Mapping Evidence on the Regulations Affecting the Accessibility, Availability, and Management of Snake Antivenom Globally: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Identifying the Research Question
2.3. Identifying Relevant Studies
2.4. Study Selection
2.5. Data Extraction and Charting
2.6. Collating, Summarizing, and Reporting Results
3. Results
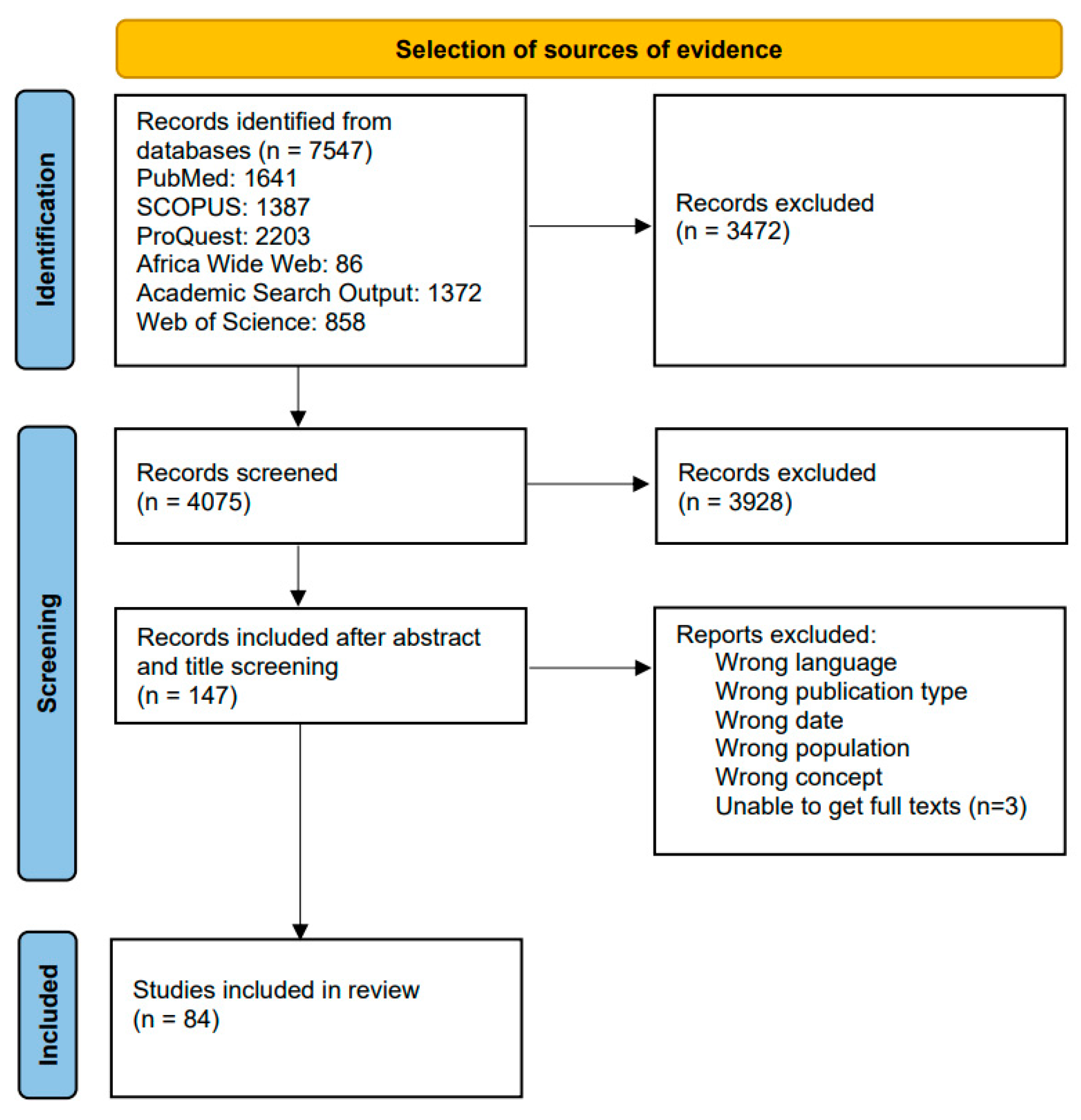
3.1. Screening Results
3.2. Descriptive Characteristics
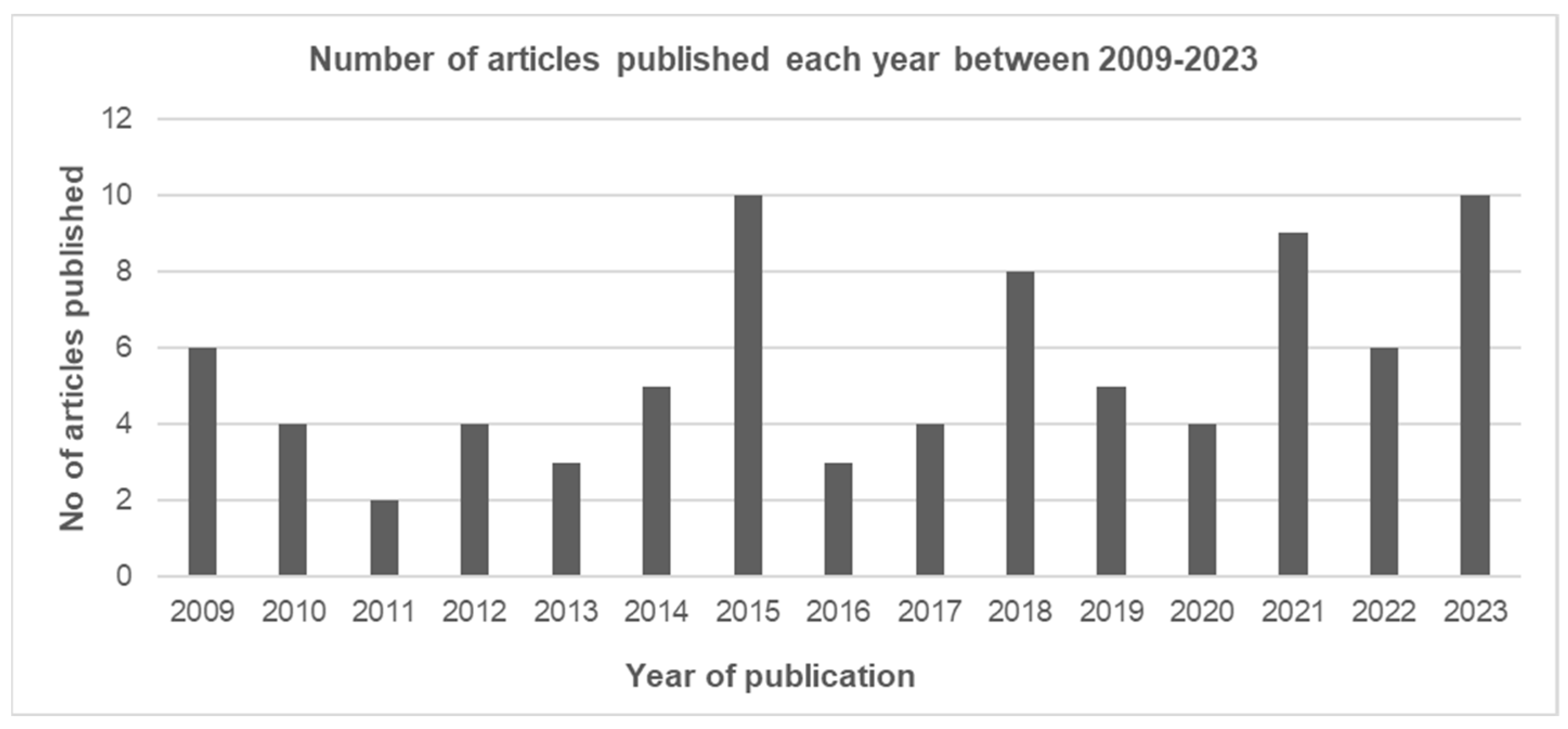
3.2.1. Year of Publication
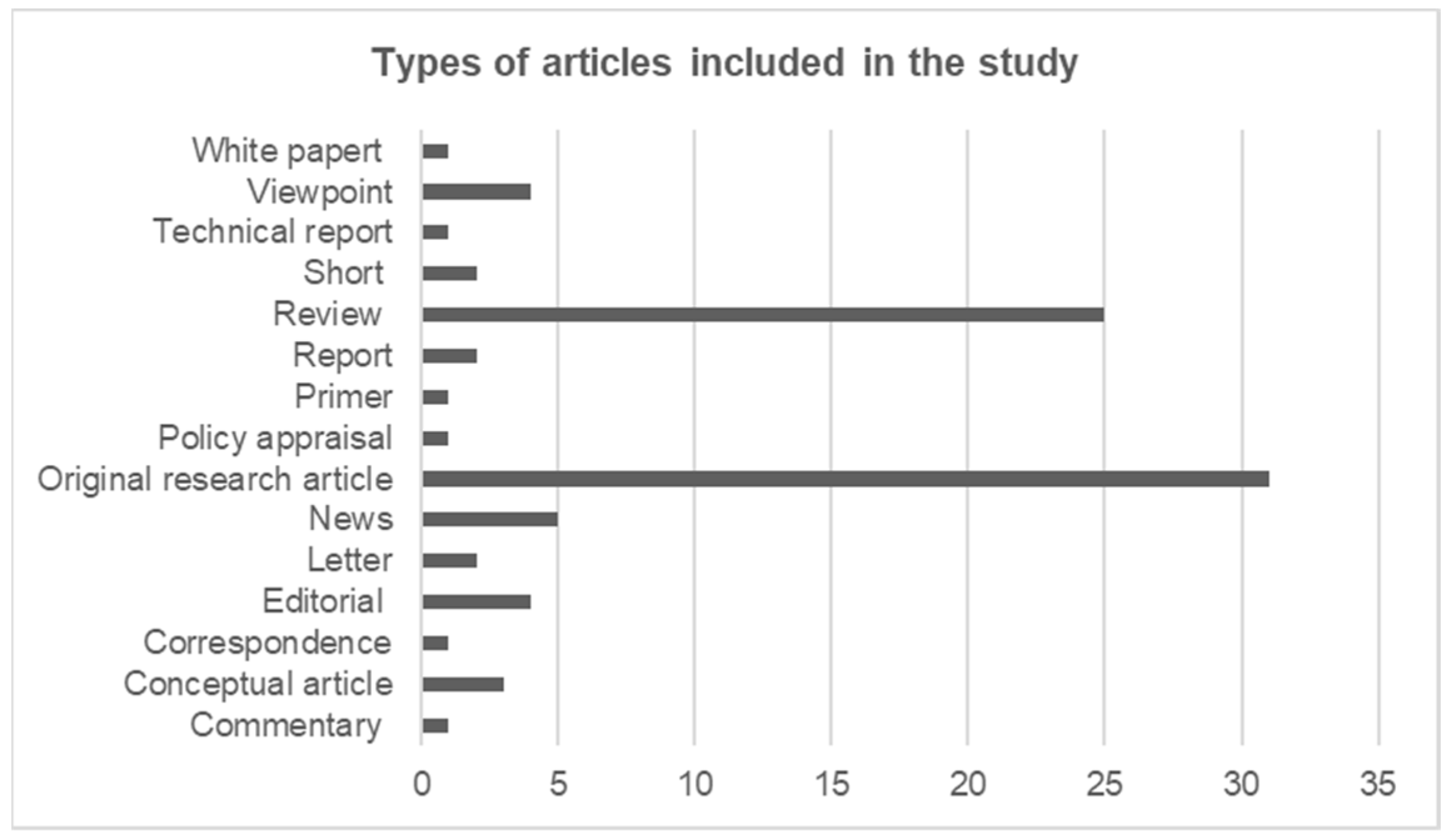
3.2.2. Type of Articles
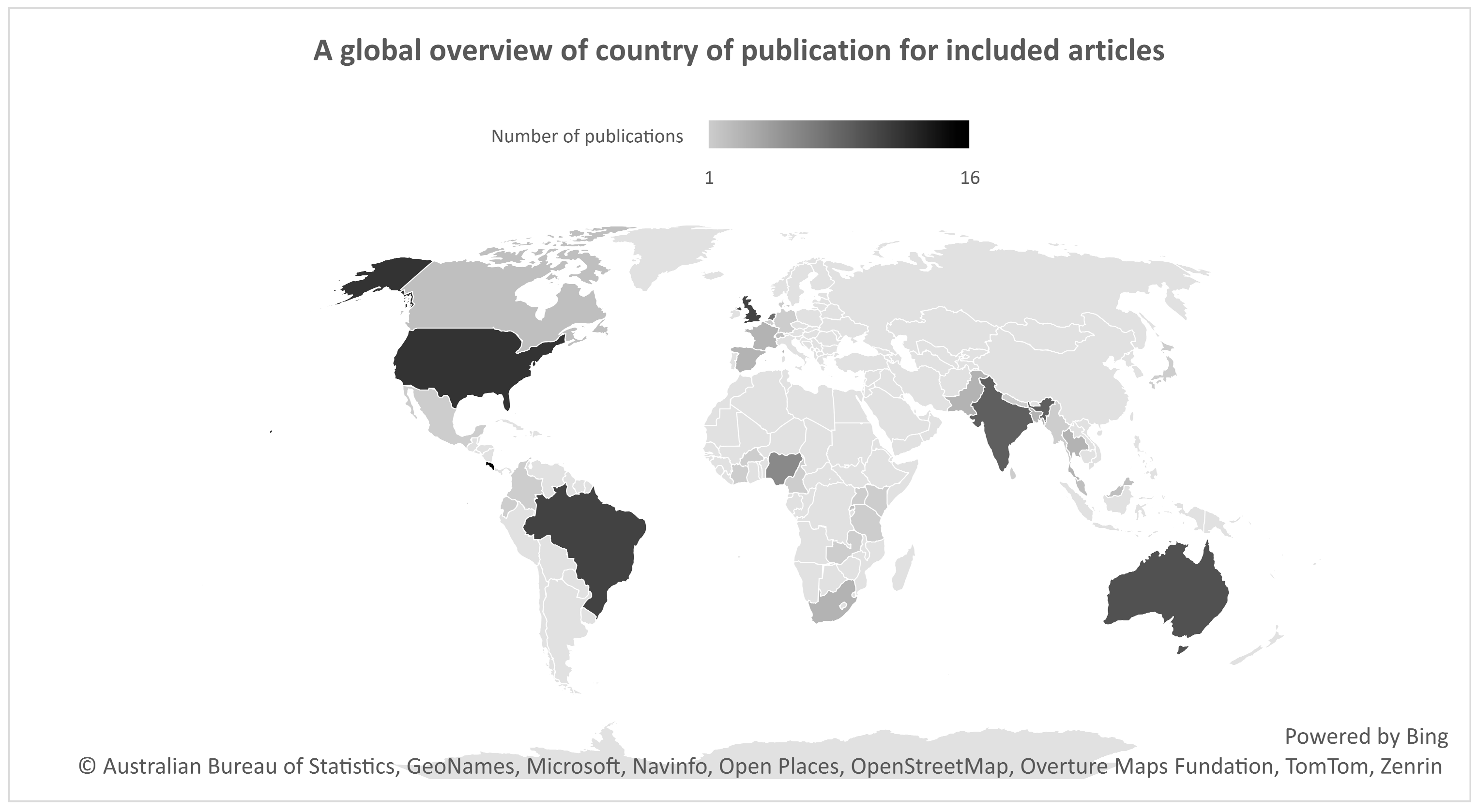
3.2.3. Country of Publication and Income Level
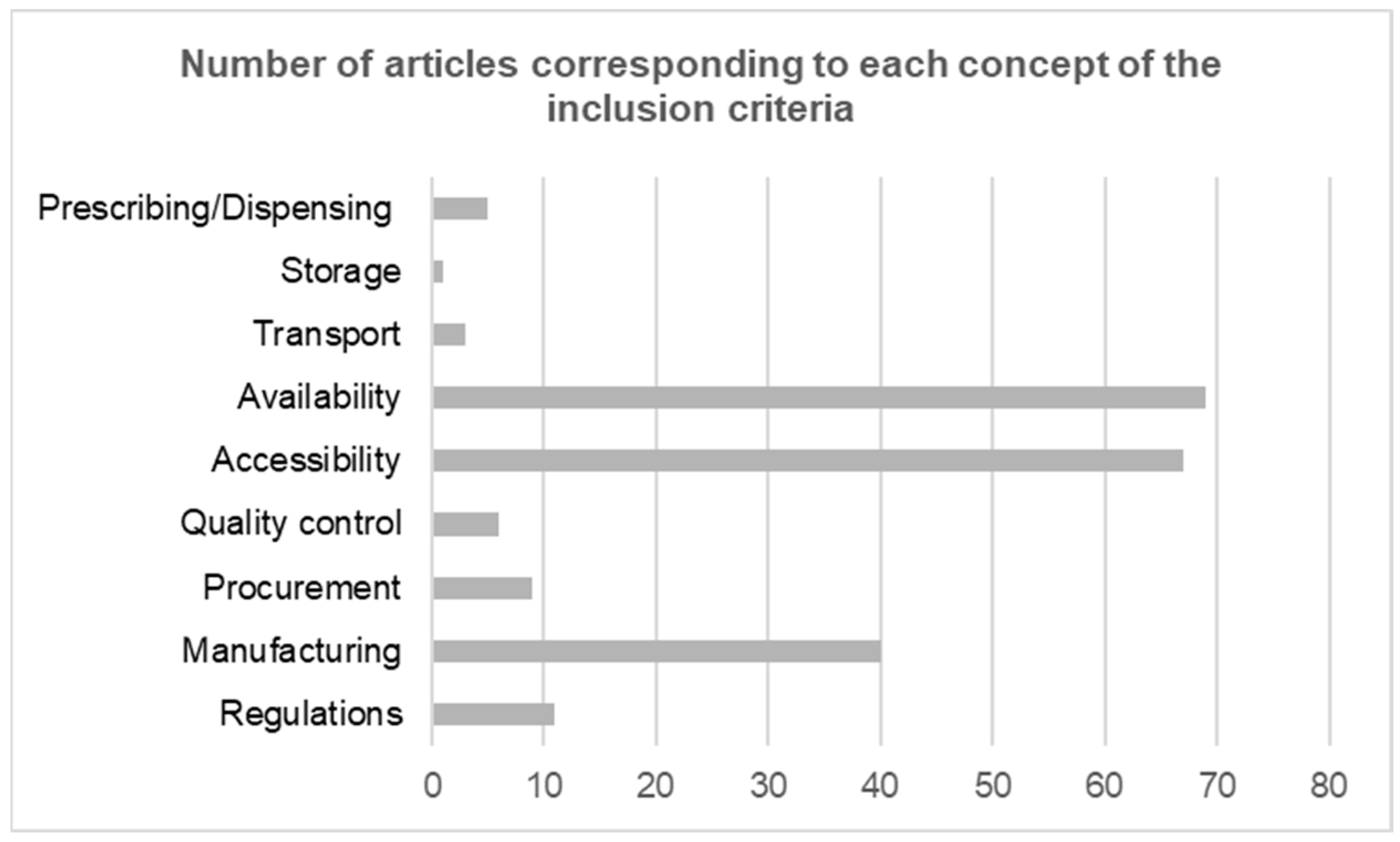
3.2.4. Concepts Included
3.3. Findings of the Study
3.3.1. Regulatory Frameworks
3.3.2. Manufacturing and Procurement
3.3.3. Availability and Access of Antivenom
4. Discussion
4.1. Strengths and Limitations of the Study
4.2. Implications of Research and Practice
4.3. Recommendations for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SBE | Snakebite envenoming |
| WHO | World Health Organization |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews |
| CAMs | Complementary and Alternative Medicines |
| JBI | Joanna Briggs Institute |
| PCC | Population, concept, context |
| MeSH | Medical subject headings |
| CENADI | National Centre for Distribution and Storage of Immunobiologicals |
| INCQS | National Institute for Quality Control in Health |
| INVIMA | National Institute for the Surveillance of Medicines and Food |
| FDA | Food and Drug Administration |
| GDP | Good distribution practice |
| GMP | Good manufacturing practice |
| LMICs | Low- and middle-income countries |
References
- Rathore, A.S.; Kumar, R.; Tiwari, O.S. Recent advancements in snake antivenom production. Int. J. Biol. Macromol. 2023, 240, 124478. [Google Scholar] [CrossRef]
- Gamulin, E.; Lukačević, S.M.; Halassy, B.; Kurtović, T. Snake Antivenoms—Toward Better Understanding of the Administration Route. Toxins 2023, 15, 398. [Google Scholar] [CrossRef]
- Afroz, A.; Siddiquea, B.N.; Shetty, A.N.; Jackson, T.N.W.; Watt, A.D.; Monteiro, W.M. Assessing knowledge and awareness regarding snakebite and management of snakebite envenoming in healthcare workers and the general population: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2023, 17, e0011048. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Ralph, R.; Faiz, M.A.; Sharma, S.K.; Ribeiro, I.; Chappuis, F. Managing snakebite. BMJ 2022, 376, e057926. [Google Scholar] [CrossRef]
- Squaiella-Baptistão, C.C.; Sant’ANna, O.A.; Marcelino, J.R.; Tambourgi, D.V. The history of antivenoms development: Beyond Calmette and Vital Brazil. Toxicon 2018, 150, 86–95. [Google Scholar] [CrossRef]
- Nikapitiya, B.; Maduwage, K. Pharmacodynamics and pharmacokinetics of snake antivenom. Sri Lanka J. Med. 2018, 27, 54. [Google Scholar] [CrossRef]
- León, G.; Herrera, M.; Vargas, M.; Arguedas, M.; Sánchez, A.; Segura, Á.; Gómez, A.; Solano, G.; Corrales-Aguilar, E.; Risner, K.; et al. Development and characterization of two equine formulations towards SARS-CoV-2 proteins for the potential treatment of COVID-19. Sci. Rep. 2021, 11, 9825. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease? PLoS Neglected Trop. Dis. 2017, 11, e0005361. [Google Scholar] [CrossRef]
- Silva, A.; Isbister, G. Current research into snake antivenoms, their mechanisms of action and applications. Biochem. Soc. Trans. 2020, 48, 537–546. [Google Scholar] [CrossRef]
- Bawaskar, H.S.; Bawaskar, P.H. The global burden of snake bite envenoming. J. R. Coll. Physicians Edinb. 2021, 51, 7–8. [Google Scholar] [CrossRef]
- Afroz, A.; Siddiquea, B.N.; Chowdhury, H.A.; Jackson, T.N.; Watt, A.D.; Habib, A.G. Snakebite envenoming: A systematic review and meta-analysis of global morbidity and mortality. PLoS Neglected Trop. Dis. 2024, 18, e0012080. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Kadam, P.; Chandrasekharuni, G.; Oliveira, I.S.; Senthilkumaran, S.; Salim, A.; Patel, K.; Sachett, J.d.A.G.; Pucca, M.B. Multifaceted community health education programs as powerful tools to mitigate snakebite-induced deaths, disabilities, and socioeconomic burden. Toxicon X 2023, 17, 100147. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.G.; Brown, N.I. The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon 2018, 150, 115–123. [Google Scholar] [CrossRef]
- Habib, A.G.; Musa, B.M.; Iliyasu, G.; Hamza, M.; Kuznik, A.; Chippaux, J.-P.; de Silva, J. Challenges and prospects of snake antivenom supply in sub-Saharan Africa. PLoS Neglected Trop. Dis. 2020, 14, e0008374. [Google Scholar] [CrossRef]
- Herzel, B.; Batavia, N.; Gavaza, P.; Phan, T.; Samones, E.; Ruha, A.-M.; Furmaga, J.; Hoyte, C.; Wolk, B.J. The Cost of Antivenom: A Cost Minimization Study using the North American Snakebite Registry. J. Med. Toxicol. 2025, 21, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Matlani, J. Snake Bites and Anti-Venom. SSRN 2024; pp. 1–4. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4701335 (accessed on 25 April 2025).
- TRS No 1004; WHO. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins, Annex 5. WHO Expert Committee on Biological Standardization: Geneva, Switzerland, 2017.
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Évid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Majeed, R.; Bester, J.; Kgarosi, K.; Strydom, M. Mapping evidence on the regulations affecting accessibility, availability and management of snake antivenom globally: A scoping review protocol. BMJ Open 2024, 14, e086964. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2020. Available online: https://jbi-global-wiki.refined.site/space/MANUAL (accessed on 20 March 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Fan, H.W.; Monteiro, W.M. History and perspectives on how to ensure antivenom accessibility in the most remote areas in Brazil. Toxicon 2018, 151, 15–23. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Reducing the impact of snakebite envenoming in Latin America and the Caribbean: Achievements and challenges ahead. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 530–537. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Fan, H.W.; Silvera, C.L.; Angulo, Y. Stability, distribution and use of antivenoms for snakebite envenomation in Latin America: Report of a workshop. Toxicon 2009, 53, 625–630. [Google Scholar] [CrossRef]
- Guerra, G.F.C.; da Silva, L.G.; Machado, C.; Fernandes, D.S. Potential geographic distribution of the genus micrurus wagler, 1824 (Serpentes: Elapidae) and antivenom supply in Rio de Janeiro State, Brazil. Oecol. Aus. 2019, 23, 496–500. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Current challenges for confronting the public health problem of snakebite envenoming in Central America. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 7. [Google Scholar] [CrossRef]
- Whitaker, R.; Whitaker, S. Venom, antivenom production and the medically important snakes of India. Curr. Sci. 2012, 103, 635–643. [Google Scholar]
- Amin, M.R. Antivenom for snake bite: Critical supply in health care settings. J. Med. 2010, 11, 57–59. [Google Scholar] [CrossRef]
- Patikorn, C.; Ismail, A.K.; Abidin, S.A.Z.; Blanco, F.B.; Blessmann, J.; Choumlivong, K.; Comandante, J.D.; Doan, U.V.; Ismail, Z.M.; Khine, Y.Y.; et al. Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries. BMJ Glob. Health 2022, 7, e007639. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Habib, A.G. Antivenom shortage is not circumstantial but structural. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P.; Massougbodji, A.; Habib, A.G. The WHO strategy for prevention and control of snakebite envenoming: A sub-Saharan Africa plan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190083. [Google Scholar] [CrossRef]
- Darryl, W.; Sartorius, B.; Hift, R. Estimating the Burden of Snakebite on Public Hospitals in KwaZulu Natal, South Africa. Wilderness Environ. Med. 2016, 27, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gampini, S.; Nassouri, S.; Chippaux, J.-P.; Semde, R. Retrospective study on the incidence of envenomation and accessibility to antivenom in Burkina Faso. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 10. [Google Scholar] [CrossRef]
- Schurer, J.M.; Murara, E.; van Oirschot, J.; Ooms, G. Antivenom for sale? Availability and affordability of snakebite medicines across public and private health facilities in Rwanda. Toxicon 2023, 234, 107292. [Google Scholar] [CrossRef]
- Texas Public Health Association. Potential Impact of Coral Snake Antivenin Shortage. TPH 2011, 63, 17–18. [Google Scholar]
- O’Malley, P.A. More Snakebites and Less Antivenom: Prescribing Burdens for Venomous Envenoming: CNS. Clin. Nurse Spec. 2019, 33, 261. [Google Scholar] [CrossRef]
- Dijkman, M.; van der Zwan, C.; de Vries, I. Establishment and first experiences of the National Serum Depot in the Netherlands. Toxicon 2012, 60, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M. Improving antivenom availability and accessibility: Science, technology, and beyond. Toxicon 2012, 60, 676–687. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Global Availability of Antivenoms: The Relevance of Public Manufacturing Laboratories. Toxins 2019, 11, 5. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Understanding and confronting snakebite envenoming: The harvest of cooperation. Toxicon 2016, 109, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Burnouf, T.; Harrison, R.A.; Calvete, J.J.; Kuch, U.; Warrell, D.A.; Williams, D.J. A multicomponent strategy to improve the availability of antivenom for treating snakebite envenoming. Bull. World Health Organ. 2014, 92, 526–532. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 56, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, R.K.; Munshi, H.; Bawaskar, H.S. National programme for prevention & control of snakebite in India: Key challenges & recommendations. Indian J. Med. Res. 2023, 157, 271–275. [Google Scholar] [PubMed]
- Shrestha, B.R.; Pandey, D.P.; Acharya, K.P.; Thapa-Magar, C.; Mohamed, F.; Isbister, G.K. Effective, polyvalent, affordable antivenom needed to treat snakebite in Nepal. Bull. World Health Organ. 2017, 95, 718–719. [Google Scholar] [CrossRef]
- Blessmann, J.; Hanlodsomphou, S.; Santisouk, B.; Krumkamp, R.; Kreuels, B.; Ismail, A.K.; Yong, M.Y.; Tan, K.Y.; Tan, C.H. Experience of using expired lyophilized snake antivenom during a medical emergency situation in Lao People’s Democratic Republic––A possible untapped resource to tackle antivenom shortage in Southeast Asia. Trop. Med. Int. Health 2023, 28, 64–70. [Google Scholar] [CrossRef]
- Potet, J.; Beran, D.; Ray, N.; Alcoba, G.; Habib, A.G.; Iliyasu, G.; Waldmann, B.; Ralph, R.; Faiz, M.A.; Monteiro, W.M.; et al. Access to antivenoms in the developing world: A multidisciplinary analysis. Toxicon X 2021, 12, 100086. [Google Scholar] [CrossRef]
- Simpson, I.D.; Blaylock, R.S. The Anti Snake Venom Crisis in Africa: A Suggested Manufacturers Product Guide. Wilderness Environ. Med. 2009, 20, 275–282. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutiérrez, J.-M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Hutson, S. Antivenoms needed, say officials, but companies won’t bite. Nat. Med. 2010, 16, 615. [Google Scholar] [CrossRef]
- Bagcchi, S. Experts call for snakebite to be re-established as a neglected tropical disease. BMJ 2015, 351, h5313. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Calvete, J.; Habib, A.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Habib, A.G. Public health aspects of snakebite care in West Africa: Perspectives from Nigeria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 27. [Google Scholar] [CrossRef]
- Harrison, R.A.; Gutiérrez, J.M. Priority actions and progress to substantially and sustainably reduce the mortality, morbidity and socioeconomic burden of tropical snakebite. Toxins 2016, 8, 351. [Google Scholar] [CrossRef]
- Scheske, L.; Ruitenberg, J.; Bissumbhar, B. Needs and availability of snake antivenoms: Relevance and application of international guidelines. Int. J. Health Policy Manag. 2015, 4, 447–457. [Google Scholar] [CrossRef]
- Schiermeier, Q. Africa braced for snakebite crisis. Nature 2015, 525, 299. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J. Snake bite: A global failure to act costs thousands of lives each year. BMJ 2015, 351, h5378. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.B. Availability of antivenom for treating adder bites in dogs. Vet. Rec. Open 2014, 174, 586. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Zanette, L.; Vigilato, M.A.N.; Pompei, J.C.A.; Martins, D.; Fan, H.W.; Latin American Network of Public Antivenom Manufacturing Laboratories (RELAPA). Appraisal of antivenom production in public laboratories in Latin America during the first semester of 2020: The impact of COVID-19. PLoS Neglected Trop. Dis. 2021, 15, e0009469. [Google Scholar] [CrossRef] [PubMed]
- Matafwali, S.K.; Vlahakis, P.A.; Daka, V.; Witika, B.A.; Nyirenda, H.T.; Chisompola, N.K.; Mwila, C. Assessment of the availability of snakebite antivenom in health facilities in Ndola District, Zambia: A cross-sectional study. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 592–594. [Google Scholar] [CrossRef]
- Ooms, G.I.; van Oirschot, J.; Okemo, D.; Waldmann, B.; Erulu, E.; Mantel-Teeuwisse, A.K.; Ham, H.A.v.D.; Reed, T.; Ainsworth, S.R. Availability, affordability and stock-outs of commodities for the treatment of snakebite in Kenya. PLoS Neglected Trop. Dis. 2021, 15, e0009702. [Google Scholar] [CrossRef]
- van Oirschot, J.; Ooms, G.I.; Waldmann, B.; Kadam, P. Snakebite incidents, prevention and care during COVID-19: Global key-informant experiences. Toxicon X 2021, 9, 100075. [Google Scholar] [CrossRef]
- Al-Abdulla, I.; Casewell, N.R.; Landon, J. Long-term physicochemical and immunological stability of a liquid formulated intact ovine immunoglobulin-based antivenom. Toxicon 2013, 64, 38–42. [Google Scholar] [CrossRef]
- Fry, B.G. Snakebite: When the Human Touch Becomes a Bad Touch. Toxins 2018, 10, 170. [Google Scholar] [CrossRef]
- Neumann, N.R.; du Plessis, A.; van Hoving, D.J.; Hoyte, C.O.; Lermer, A.; Wittels, S.; Marks, C. Antivenom supply and demand: An analysis of antivenom availability and utilization in South Africa. Afr. J. Emerg. Med. 2023, 13, 245–249. [Google Scholar] [CrossRef]
- Isbister, G.K. Antivenom availability, delays and use in Australia. Toxicon X 2023, 17, 100145. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.A.D.; Silva, D.R.X.; e Silva, M.G. Geographical accessibility to the supply of antiophidic sera in Brazil: Timely access possibilities. PLoS ONE 2022, 17, e0260326. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Teixeira, C.F.; Fan, H.W. Instituto Butantan and Instituto Clodomiro Picado: A long-standing partnership in science, technology, and public health. Toxicon 2021, 202, 75–81. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Mahmood, M.A.; Alfred, S.; Thwin, K.T.; Kyaw, K.M.; Zaw, A.; Warrell, D.; Cumming, R.; Moody, J.; Eagles, D.; et al. A comprehensive approach to managing a neglected, neglected tropical disease; The Myanmar Snakebite Project (MSP). Toxicon X 2019, 1, 100001. [Google Scholar] [CrossRef]
- Yoshimura, K.; Hossain, M.; Tojo, B.; Tieu, P.; Trinh, N.N.; Huy, N.T.; Sato, M.; Moji, K.; Monteiro, W.M. Barriers to the hospital treatment among Bede snake charmers in Bangladesh with special reference to venomous snakebite. PLoS Neglected Trop. Dis. 2023, 17, e0011576. [Google Scholar] [CrossRef]
- Beck, T.P.; Tupetz, A.; Farias, A.S.; Silva-Neto, A.; Rocha, T.; Smith, E.R.; Murta, F.; Dourado, F.S.; Cardoso, S.; Ramos, T.A.; et al. Mapping of clinical management resources for snakebites and other animal envenomings in the Brazilian Amazon. Toxicon X 2022, 16, 100137. [Google Scholar]
- Suchonwanich, N.; Wananukul, W. Improving access to antidotes and antivenoms, Thailand. Bull. World Health Organ. 2018, 96, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.D.; Jacobsen, I.M. Antisnake Venom Production Crisis—Who Told Us It Was Uneconomic and Unsustainable? Wilderness Environ. Med. 2009, 20, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Janke, R.; Bermúdez-Méndez, E.; Ledsgaard, L.; Barbosa, J.E.; Laustsen, A.H. History of envenoming therapy and current perspectives. Front. Immunol. 2019, 10, 1598. [Google Scholar] [CrossRef]
- Salim, A.; Williams, J.; Wahab, S.A.; Adeshokan, T.; Almeida, J.R.; Williams, H.F.; Vaiyapuri, R.; Senthilkumaran, S.; Thirumalaikolundusubramanian, P.; Patel, K.; et al. Identifying key factors contributing to treatment costs for snakebite envenoming in private tertiary healthcare settings in Tamil Nadu, India. PLoS Neglected Trop. Dis. 2023, 17, e0011699. [Google Scholar] [CrossRef]
- Soopairin, S.; Patikorn, C.; Taychakhoonavudh, S.; de Morais-Zani, K. Antivenom preclinical efficacy testing against Asian snakes and their availability in Asia: A systematic review. PLoS ONE 2023, 18, e0288723. [Google Scholar] [CrossRef]
- Solano, G.; Cunningham, S.; Edge, R.J.; Duran, G.; Sanchez, A.; Villalta, M.; Clare, R.H.; Wilkinson, M.C.; Marriott, A.E.; Abada, C.; et al. African polyvalent antivenom can maintain pharmacological stability and ability to neutralise murine venom lethality for decades post-expiry: Evidence for increasing antivenom shelf life to aid in alleviating chronic shortages. BMJ Glob. Health 2024, 9, e014813. [Google Scholar] [CrossRef]
- J, P. Debate-Pro: Manufacturers should assess the long-term stability of their antivenoms. Emerg. Med. J. 2024, 41, 560. [Google Scholar]
- Patikorn, C.; Ismail, A.K.; Abidin, S.A.Z.; Othman, I.; Chaiyakunapruk, N.; Taychakhoonavudh, S.; Maduwage, K.P. Potential economic and clinical implications of improving access to snake antivenom in five ASEAN countries: A cost-effectiveness analysis. PLoS Neglected Trop. Dis. 2022, 16, e0010915. [Google Scholar] [CrossRef]
- Dalhat, M.M.; Potet, J.; Mohammed, A.; Chotun, N.; Tesfahunei, H.A.; Habib, A.G. Availability, accessibility and use of antivenom for snakebite envenomation in Africa with proposed strategies to overcome the limitations. Toxicon X 2023, 18, 100152. [Google Scholar] [CrossRef] [PubMed]
- Potet, J.; Smith, J.; McIver, L.; Chippaux, J.-P. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Neglected Trop. Dis. 2019, 13, e0007551. [Google Scholar] [CrossRef]
- Chaisakul, J.; Rusmili, M.R.A.; Alsolaiss, J.; Albulescu, L.-O.; Harrison, R.A.; Othman, I.; Casewell, N.R. In Vitro Immunological Cross-Reactivity of Thai Polyvalent and Monovalent Antivenoms with Asian Viper Venoms. Toxins 2020, 12, 766. [Google Scholar] [CrossRef]
- Kumar, R.; Rathore, A.S. Snakebite Management: The Need of Reassessment, International Relations, and Effective Eco-nomic Measures to Reduce the Considerable SBE Burden. J. Epidemiol. Glob. Health 2024, 14, 586–612. [Google Scholar] [CrossRef]
- Justo, N.; Espinoza, M.A.; Ratto, B.; Nicholson, M.; Rosselli, D.; Ovcinnikova, O.; Martí, S.G.; Ferraz, M.B.; Langsam, M.; Drummond, M.F. Real-World Evidence in Healthcare Decision Making: Global Trends and Case Studies From Latin America. Value Health 2019, 22, 739–749. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Fan, H.W. Improving the control of snakebite envenomation in Latin America and the Caribbean: A discussion on pending issues. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 523–526. [Google Scholar] [CrossRef]
- Montoya-Vargas, W.; Gutiérrez, J.M.; Quesada-Morúa, M.S.; Morera-Huertas, J.; Rojas, C.; Leon-Salas, A. Preliminary assessment of antivenom availability and management in the public health system of Costa Rica: An analysis based on a survey to pharmacists in public health facilities. Toxicon X 2022, 16, 100139. [Google Scholar] [CrossRef]
- Willyard, C. A Fanged Crises. Nature 2023, 621, S40–S47. [Google Scholar] [CrossRef]
| Inclusion | Exclusion | ||
|---|---|---|---|
| Population | Snake antivenom |
|
|
| Concept | Accessibility |
|
|
| Context | Global |
|
| Theme | Key Findings |
|---|---|
| Regulatory frameworks |
|
| Manufacturing and procurement |
|
| Availability and access |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, R.; Bester, J.; Kgarosi, K.; Strydom, M. Mapping Evidence on the Regulations Affecting the Accessibility, Availability, and Management of Snake Antivenom Globally: A Scoping Review. Trop. Med. Infect. Dis. 2025, 10, 228. https://doi.org/10.3390/tropicalmed10080228
Majeed R, Bester J, Kgarosi K, Strydom M. Mapping Evidence on the Regulations Affecting the Accessibility, Availability, and Management of Snake Antivenom Globally: A Scoping Review. Tropical Medicine and Infectious Disease. 2025; 10(8):228. https://doi.org/10.3390/tropicalmed10080228
Chicago/Turabian StyleMajeed, Ramsha, Janette Bester, Kabelo Kgarosi, and Morné Strydom. 2025. "Mapping Evidence on the Regulations Affecting the Accessibility, Availability, and Management of Snake Antivenom Globally: A Scoping Review" Tropical Medicine and Infectious Disease 10, no. 8: 228. https://doi.org/10.3390/tropicalmed10080228
APA StyleMajeed, R., Bester, J., Kgarosi, K., & Strydom, M. (2025). Mapping Evidence on the Regulations Affecting the Accessibility, Availability, and Management of Snake Antivenom Globally: A Scoping Review. Tropical Medicine and Infectious Disease, 10(8), 228. https://doi.org/10.3390/tropicalmed10080228






