Seroprevalence of West Nile Virus in Blood Donors in Mainland Portugal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data and Sample Collection
2.3. Serological Study
2.3.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3.2. Virus Neutralization Test (VNT)
2.4. Statistical Analysis
3. Results
3.1. Serological Results
3.2. Associations Between Sociodemographic Variables and Positive/Borderline Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E.; et al. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- World Health Organisation. Dengue and Severe Dengue 2022; WHO Fact Sheet; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- Erazo, D.; Grant, L.; Ghisbain, G.; Marini, G.; Colón-González, F.J.; Wint, W.; Rizzoli, A.; Van Bortel, W.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Contribution of climate change to the spatial expansion of West Nile virus in Europe. Nat. Commun. 2024, 15, 1196. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile virus: Biology, transmission, and human infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Young, J.J.; Haussig, J.M.; Aberle, S.W.; Pervanidou, D.; Riccardo, F.; Sekulić, N.; Bakonyi, T.; Gossner, C.M. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Eurosurveillance 2021, 26, 2001095. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, e96. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Epidemiological Update: West Nile Virus Transmission Season in Europe; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Orru’, S.; Reissinger, A.; Filomena, A.; Heitmann, A.; Funk, M.B.; Schmidt-Chanasit, J.; Kreß, J.; Scheiblauer, H.; Cadar, D.; Fiedler, S.A. Assessment of the effectiveness of West Nile virus screening by analysing suspected positive donations among blood donors, Germany, 2020 to 2023. Eurosurveillance 2025, 30, 2400373. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Barzon, L.; Squarzon, L.; Cattai, M.; Franchin, E.; Pagni, S.; Cusinato, R.; Palù, G. West Nile virus infection in Veneto region, Italy, 2008–2009. Eurosurveillance 2009, 14, 19289. [Google Scholar] [CrossRef]
- Pelz, J.O.; Mühlberg, C.; Friedrich, I.; Weidhase, L.; Zimmermann, S.; Maier, M.; Pietsch, C. A Specific Pattern of Routine Cerebrospinal Fluid Parameters Might Help to Identify Cases of West Nile Virus Neuroinvasive Disease. Viruses 2024, 16, 341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ergünay, K.; Aydoğan, S.; Menemenlioğlu, D.; Sener, B.; Lederer, S.; Steinhagen, K.; Hasçelik, G.; Pinar, A.; Ozkul, A.; Us, D. Ankara bölgesinde nedeni bilinmeyen merkezi sinir sistemi enfeksiyonlarinda Bati Nil virusunun araştirilmasi [Investigation of West Nile virus in central nervous system infections of unknown etiology in Ankara, Turkey]. Mikrobiyol. Bul. 2010, 44, 255–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacIntyre, C.; Lourens, C.; Mendes, A.; de Villiers, M.; Avenant, T.; du Plessis, N.M.; Leendertz, F.H.; Venter, M. West Nile Virus, an Underdiagnosed Cause of Acute Fever of Unknown Origin and Neurological Disease among Hospitalized Patients in South Africa. Viruses 2023, 15, 2207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanichanan, J.; Salazar, L.; Wootton, S.H.; Aguilera, E.; Garcia, M.N.; Murray, K.O.; Hasbun, R. Use of Testing for West Nile Virus and Other Arboviruses. Emerg. Infect. Dis. 2016, 22, 1587–1593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frías, M.; Caballero-Gómez, J.; Vázquez, A.; Madrigal, E.; Ruiz-Fons, F.; Gallo, M.; Herrero, L.; Jarilla, M.; García-Bocanegra, I.; Rivero, A.R.-J.A. Serosurvey of blood donors to assess West Nile virus exposure, South-Central Spain. Emerg. Infect. Dis. 2024, 30, 1496–1498. [Google Scholar] [CrossRef]
- Piron, M.; Plasencia, A.; Fleta-Soriano, E.; Martinez, A.; Martinez, J.P.; Torner, N.; Sauleda, S.; Meyerhans, A.; Escalé, J.; Trilla, A.; et al. Low seroprevalence of West Nile virus in blood donors from Catalonia, Spain. Vector-Borne Zoonotic Dis. 2015, 15, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Wittel, M.; Ruiz-Pérez, M.; Del Toro, M.D.; Aznar, J.; Muniain, Á.; De Ory, F.; Domingo, C.; Pachón, J. West Nile virus past infections in the general population of Southern Spain. Enferm. Infecc. Microbiol. Clin. 2007, 25, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Constant, O.; Gil, P.; Barthelemy, J.; Bolloré, K.; Foulongne, V.; Desmetz, C.; Leblond, A.; Desjardins, I.; Pradier, S.; Joulié, A.; et al. One Health surveillance of West Nile and Usutu viruses: A repeated cross-sectional study exploring seroprevalence and endemicity in Southern France, 2016 to 2020. Eurosurveillance 2022, 27, 2200068. [Google Scholar] [CrossRef]
- Charrel, R.N.; De Lamballerie, X.; Durand, J.P.; Gallian, P.; Attoui, H.; Biagini, P.; De Micco, P. Prevalence of antibody against West Nile virus in volunteer blood donors living in southeastern France. Transfusion 2001, 41, 1320–1321. [Google Scholar] [CrossRef] [PubMed]
- Remoli, M.E.; Fiorentini, C.; Marchi, A.; Di Renzi, S.; Vonesch, N.; Peri, V.M.; Bastianini, L.; Rossi, S.; Bartoccini, G.; Kuttappasery, M.L.; et al. Seroprevalence survey of arboviruses in workers from Tuscany, Italy. Med. Lav. 2018, 109, 125–131. [Google Scholar] [CrossRef]
- Marchi, S.; Montomoli, E.; Viviani, S.; Giannecchini, S.; Stincarelli, M.A.; Lanave, G.; Camero, M.; Alessio, C.; Coluccio, R.; Trombetta, C.M. West Nile virus seroprevalence in the Italian Tuscany region from 2016 to 2019. Pathogens 2021, 10, 844. [Google Scholar] [CrossRef]
- Faggioni, G.; De Santis, R.; Pomponi, A.; Grottola, A.; Serpini, G.F.; Meacci, M.; Gennari, W.; Tagliazucchi, S.; Pecorari, M.; Monaco, F.; et al. Prevalence of Usutu and West Nile virus antibodies in human sera, Modena, Italy, 2012. J. Med. Virol. 2018, 90, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Pierro, A.; Lunghi, G.; Farina, C.; Toschi, V.; Matinato, C.; Orlandi, A.; Zoccoli, A.; Almini, D.; Landini, M.P.; et al. Seroprevalence of West Nile virus antibodies in blood donors living in the metropolitan area of Milan, Italy, 2009–2011. New Microbiol. 2013, 36, 81–83. [Google Scholar] [PubMed]
- Pierro, A.; Gaibani, P.; Manisera, C.; Dirani, G.; Rossini, G.; Cavrini, F.; Ghinelli, F.; Ghinelli, P.; Finarelli, A.C.; Mattivi, A.; et al. Seroprevalence of west nile virus-specific antibodies in a cohort of blood donors in Northeastern Italy. Vector-Borne Zoonotic Dis. 2011, 11, 1605–1607. [Google Scholar] [CrossRef] [PubMed]
- Filipe, A.R. Anticorpos contra arbovírus na população de Portugal. O Médico 1973, 1138, 731–732. [Google Scholar]
- Lourenço, J.; Barros, S.C.; Zé-Zé, L.; Damineli, D.S.C.; Giovanetti, M.; Osório, H.C.; Amaro, F.; Henriques, A.M.; Ramos, F.; Luís, T.; et al. West Nile virus transmission potential in Portugal. Commun. Biol. 2022, 5, 6. [Google Scholar] [CrossRef]
- Geraldes, M.A.; Cunha, M.V.; Godinho, C.; de Lima, R.F.; Giovanetti, M.; Lourenço, J. The historical ecological background of West Nile virus in Portugal indicates One Health opportunities. Sci. Total Environ. 2024, 944, 173875. [Google Scholar] [CrossRef]
- Maroco, D.; Parreira, R.; Dos Santos, F.A.; Lopes, Â.; Simões, F.; Orge, L.; Seabra, S.G.; Fagulha, T.; Brazio, E.; Henriques, A.M.; et al. Tracking the Pathways of West Nile Virus: Phylogenetic and Phylogeographic Analysis of a 2024 Isolate from Portugal. Microorganisms 2025, 13, 585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almeida, A.P.; Freitas, F.B.; Novo, M.T.; Sousa, C.A.; Rodrigues, J.C.; Alves, R.; Esteves, A. Mosquito surveys and West Nile virus screening in two different areas of southern Portugal, 2004–2007. Vector Borne Zoonotic Dis. 2010, 10, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Centro de Estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac, Relatório REVIVE 2023—Culicídeos, Ixodídeos e Flebótomos: Rede de Vigilância de Vetores; Instituto Nacional de Saúde Doutor Ricardo Jorge: Lisboa, Portugal, 2024.
- Martinho, J.; Osório, H.; Amaro, F.; Silva, M.; Zé-Zé, L.; Alves, M.J.; Nunes, B.; Soares, P. Distribution of the Culex pipiens mosquito in mainland Portugal: A geospatial modelling study. Eur J Public Health. 2024, 34 (Suppl. 3), ckae144-1417. [Google Scholar] [CrossRef]
- Rocha, R.; Gonçalves, L.; Conceição, C.; Andrade, P.; Cristóvão, J.M.; Condeço, J.; Delgado, B.; Caeiro, C.; Kuzmenko, T.; Vasconcelos, E.; et al. Prevalence of asymptomatic Leishmania infection and knowledge, perceptions, and practices in blood donors in mainland Portugal. Parasit. Vectors 2023, 16, 357. [Google Scholar] [CrossRef]
- Instituto Português do Sangue e da Transplantação I. Relatório de Atividade Transfusional e Sistema Português de Hemovigilância 2022; Instituto Português do Sangue e da Transplantação I: Lisboa, Portugal, 2023; Available online: https://www.hemovigilancia.net/files/RA_2022.pdf (accessed on 4 June 2025).
- Fundação Francisco Manuel dos Santos. PORDATA. O que são NUTS? Available online: https://www.pordata.pt/o+que+sao+nuts (accessed on 4 June 2025).
- Instituto Nacional de Estatística. Censos 2021 Resultados Definitivos—Portugal; Instituto Nacional de Estatística: Lisboa, Portugal, 2022; Available online: https://www.ine.pt/ngt_server/attachfileu.jsp?look_parentBoui=586659861&att_display=n&att_download=y (accessed on 4 June 2025).
- ESCO. Classification of Occupations. Available online: https://esco.ec.europa.eu/en/classification/occupation_main (accessed on 4 June 2025).
- Programa de Desenvolvimento Rural 2014–2020. Freguesias Rurais PDR2020 (Nova Divisão Administrativa Freguesias 2013). Available online: https://www.gpp.pt/images/Estatisticas_e_analises/Estatisticas/associadasmedidasapoio/Territorios_Rurais.pdf (accessed on 4 June 2025).
- Hierholzer, J.C.; Killington, R.A.; Kangro, H.O.; Mahy, B.W.J. Virology Methods Manual; Section I: Classical Techniques; Academic Press: Cambridge, MA, USA, 1996; pp. 25–46. [Google Scholar]
- Sargent, E.; Ausvet. Epitools Epidemiological Calculators. 2018. Available online: https://epitools.ausvet.com.au/ (accessed on 4 June 2025).
- Greiner, M.; Gardner, I.A. Application of diagnostic tests in veterinary epidemiologic studies. Prev. Vet. Med. 2000, 45, 43–59. [Google Scholar] [CrossRef]
- Fagerland, M.W.; Hosmer, D.W. A generalized Hosmer-Lemeshow goodness-of-fit test for multinomial logistic regression models. Stata J. 2012, 12, 447–453. [Google Scholar] [CrossRef]
- Sanchini, A.; Donoso-Mantke, O.; Papa, A.; Sambri, V.; Teichmann, A.; Niedrig, M. Second international diagnostic accuracy study for the serological detection of West Nile virus infection. PLoS Negl. Trop. Dis. 2013, 7, e2184. [Google Scholar] [CrossRef] [PubMed]
- Berneck, B.S.; Rockstroh, A.; Barzon, L.; Sinigaglia, A.; Vocale, C.; Landini, M.P.; Rabenau, H.F.; Schmidt-Chanasit, J.; Ulbert, S. Serological differentiation of West Nile virus- and Usutu virus-induced antibodies by envelope proteins with modified cross-reactive epitopes. Transbound. Emerg. Dis. 2022, 69, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Endale, A.; Medhin, G.; Darfiro, K.; Kebede, N.; Legesse, M. Magnitude of antibody cross-reactivity in medically important mosquito-borne flaviviruses: A systematic review. Infect Drug Resist. 2021, 14, 4291–4299. [Google Scholar] [CrossRef]
- Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell. Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef]
- Fontoura-Gonçalves, C.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Costa, J.B.; de Mello, G.; Gonçalves, D.; Alves, P.C.; Höfle, U.; Queirós, J. Dynamics of Bagaza, West Nile, and Usutu Viruses in Red-Legged Partridges, Portugal, 2018–2022. Emerg. Infect. Dis. 2025, 31, 824–828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dos Santos, F.A.A.; Barros, S.C.; Fagulha, T.; Ramos, F.; Henriques, A.M.; Duarte, A.; Magalhães, A.; Luís, T.; Duarte, M.D. First detection of Bagaza virus in Common magpies (Pica pica), Portugal 2023. Sci. Rep. 2024, 14, 19452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mansfield, K.L.; Horton, D.L.; Johnson, N.; Li, L.; Barrett, A.D.T.; Smith, D.J.; Galbraith, S.E.; Solomon, T.; Fooks, A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011, 92, 2821–2829. [Google Scholar] [CrossRef]
- Direção-Geral da Alimentação e Veterinária. Doenças de Declaração Obrigatória; Direção-Geral da Alimentação e Veterinária: Lisboa, Portugal, 2024. [Google Scholar]
- Direção-Geral da Alimentação e Veterinária. Nota Informativa—Febre do Nilo Ocidental; Direção-Geral da Alimentação e Veterinária: Lisboa, Portugal, 2024. [Google Scholar]
- Castro-Scholten, S.; Caballero-Gómez, J.; Bravo-Barriga, D.; Llorente, F.; Cano-Terriza, D.; Jiménez-Clavero, M.Á.; Jiménez-Martín, D.; Camacho-Sillero, L.; García-Bocanegra, I. Exposure to West Nile virus in wild lagomorphs in Spanish mediterranean ecosystems. Zoonoses Public Health 2024, 72, 207–214. [Google Scholar] [CrossRef]
- Purpari, G. Importance of dogs as sentinels of West Nile Virus activity in urban and suburban areas. Int. J. Infect. Dis. 2012, 16, e270. [Google Scholar] [CrossRef][Green Version]
- Tamba, M.; Bonilauri, P.; Galletti, G.; Casadei, G.; Santi, A.; Rossi, A.; Calzolari, M. West Nile virus surveillance using sentinel birds: Results of eleven years of testing in corvids in a region of northern Italy. Front. Vet. Sci. 2024, 11, 1407271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jean, C.M.; Honarmand, S.; Louie, J.K.; Glaser, C.A. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg. Infect. Dis. 2007, 13, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.R.; Murray, K.O. Risk factors for West Nile virus infection and disease in populations and individuals. Expert Rev. Anti Infect. Ther. 2015, 13, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Poças, J.M.D.; Luz, T.; Amaro, F.; Zé-Zé, L.; Osório, H. West Nile virus (Flavivirus) infection in Portugal. Considerations about a clinical case with febrile syndrome and rash. Rev. Port. Doenças Infecc. 2012, 8, 46–51. [Google Scholar]
- Humphry, R.W.; Cameron, A.; Gunn, G.J. A practical approach to calculate sample size for herd prevalence surveys. Prev. Vet. Med. 2004, 65, 173–188. [Google Scholar] [CrossRef]
- Aliaga, L.; Ceballos, J.; Sampedro, A.; Cobo, F.; López-Nevot, M.Á.; Merino-Espinosa, G.; Morillas-Márquez, F.; Martín-Sánchez, J. Asymptomatic Leishmania infection in blood donors from the Southern of Spain. Infection 2019, 47, 739–747. [Google Scholar] [CrossRef]
- Pérez-Cutillas, P.; Goyena, E.; Chitimia, L.; De la Rúa, P.; Bernal, L.J.; Fisa, R.; Riera, C.; Iborra, A.; Murcia, L.; Segovia, M.; et al. Spatial distribution of human asymptomatic Leishmania infantum infection in southeast Spain: A study of environmental, demographic and social risk factors. Acta Trop. 2015, 146, 127–134. [Google Scholar] [CrossRef]
- Martín-Sánchez, J.; Rodríguez-Granger, J.; Morillas-Márquez, F.; Merino-Espinosa, G.; Sampedro, A.; Aliaga, L.; Corpas-López, V.; Tercedor-Sánchez, J.; Aneiros-Fernández, J.; Acedo-Sánchez, C.; et al. Leishmaniasis due to Leishmania infantum: Integration of human, animal and environmental data through a one health approach. Transbound Emerg Dis. 2020, 67, 2423–2434. [Google Scholar] [CrossRef]
- SPMS. Doenças de Declaração Obrigatória. Portal da Transparência. 2018. Available online: https://dados.gov.pt/pt/datasets/doencas-de-declaracao-obrigatoria (accessed on 22 February 2023).
| Global | Norte | Centro | OVT | GL | PS | Alentejo | Algarve | |
|---|---|---|---|---|---|---|---|---|
| Total | 100 | 33.6 | 14.7 | 8.1 | 19.4 | 7.6 | 10.0 | 6.6 |
| (3572/3572) | (1201/3572) | (527/3572) | (289/3572) | (692/3572) | (270/3572) | (358/3572) | (235/3572) | |
| Median age (years) | 41 | 39 | 40 | 41 | 41 | 44 | 43 | 42 |
| (IQR) | (31–48) | (30–47) | (29–47) | (29–49) | (30–50) | (34–49) | (35–50) | (35–50) |
| Male sex (%) | 49.8 | 47.3 | 47.1 | 50.9 | 48.7 | 44.8 | 61.7 | 57.5 |
| (1774/3564) | (567/1198) | (248/526) | (147/289) | (336/690) | (121/270) | (221/358) | (134/233) | |
| Education level a | ||||||||
| Basic (1–4) | 1.7 | 1.6 | 1,4 | 3.1 | 1.0 | 1.1 | 3.4 | 1.7 |
| (60/3483) | (18/1161) | (7/514) | (9/286) | (7/677) | (3/265) | (12/350) | (4/230) | |
| Basic (5–9) | 16.6 | 20.0 | 16.3 | 18.2 | 9.6 | 14.0 | 19.7 | 17.0 |
| (578/3483) | (232/1161) | (84/514) | (52/286) | (65/677) | (37/265) | (69/350) | (39/230) | |
| Secondary (10–12) | 44.0 | 43.2 | 42.4 | 49.3 | 39.6 | 39.6 | 51.1 | 51.3 |
| (1531/3483) | (502/1161) | (218/514) | (141/286) | (268/677) | (105/265) | (179/350) | (118/230) | |
| Bachelor’s | 26.0 | 23.6 | 28.8 | 22.7 | 31.9 | 32.5 | 20.0 | 20.9 |
| (907/3483) | (274/1161) | (148/514) | (65/286) | (216/677) | (86/265) | (70/350) | (48/230) | |
| MSc/PhD | 11.7 | 11.6 | 11.1 | 6.6 | 17.9 | 12.8 | 5.7 | 9.1 |
| (407/3483) | (135/1161) | (57/514) | (19/286) | (121/677) | (34/265) | (20/350) | (21/230) | |
| Occupation b | ||||||||
| Student | 9.9 | 9.3 | 13.6 | 12.4 | 12.6 | 5.5 | 5.7 | 4.3 |
| (283/2866) | (89/960) | (59/435) | (30/241) | (69/546) | (12/219) | (16/279) | (8/186) | |
| Unemployed | 3.5 | 4.5 | 2.8 | 3.3 | 3.7 | 2.7 | 1.4 | 3.2 |
| (99/2866) | (43/960) | (12/435) | (8/241) | (20/546) | (6/219) | (4/279) | (6/186) | |
| Retired | 1.7 | 1.0 | 0.7 | 1.7 | 2.9 | 1.4 | 3.2 | 2.7 |
| (50/2866) | (10/960) | (3/435) | (4/241) | (16/546) | (3/219) | (9/279) | (5/186) | |
| Armed forces (0) | 1.9 | 1.4 | 1.6 | 3.3 | 1.1 | 2.7 | 2.5 | 3.8 |
| (54/2866) | (13/960) | (7/435) | (8/241) | (6/546) | (6/219) | (7/279) | (7/186) | |
| Managers, professionals and technicians (1–3) | 39.4 | 37.2 | 37.7 | 31.5 | 50.0 | 49.3 | 33.0 | 31.2 |
| (1128/2866) | (357/960) | (164/435) | (76/241) | (273/546) | (108/219) | (92/279) | (58/186) | |
| Clerical support, service and sales (4–5) | 25.7 | 24.3 | 22.5 | 26.1 | 20.0 | 24.2 | 35.1 | 44.6 |
| (737/2866) | (233/960) | (98/435) | (63/241) | (109/546) | (53/219) | (98/279) | (83/186) | |
| Agriculture, craft, industry and elementary (6–9) | 18.0 | 22.4 | 21.1 | 21.6 | 9.7 | 14.2 | 19.0 | 10.2 |
| (515/2866) | (215/960) | (92/435) | (52/241) | (53/546) | (31/219) | (53/279) | (19/186) | |
| Others | ||||||||
| Regular contact with domestic animals | 70.8 | 70.5 | 79.4 | 74.9 | 61.4 | 68.6 | 74.3 | 73.3 |
| (2410/3404) | (799/1134) | (397/500) | (209/279) | (409/666) | (179/261) | (252/339) | (165/225) | |
| Practice of outdoor activities during nighttime | 24.6 | 19.9 | 29.2 | 26.9 | 23.2 | 23.4 | 33.0 | 27.6 |
| (798/3248) | (217/1088) | (138/473) | (71/264) | (148/637) | (59/252) | (107/324) | (58/210) |
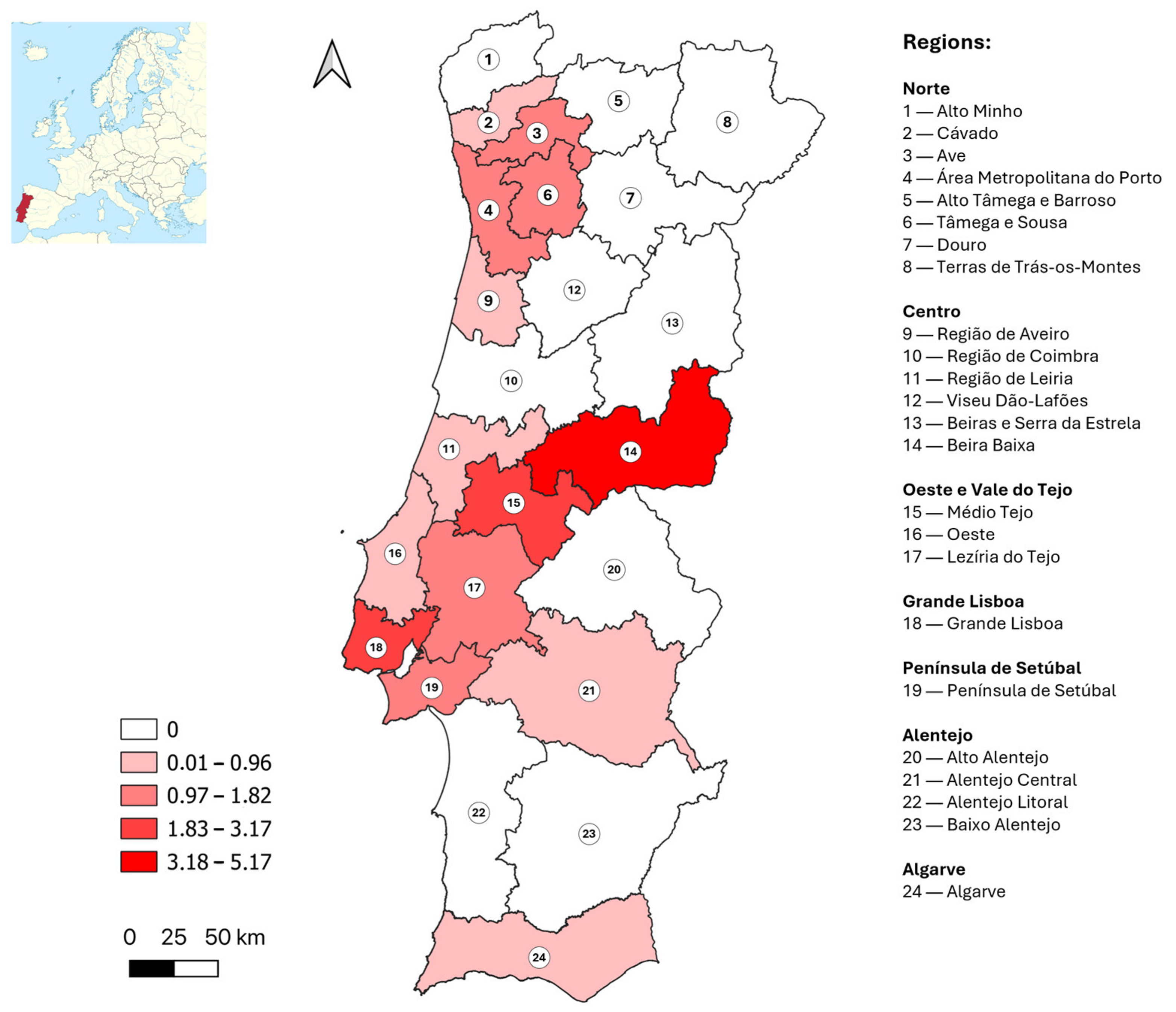
| Region | Sampling Sites (n) | Total Samples (n) | Positive or Borderline Samples, ELISA (n) | Positive Samples, VNT (n) | Crude Prevalence, VNT (%) | Adjusted Prevalence, VNT (%) | 95% Confidence Interval |
|---|---|---|---|---|---|---|---|
| Norte | 149 | 1201 | 17 | 13 | 1.1 | 1.1 | 0.6–1.8 |
| Alto Minho | 12 | 68 | 0 | 0 | 0.0 | 0.0 | 0.0–5.3 |
| Cávado | 17 | 148 | 3 | 1 | 0.7 | 0.8 | 0.1–3.7 |
| Ave | 16 | 143 | 2 | 2 | 1.4 | 1.4 | 0.4–5.0 |
| Área Metropolitana do Porto | 60 | 555 | 10 | 8 | 1.4 | 1.5 | 0.7–2.8 |
| Alto Tâmega e Barroso | 4 | 29 | 0 | 0 | 0.0 | 0.0 | 0.0–11.7 |
| Tâmega e Sousa | 23 | 151 | 2 | 2 | 1.3 | 1.4 | 0.4–4.8 |
| Douro | 13 | 75 | 0 | 0 | 0.0 | 0.0 | 0.0–4.9 |
| Terras de Trás-os-Montes | 4 | 32 | 0 | 0 | 0.0 | 0.0 | 0.0–10.7 |
| Centro | 91 | 527 | 7 | 4 | 0.8 | 0.6 | 0.2–1.7 |
| Região de Aveiro | 19 | 134 | 2 | 1 | 0.7 | 0.9 | 0.1–4.2 |
| Região de Coimbra | 21 | 108 | 1 | 0 | 0.0 | 0.0 | 0.0–3.4 |
| Região de Leiria | 14 | 99 | 1 | 1 | 1.0 | 0.9 | 0.2–5.1 |
| Viseu Dão-Lafões | 15 | 65 | 0 | 0 | 0.0 | 0.0 | 0.0–5.6 |
| Beira Baixa | 7 | 40 | 2 | 2 | 5.0 | 5.2 | 1.4–16.5 |
| Beiras e Serra da Estrela | 15 | 81 | 1 | 0 | 0.0 | 0.0 | 0.0–4.5 |
| Oeste e Vale do Tejo | 38 | 289 | 5 | 3 | 1.0 | 1.1 | 0.4–3.0 |
| Oeste | 12 | 139 | 1 | 1 | 0.7 | 0.9 | 0.1–4.2 |
| Médio Tejo | 16 | 64 | 2 | 1 | 1.6 | 2.2 | 0.3–8.9 |
| Lezíria do Tejo | 10 | 86 | 2 | 1 | 1.2 | 1.2 | 0.2–6.3 |
| Grande Lisboa | 16 | 692 | 28 | 20 | 2.9 | 3.0 | 2.0–4.6 |
| Península de Setúbal | 9 | 270 | 7 | 4 | 1.5 | 1.4 | 0.6–3.6 |
| Alentejo | 42 | 358 | 5 | 1 | 0.3 | 0.2 | 0.0–1.6 |
| Alentejo Litoral | 10 | 69 | 0 | 0 | 0.0 | 0.0 | 0.0–5.3 |
| Baixo Alentejo | 8 | 100 | 1 | 0 | 0.0 | 0.0 | 0.0–3.7 |
| Alto Alentejo | 9 | 55 | 0 | 0 | 0.0 | 0.0 | 0.0–6.5 |
| Alentejo Central | 15 | 134 | 4 | 1 | 0.7 | 0.7 | 0.1–4.1 |
| Algarve | 2 | 235 | 7 | 2 | 0.9 | 0.7 | 0.2–2.8 |
| Total | 347 | 3572 | 76 | 47 | 1.3 | 1.4 | 1.0–1.8 |
| Result of WNV Serology | ||||
|---|---|---|---|---|
| Variables | Categories | ELISA and VNT Positive | ELISA Negative | p Value |
| Sex | Male | 51.1 (24/47) | 49.7 (1734/3488) | 0.854 (χ2 = 0.34, df = 1) |
| Female | 48.9 (23/47) | 50.3 (1754/3488) | ||
| Age (years) | 18–24 | 10.6 (5/47) | 12.9 (442/3413) | 0.381 (χ2 = 4.19, df = 4) |
| 25–34 | 27.7 (13/47) | 20.2 (688/3413) | ||
| 35–44 | 21.3 (10/47) | 28.7 (979/3413) | ||
| 45–54 | 34.0 (16/47) | 27.0 (921/3413) | ||
| 55–65 | 6.4 (3/47) | 11.2 (383/3413) | ||
| Level of education a | 1–4 | 0.0 (0/47) | 1.7 (59/3407) | 0.125 (FET = 6.79) |
| 5–9 | 6.4 (3/47) | 16.8 (573/3407) | ||
| 10–12 | 46.8 (22/47) | 43.9 (1495/3407) | ||
| Bachelor’s | 25.5 (12/47) | 25.9 (884/3407) | ||
| MSc/PhD | 21.3 (10/47) | 11.6 (396/3407) | ||
| Occupation b | Student | 7.7 (5/65) | 9.9 (278/2801) | |
| Retired | 4.6 (3/65) | 1.7 (47/2801) | ||
| Unemployed | 3.1 (2/65) | 3.5 (97/2801) | ||
| 0 | 8.8 (3/34) | 2.1 (49/2379) | 0.066 (FET = 6.81) | |
| 1–3 | 52.9 (18/34) | 46.2 (1100/2379) | ||
| 4–5 | 20.6 (7/34) | 30.4 (724/2379) | ||
| 6–9 | 17.6 (6/34) | 21.3 (506/2379) | ||
| Travel abroad (<2 years previously) | Yes | 29.8 (14/47) | 23.7 (815/3437) | 0.331 (χ2 = 0.94, df = 1) |
| No | 70.2 (33/47) | 76.3 (2622/3437) | ||
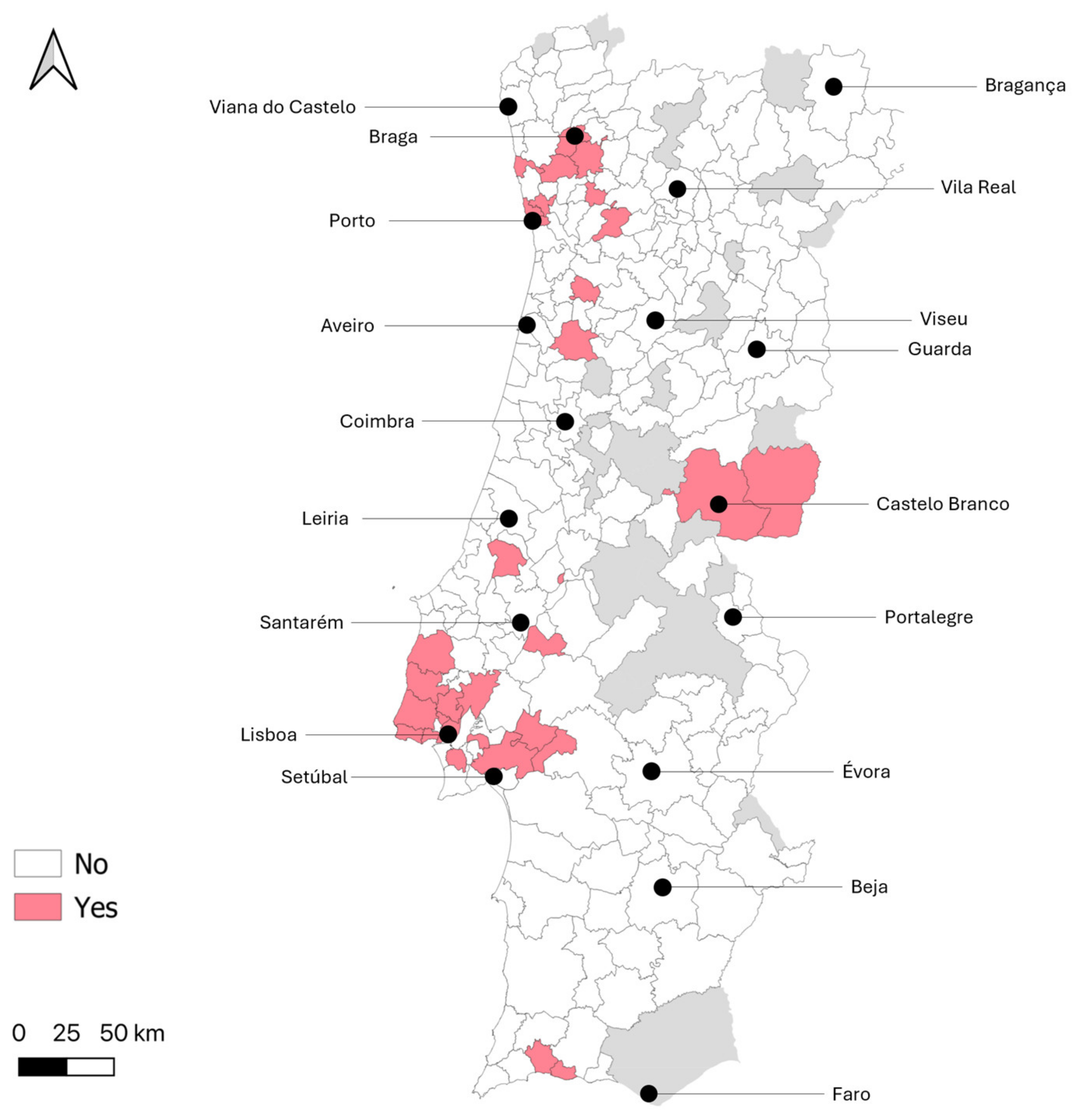
| Type of parish of residence | Non-rural | 76.6 (36/47) | 57.6 (2012/3492) | 0.009 * (χ2 = 6.85, df = 1) |
| Rural | 23.4 (11/47) | 42.4 (1480/3492) | ||
| Regular contact with domestic animals | Yes | 51.1 (23/45) | 71.0 (2366/3331) | 0.004 * (χ2 = 8.52, df = 1) |
| No | 48.9 (22/45) | 29.0 (965/3331) | ||
| Regular contact with wild animals | Yes | 4.7 (2/43) | 4.1 (131/3159) | 0.699 (FET = 0.50) |
| No | 95.3 (41/43) | 95.9 (3028/3159) | ||
| Practice of outdoor activities during nighttime | Yes | 22.7 (10/44) | 24.4 (777/3178) | 0.792 (χ2 = 0.07, df = 1) |
| No | 77.3 (34/44) | 75.6 (2401/3178) | ||
| Use of nets in windows/doors | Yes (all/some) | 13.3 (6/45) | 24.0 (799/3331) | 0.096 (χ2 = 2.78, df = 1) |
| None | 86.7 (39/45) | 76.0 (2532/3331) | ||
| NUTS2 region of residence | Norte | 27.7 (13/47) | 33.9 (1184/3496) | 0.011 * (FET = 15.36) |
| Centro | 8.5 (4/47) | 14.9 (520/3496) | ||
| Oeste e Vale do Tejo | 6.4 (4/47) | 8.1 (284/3496) | ||
| Grande Lisboa | 42.6 (20/47) | 19.0 (664/3496) | ||
| Península de Setúbal | 8.5 (4/47) | 7.5 (263/3496) | ||
| Alentejo | 2.1 (1/47) | 10.1 (353/3496) | ||
| Algarve | 4.3 (2/47) | 6.5 (228/3496) | ||
| Potential Risk Factor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| % in Sample | Crude OR | 95% CI | Adjusted OR | 95% CI | p-Value | |
| Age ≥ 45 years old | 38.3 | 1.10 | 0.61–1.97 | 1.04 | 0.56–1.93 | 0.607 |
| Male sex | 49.8 | 1.06 | 0.59–1.88 | 1.00 | 0.55–1.83 | 0.953 |
| Residing in Grande Lisboa region | 19.4 | 3.16 | 1.76–5.67 | 2.18 | 1.15–4.12 | 0.017 * |
| Residing in a non-rural parish | 57.9 | 2.41 | 1.22–4.74 | 1.37 | 0.64–2.93 | 0.672 |
| Higher education level | 37.7 | 1.46 | 0.82–2.60 | 1.28 | 0.69–2.38 | 0.826 |
| No use of nets in windows/doors | 76.1 | 2.05 | 0.87–4.86 | 1.72 | 0.72–4.12 | 0.815 |
| No regular contact with domestic animals | 29.2 | 2.35 | 1.30–4.23 | 1.53 | 0.74–3.16 | 0.310 |
| Constant | 0.018 | <0.001 | ||||
| Hosmer and Lemeshow Test | Sig. = 0.945 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, R.; Kurum, E.; Charrel, R.; Ayhan, N.; Maia, C. Seroprevalence of West Nile Virus in Blood Donors in Mainland Portugal. Trop. Med. Infect. Dis. 2025, 10, 229. https://doi.org/10.3390/tropicalmed10080229
Rocha R, Kurum E, Charrel R, Ayhan N, Maia C. Seroprevalence of West Nile Virus in Blood Donors in Mainland Portugal. Tropical Medicine and Infectious Disease. 2025; 10(8):229. https://doi.org/10.3390/tropicalmed10080229
Chicago/Turabian StyleRocha, Rafael, Elif Kurum, Rémi Charrel, Nazli Ayhan, and Carla Maia. 2025. "Seroprevalence of West Nile Virus in Blood Donors in Mainland Portugal" Tropical Medicine and Infectious Disease 10, no. 8: 229. https://doi.org/10.3390/tropicalmed10080229
APA StyleRocha, R., Kurum, E., Charrel, R., Ayhan, N., & Maia, C. (2025). Seroprevalence of West Nile Virus in Blood Donors in Mainland Portugal. Tropical Medicine and Infectious Disease, 10(8), 229. https://doi.org/10.3390/tropicalmed10080229







