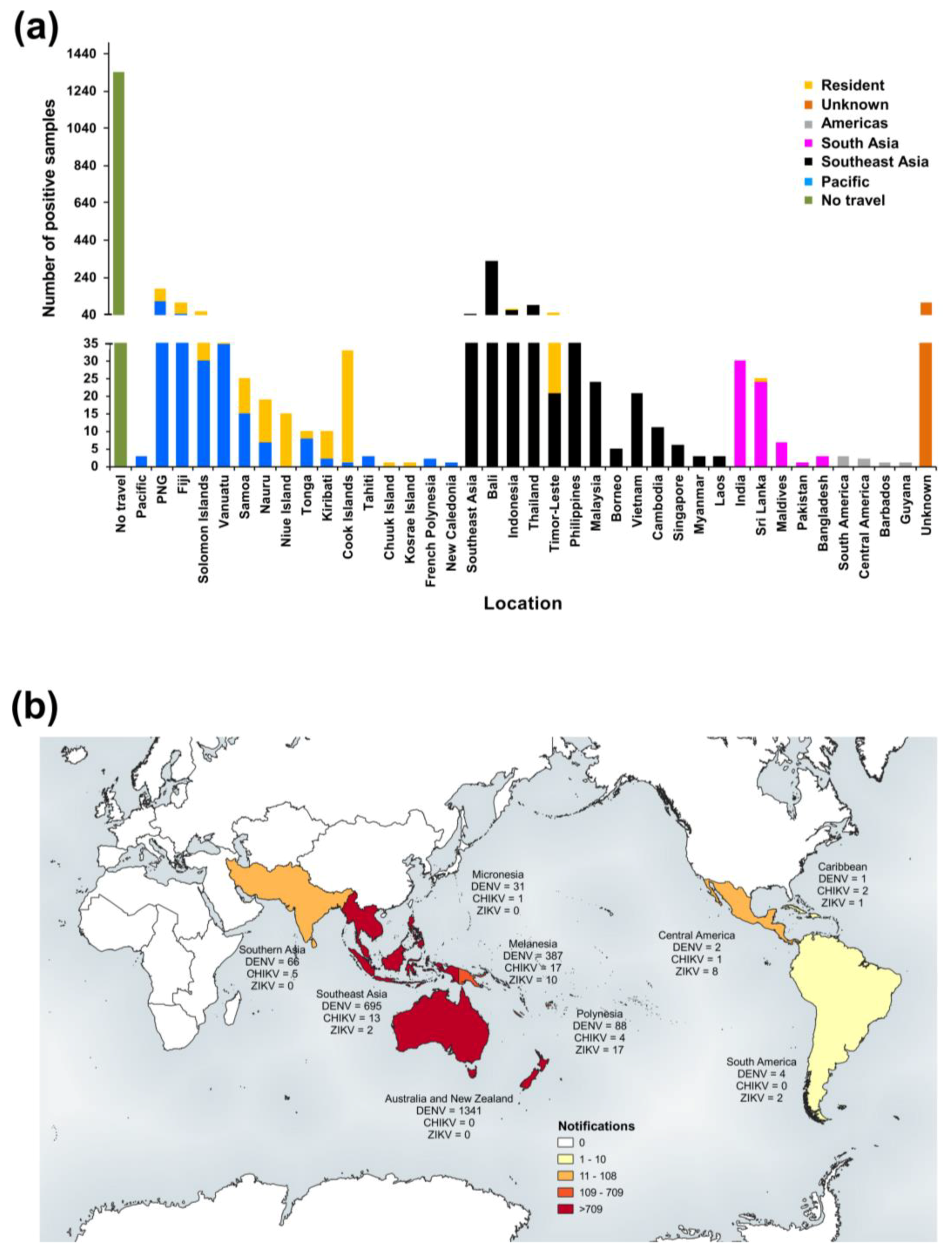
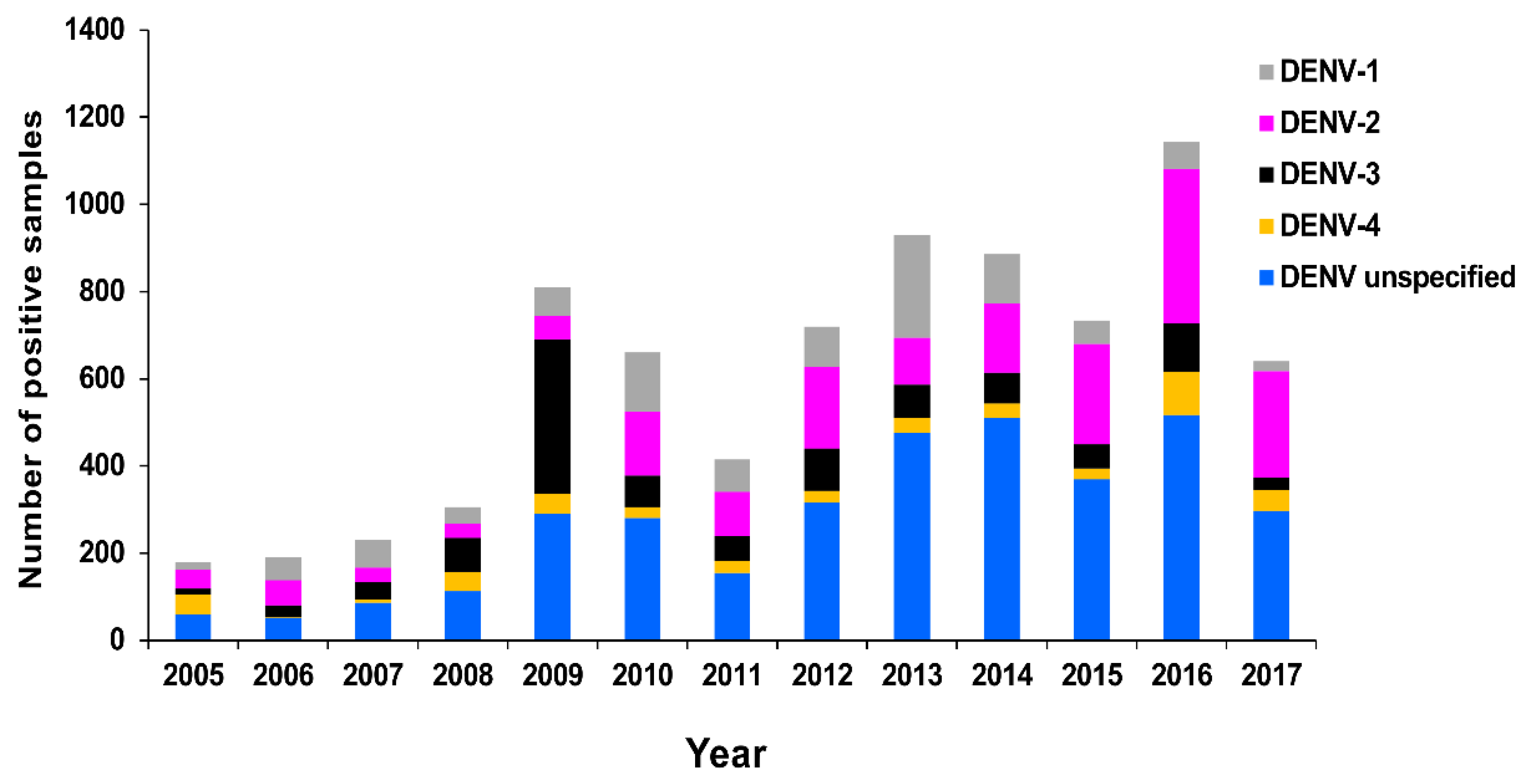
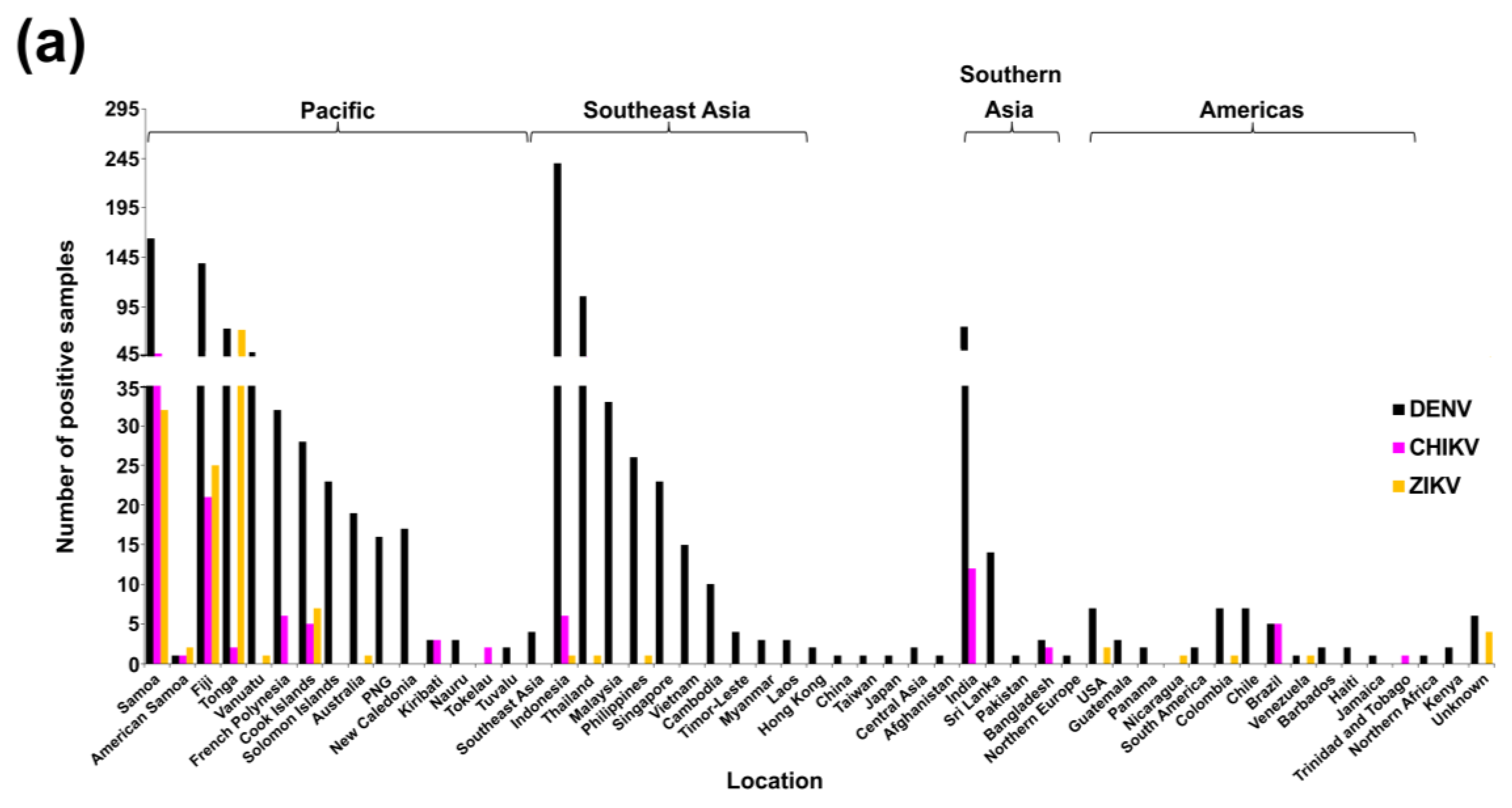
3.2. Confirmed Cases
3.2.1. PHV
RT-rtPCR and RT-PCR
DENV Serology
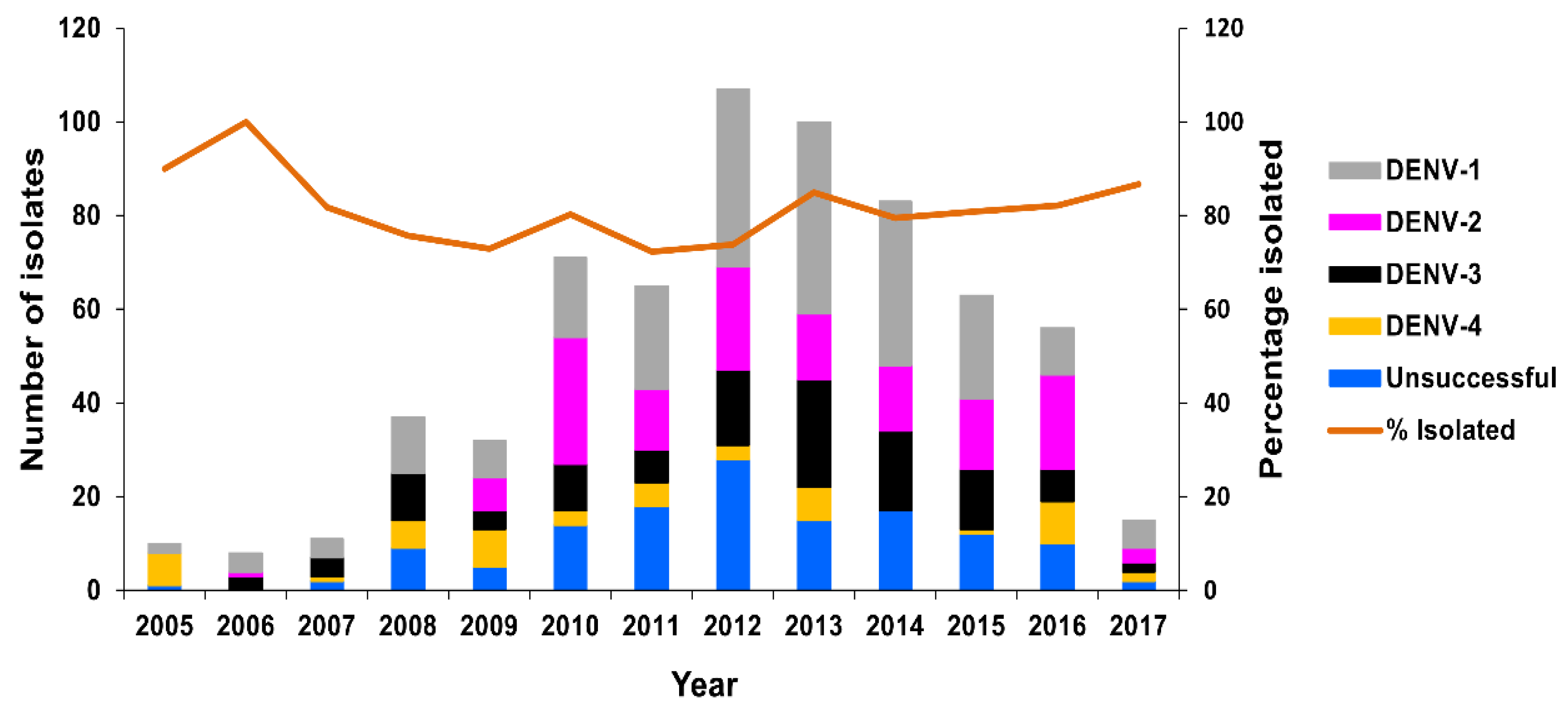
Virus Culture
Molecular Genotyping
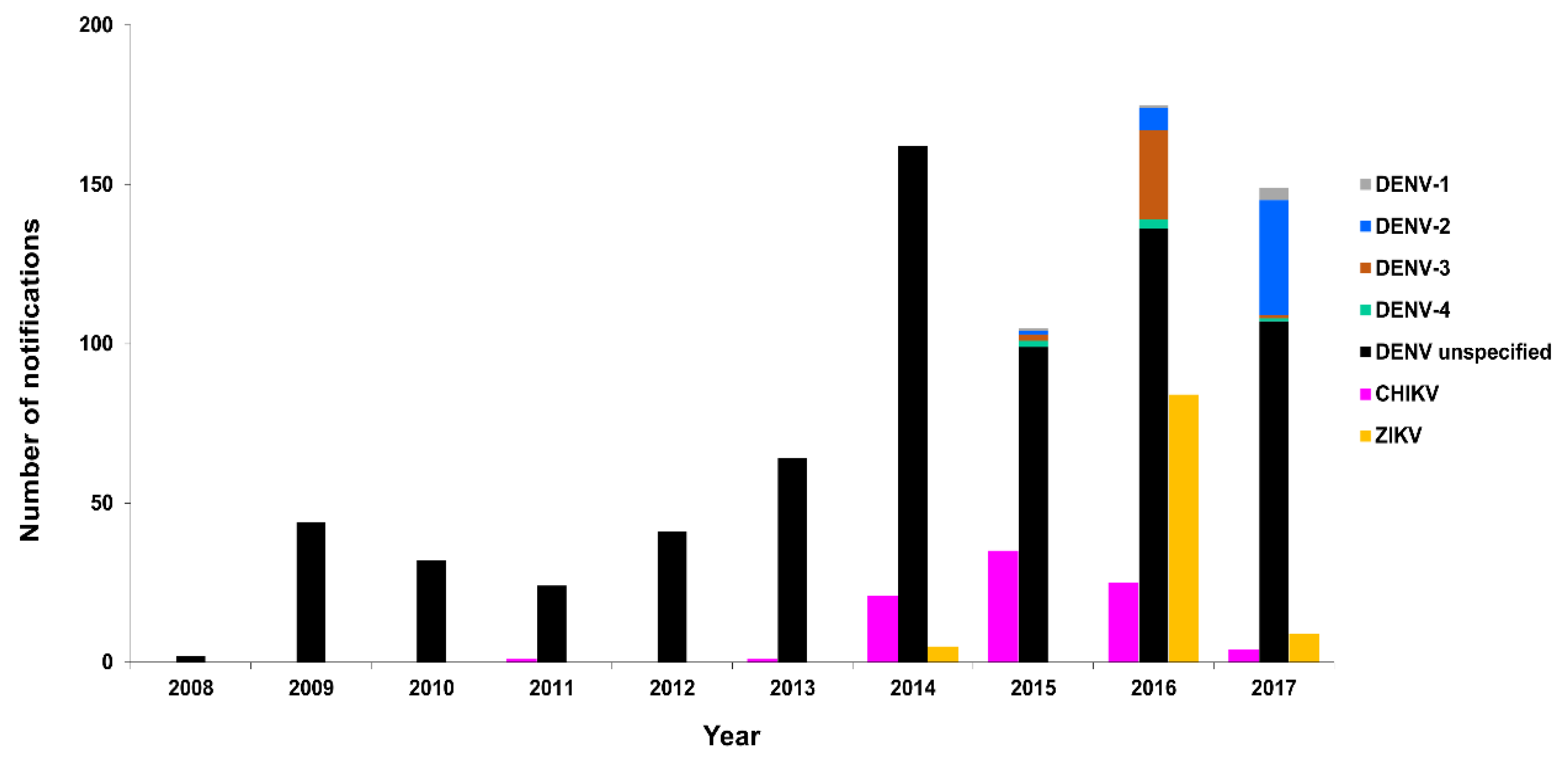
3.2.2. New Zealand
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Dengue and Severe Dengue Fact Sheet. 2017. Available online: http://www.who.int/mediacenter/factsheets/fs117/en/ (accessed on 19 February 2018).
- Calisher, C.H.; Karabatsos, N. Arbovirus serogroups: Definition and geographic distribution. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1988; Volume I, pp. 19–57. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [PubMed]
- Mackenzie, J.S.; la Brooy, J.T.; Hueston, L.; Cunningham, A.L. Dengue in Australia. J. Med. Microbiol. 1996, 45, 159–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Western Pacific Regional Action Plan for Dengue Prevention and Control. World Health Organization Regional Office for the Western Pacific (2016). Manila, Philippines 2017, Licence: CC BY-NC-SA 3.0 IGO. Available online: http://iris.wpro.who.int/bitstream/handle/10665.1/13599/9789290618256-eng.pdf?ua=1 (accessed on 7 March 2018).
- Moore, P.R.; van den Hurk, A.F.; Mackenzie, J.S.; Pyke, A.T. Dengue viruses in Papua New Guinea: Evidence of endemicity and phylogenetic variation, including the evolution of new genetic lineages. Emerg. Microbes Infect. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Kitau, R.; Samiak, L.; Guldan, G.; Machine, E. The public health challenge of dengue fever in Papua New Guinea. Pac. J. Med. Sci. 2016, 16, 20–26. [Google Scholar]
- Ng, L.C. Challenges in dengue surveillance and control. Western Pac. Surveill. Response J. 2011, 2, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Daly, M.T.; Cameron, J.N.; Moore, P.R.; Taylor, C.T.; Hewitson, G.R.; Humphreys, J.L.; Gair, R. Imported Zika virus infection from the Cook Islands into Australia, 2014. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Moore, P.R.; Hall-Mendelin, S.; McMahon, J.L.; Harrower, B.J.; Constantino, T.R.; van den Hurk, A.F. Isolation of Zika virus imported from Tonga into Australia. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Roche, C.; Aubry, M.; Teissier, A.; Lastere, S.; Daudens, E.; Mallet, H.P.; Musso, D.; Aaskov, J. Recent emergence of dengue virus serotype 4 in French Polynesia results from multiple introductions from other South Pacific islands. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuegoonpipat, A.; Berlioz-Arthaud, A.; Chow, V.; Endy, T.; Lowry, K.; Mai le, Q.; Ninh, T.U.; Pyke, A.; Reid, M.; Reynes, J.M.; et al. Sustained transmission of dengue virus type 1 in the Pacific due to repeated introductions of different Asian strains. Virology 2004, 329, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Horwood, P.F.; Reimer, L.J.; Dagina, R.; Susapu, M.; Bande, G.; Katusele, M.; Koimbu, G.; Jimmy, S.; Ropa, B.; Siba, P.M.; et al. Outbreak of chikungunya virus infection, Vanimo, Papua New Guinea. Emerg. Infect. Dis. 2013, 19, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Northill, J.A.; Pyke, A.T. Sources of dengue viruses imported into Queensland, Australia, 2002-2010. Emerg. Infect. Dis. 2012, 18, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T. The origins of dengue outbreaks in northern Queensland, Australia, 1990–2017. Microbiol. Aus. 2018, 39. [Google Scholar] [CrossRef]
- Laird, M.; Calder, L.; Thornton, R.C.; Syme, R.; Holder, P.W.; Mogi, M. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. J. Am. Mosq. Control. Assoc. 1994, 10, 14–23. [Google Scholar] [PubMed]
- Border Health Newsletter, New Zealand Biosecure. January 2018. Available online: https://www.smsl.co.nz/site/southernmonitoring/files/Newsletters/2018/2018_1_NZB%20BH%20newsletter%20January%2018-1.pdf (accessed on 5 March 2018).
- Pyke, A.T.; Moore, P.R.; Taylor, C.T.; Hall-Mendelin, S.; Cameron, J.N.; Hewitson, G.R.; Pukallus, D.S.; Huang, B.; Warrilow, D.; van den Hurk, A.F. Highly divergent dengue virus type 1 genotype sets a new distance record. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Holmes, E.C.; Fokam, E.B.; Faye, O.; Diallo, M.; Sall, A.A.; Weaver, S.C. Evolutionary processes among sylvatic dengue type 2 viruses. J. Virol. 2007, 81, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Weaver, S.C. The history and evolution of human dengue emergence. Adv. Virus Res. 2008, 72, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.; Palacios, G.; Martinez, J.A.; Vazquez, A.; Savji, N.; De Ory, F.; Sanchez-Seco, M.P.; Martin, D.; Lipkin, W.I.; Tenorio, A. First report of sylvatic DENV-2-associated dengue hemorrhagic fever in West Africa. PLoS Negl. Trop. Dis. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Saluzzo, J.F.; Cornet, M.; Castagnet, P.; Rey, C.; Digoutte, J.P. Isolation of dengue 2 and dengue 4 viruses from patients in Senegal. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 5. [Google Scholar] [CrossRef]
- Cardosa, J.; Ooi, M.H.; Tio, P.H.; Perera, D.; Holmes, E.C.; Bibi, K.; Abdul Manap, Z. Dengue virus serotype 2 from a sylvatic lineage isolated from a patient with dengue hemorrhagic fever. PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef] [PubMed]
- Teoh, B.T.; Sam, S.S.; Abd-Jamil, J.; AbuBakar, S. Isolation of ancestral sylvatic dengue virus type 1, Malaysia. Emerg. Infect. Dis. 2010, 16, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Huang, B.; Warrilow, D.; Moore, P.R.; McMahon, J.; Harrower, B. Complete genome sequence of a highly divergent dengue virus type 2 strain, imported into Australia from Sabah, Malaysia. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T. Second dengue infection with a highly divergent sylvatic dengue virus strain, Brisbane, Queensland, Australia. ProMED-mail 2016, 7th May: 20160507.4207603. Available online: http://www.promedmail.org (accessed on 5 April 2017).
- Jackson, B. Utilization management: The role of reference laboratories. In Utilization Management in the Clinical Laboratory and Other Ancillary Services; Lewandrowski, K., Ed.; Springer International Publishing: Basel, Switzerland, 2017; pp. 171–176. [Google Scholar]
- Queensland Dengue Management Plan, 2015–2020. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0022/444433/dengue-mgt-plan.pdf (accessed on 27 June 2018).
- Zhang, F.C.; Li, X.F.; Deng, Y.Q.; Tong, Y.G.; Qin, C.F. Excretion of infectious Zika virus in urine. Lancet Infect. Dis. 2016, 16, 641–642. [Google Scholar] [CrossRef]
- Taylor, C.T.; Mackay, I.M.; McMahon, J.L.; Wheatley, S.L.; Moore, P.R.; Finger, M.J.; Hewitson, G.R.; Moore, F.A. Detection of specific ZIKV IgM in travelers, using a 2 multiplexed flavivirus microsphere immunoassay. Viruses 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Calisher, C.H.; Gubler, D.J.; Chang, G.J.; Vorndam, A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 545–551. [Google Scholar] [PubMed]
- van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Smith, G.A.; Mackenzie, J.S. Vector competence of Australian mosquitoes for chikungunya virus. Vector Borne Zoonotic Dis. 2010, 10, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Smith, I.L.; Van Den Hurk, A.F.; Northill, J.A.; Chuan, T.F.; Westacott, A.J.; Smith, G.A. Detection of Australasian flavivirus encephalitic viruses using rapid fluorogenic TaqMan RT-PCR assays. J. Virol. Methods 2004, 117, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hall-Mendelin, S.; Pyke, A.T.; Moore, P.R.; Mackay, I.M.; McMahon, J.L.; Ritchie, S.A.; Taylor, C.T.; Moore, F.A.; van den Hurk, A.F. Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of Zika virus in Australia. PLoS Negl. Trop. Dis. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Northill, J.A.; Pyke, A.; Smith, G.A. Single rapid TaqMan fluorogenic probe based PCR assay that detects all four dengue serotypes. J. Med. Virol. 2002, 66, 524–528, Erratum in 2003, 71, 473. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.D.; Wu, S.J.; Dion-Schultz, A.; Mangold, B.E.; Peruski, L.F.; Watts, D.M.; Porter, K.R.; Murphy, G.R.; Suharyono, W.; King, C.C.; et al. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 2001, 39. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Pyke, A.T.; Northill, J.A.; Mackay, I.M. Dengue virus type 2 (DENV-2) prMG-multiplex taqMan assay. Protocols.io 2018. [Google Scholar] [CrossRef]
- Mackay, I.M.; Northill, J.A.; Pyke, A.T. Dengue virus type 2 (DENV-2) capsid-Thai taqMan assay. Protocols.io 2018. [Google Scholar] [CrossRef]
- Pyke, A.T.; Williams, D.T.; Nisbet, D.J.; van den Hurk, A.F.; Taylor, C.T.; Johansen, C.A.; Macdonald, J.; Hall, R.A.; Simmons, R.J.; Mason, R.J.; et al. The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am. J. Trop. Med. Hyg. 2001, 65, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.R.; Roehrig, J.T. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of western equine encephalitis virus. Virology 1985, 142, 334–346. [Google Scholar] [CrossRef]
- Goh, L.Y.; Hobson-Peters, J.; Prow, N.A.; Gardner, J.; Bielefeldt-Ohmann, H.; Pyke, A.T.; Suhrbier, A.; Hall, R.A. Neutralizing monoclonal antibodies to the E2 protein of chikungunya virus protects against disease in a mouse model. Clin. Immunol. 2013, 149, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pyke, A.T.; McMahon, J.; Warrilow, D. Complete coding sequence of a case of chikungunya virus imported into Australia. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Moore, P.R.; McMahon, J. New insights into chikungunya virus emergence and spread from Southeast Asia. Emerg. Microbes Infect. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Simmons, R.; Smith, I. Development of immunoglobulin M capture enzyme-linked immunosorbent assay to differentiate human flavivirus infections occurring in Australia. Clin. Diagn. Lab. Immunol. 2005, 12, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Musso, D. Emerging arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [Google Scholar] [CrossRef]
- Musso, D.; Cao-Lormeau, V.M.; Gubler, D.J. Zika virus: Following the path of dengue and chikungunya? Lancet 2015, 386, 243–244. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F.; et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Rouzeyrol, M.; Caro, V.; Guillaumot, L.; Vazeille, M.; D’Ortenzio, E.; Thiberge, J.M.; Baroux, N.; Gourinat, A.C.; Grandadam, M.; Failloux, A.B. Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific region). Vector Borne Zoonotic Dis. 2012, 12, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Cassadou, S.; Boucau, S.; Petit-Sinturel, M.; Huc, P.; Leparc-Goffart, I.; Ledrans, M. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo Rdo, S.; Kraemer, M.U.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Notifiable Conditions Annual Reporting, Queensland Government. 2018. Available online: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/surveillance/reports/notifiable/annual (accessed on 9 February 2018).
- Ritchie, S.A.; Pyke, A.T.; Hall-Mendelin, S.; Day, A.; Mores, C.N.; Christofferson, R.C.; Gubler, D.J.; Bennett, S.N.; van den Hurk, A.F. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Chen, R.; Puri, V.; Fedorova, N.; Lin, D.; Hari, K.L.; Jain, R.; Rodas, J.D.; Das, S.R.; Shabman, R.S.; Weaver, S.C. Comprehensive genome scale phylogenetic study provides new insights on the global expansion of chikungunya virus. J. Virol. 2016, 90, 10600–10611. [Google Scholar] [CrossRef] [PubMed]
- Harrower, J.; Kiedrzynski, T.; Baker, S.; Upton, A.; Rahnama, F.; Sherwood, J.; Huang, Q.S.; Todd, A.; Pulford, D. Sexual transmission of Zika virus and persistence in semen, New Zealand, 2016. Emerg. Infect. Dis. 2016, 22, 1855–1857. [Google Scholar] [CrossRef] [PubMed]
- Brookes, D.L.; Tropical Public Health Services, Cairns, Queensland, Australia; Humphreys, J.L.; Tropical Public Health Unit, Townsville, Queensland, Australia. Personal communication, 2018.
- Pacific: Dengue Outbreak—October 2016. Available online: https://reliefweb.int/disaster/ep-2016-000112-slb/thumb#content_top (accessed on 4 April 2018).
- Dunbar, S.A.; Hoffmeyer, M.R. Microsphere-based multiplex immunoassays: Development and applications using Luminex® XMap® Technology. In The Immunoassay Handbook, 4th ed.; Wild, D.G., Ed.; Elsevier Ltd.: Oxford, UK, 2013; pp. 157–174. [Google Scholar]
- Hall-Mendelin, S.; Pyke, A.T.; Moore, P.R.; Ritchie, S.A.; Moore, F.A.J.; van den Hurk, A.F. Characterization of a western Pacific Zika virus strain in Australian Aedes aegypti. Vector Borne Zoonotic Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Beebe, N.W.; Cooper, R.D.; Mottram, P.; Sweeney, A.W. Australia’s dengue risk driven by human adaptation to climate change. PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Nicholson, J.; Beebe, N.W.; Davis, J.; Muzari, O.M.; Russell, R.C.; Devine, G.J.; Ritchie, S.A. Ten years of the tiger: Aedes albopictus presence in Australia since its discovery in the Torres Strait in 2005. One Health 2016, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Watterson, D.; Hall, R.A.; Davis, S.S.; Weir, R.; Kurucz, N.; Whelan, P.; Allcock, R.; Hall-Mendelin, S.; O’Brien, C.A.; Hobson-Peters, J. A new species of mesonivirus from the Northern Territory, Australia. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Public Health Surveillance, Episurv, New Zealand Ministry of Health. 2018. Available online: https://surv.esr.cri.nz/episurv/index.php (accessed on 28 February 2018).
- One Health Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/onehealth/index.html (accessed on 19 April 2018).
- Pyke, A.T.; Warrilow, D. Archival collections are important in the study of the biology, diversity, and evolution of arboviruses. Evol. Bioinform. Online 2016, 12, 27–30. [Google Scholar] [CrossRef] [PubMed]
| Virus | RT-rtPCR/RT-PCR a,b | Genome Region c,d,e,f |
|---|---|---|
| DENV 1–4 | DU5 MGB2017 a | 3′UTR |
| DENV-1 | DENV-1 TM2017a | 3′UTR |
| DENV-2 | DENV-2 MGBa | 3′UTR |
| DENV-3 | DENV-3 TM2017a | 3′UTR |
| DENV-4 | DENV-4 TM2017a | 3′UTR |
| DENV 1–4 | DENV 1–4 nested b [34] | C-prM |
| ZIKV | ZIKV Pacific Ea [11] | E |
| ZIKV | ZIKV Pacific NS1 a [11] | NS1 |
| CHIKV | CHIKV MA a [35] | E1 |
| Queensland | NZ | |||||
|---|---|---|---|---|---|---|
| Virus | Years Reported a | Total b | Mean c | Years Reported a | Total b | Mean c |
| DENV | 2005–2017 (13) | 4037 | 311 | 2008–2017 (10) | 798 | 80 |
| CHIKV | 2008–2017 (10) | 84 | 8 | 2011–2017 (7) | 87 | 12 |
| ZIKV | 2014–2017 (4) | 51 | 13 | 2014–2017 (4) | 98 | 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyke, A.T.; Gunn, W.; Taylor, C.; Mackay, I.M.; McMahon, J.; Jelley, L.; Waite, B.; May, F. On the Home Front: Specialised Reference Testing for Dengue in the Australasian Region. Trop. Med. Infect. Dis. 2018, 3, 75. https://doi.org/10.3390/tropicalmed3030075
Pyke AT, Gunn W, Taylor C, Mackay IM, McMahon J, Jelley L, Waite B, May F. On the Home Front: Specialised Reference Testing for Dengue in the Australasian Region. Tropical Medicine and Infectious Disease. 2018; 3(3):75. https://doi.org/10.3390/tropicalmed3030075
Chicago/Turabian StylePyke, Alyssa T., Wendy Gunn, Carmel Taylor, Ian M. Mackay, Jamie McMahon, Lauren Jelley, Ben Waite, and Fiona May. 2018. "On the Home Front: Specialised Reference Testing for Dengue in the Australasian Region" Tropical Medicine and Infectious Disease 3, no. 3: 75. https://doi.org/10.3390/tropicalmed3030075
APA StylePyke, A. T., Gunn, W., Taylor, C., Mackay, I. M., McMahon, J., Jelley, L., Waite, B., & May, F. (2018). On the Home Front: Specialised Reference Testing for Dengue in the Australasian Region. Tropical Medicine and Infectious Disease, 3(3), 75. https://doi.org/10.3390/tropicalmed3030075






