Epidemiological Analysis of the COVID-19 Clusters in the Early Stages of the Epidemic in Shanghai, China: Pandemic-to-Epidemic Response Shift
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Statistical Analysis and Study Definitions
2.2.1. Epidemiology of Clusters
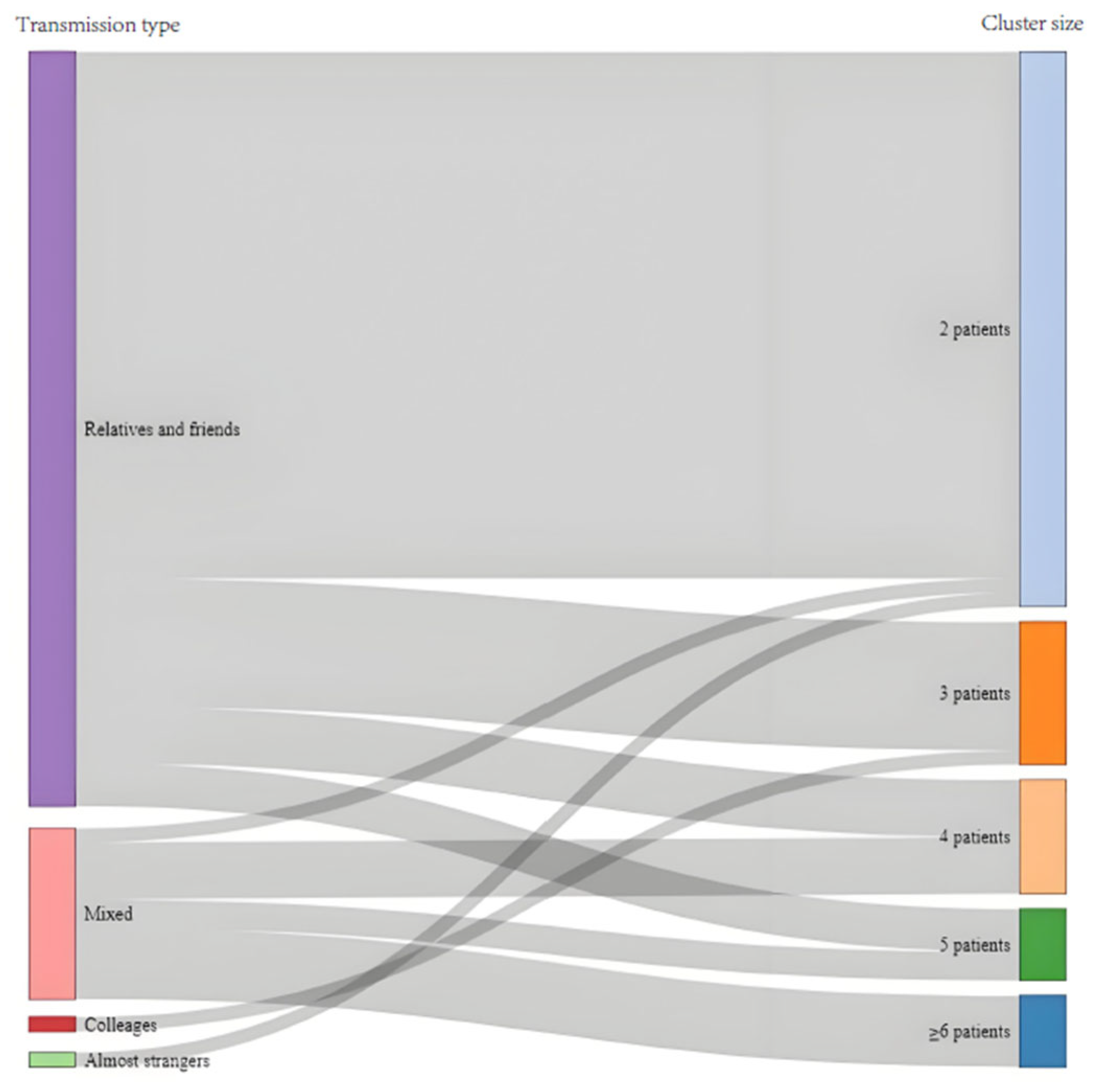
2.2.2. Analysis on the Transmission Features of Clusters
2.3. Ethics Approval
3. Results
3.1. Epidemiological Description of Clusters in Shanghai
3.2. Transmission Features of Clusters
3.3. Individual Risk Factors of Contagiousness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahir, M.J.; Sawal, I.; Essar, M.Y.; Jabbar, A.; Ullah, I.; Ahmed, A. Disease X: A hidden but inevitable creeping danger. Infect. Control Hosp. Epidemiol. 2022, 43, 1758–1759. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, Z.-L. The First Disease X is Caused by a Highly Transmissible Acute Respiratory Syndrome Coronavirus. Virol. Sin. 2020, 35, 263–265. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Si, H.; Shi, Z. Emergence of SARS and COVID-19 and preparedness for the next emerging disease X. Front. Med. 2024, 18, 1–18. [Google Scholar] [CrossRef]
- Awasthi, R. Disease X: Beyond Fear, Toward Preparedness. Infect. Disord. Drug Targets 2024, 24, e230124226003. [Google Scholar] [CrossRef]
- Ulrichs, T.; Rolland, M.; Wu, J.; Nunes, M.C.; El Guerche-Séblain, C.; Chit, A. Changing epidemiology of COVID-19: Potential future impact on vaccines and vaccination strategies. Expert Rev. Vaccines 2024, 23, 510–522. [Google Scholar] [CrossRef]
- COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 6 May 2025).
- From Emergency Response to Long-Term COVID-19 Disease Management: Sustaining Gains Made During the COVID-19 Pandemic. Available online: https://www.who.int/publications/i/item/WHO-WHE-SPP-2023.1 (accessed on 6 May 2025).
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 9 May 2025).
- Katzourakis, A. COVID-19: Endemic doesn’t mean harmless. Nature 2022, 601, 485. [Google Scholar] [CrossRef]
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef]
- Stein, R. Is COVID Endemic Yet? Yep, Says the CDC. Here’s What That Means. NPR. 9 August 2024. Available online: https://www.npr.org/sections/shots-health-news/2024/08/09/nx-s1-5060398/covid-endemic-cdc-summer-surge (accessed on 6 May 2025).
- Biancolella, M.; Colona, V.L.; Mehrian-Shai, R.; Watt, J.L.; Luzzatto, L.; Novelli, G.; Reichardt, J.K.V. COVID-19 2022 update: Transition of the pandemic to the endemic phase. Hum. Genom. 2022, 16, 19. [Google Scholar] [CrossRef]
- Cohen, L.E.; Spiro, D.J.; Viboud, C. Projecting the SARS-CoV-2 transition from pandemicity to endemicity: Epidemiological and immunological considerations. PLoS Pathog. 2022, 18, e1010591. [Google Scholar] [CrossRef]
- Antia, R.; Halloran, M.E. Transition to endemicity: Understanding COVID-19. Immunity 2021, 54, 2172–2176. [Google Scholar] [CrossRef]
- Fouda, B.; Tram, H.P.B.; Makram, O.M.; Abdalla, A.S.; Singh, T.; Hung, I.-C.; Raut, A.; Hemmeda, L.; Alahmar, M.; ElHawary, A.S.; et al. Identifying SARS-CoV2 transmission cluster category: An analysis of country government database. J. Infect. Public Health 2021, 14, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ang, Z.Y.; Balqis-Ali, N.Z.; Jailani, A.-S.; Kong, Y.-L.; Sharif, S.M.; Fun, W.H. COVID-19 clusters in Malaysia: Characteristics, detection methods and modes of early transmission. West. Pac. Surveill. Response J. WPSAR 2023, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Huang, T.; Yuan, H.; Li, M.; Huang, X.; Yang, C.; Zhou, X.; Cheng, X.; Su, Q.; Wu, X. Epidemiological analysis of 67 local COVID-19 clusters in Sichuan Province, China. BMC Public Health 2020, 20, 1525. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sharma, A.; Sharma, D.K.; Nirola, M.; Dhungel, P.; Patel, A.; Singh, H.; Gupta, A. Tracing the COVID-19 spread pattern in India through a GIS-based spatio-temporal analysis of interconnected clusters. Sci. Rep. 2024, 14, 847. [Google Scholar] [CrossRef]
- Ladoy, A.; Opota, O.; Carron, P.-N.; Guessous, I.; Vuilleumier, S.; Joost, S.; Greub, G. Size and duration of COVID-19 clusters go along with a high SARS-CoV-2 viral load: A spatio-temporal investigation in Vaud state, Switzerland. Sci. Total Environ. 2021, 787, 147483. [Google Scholar] [CrossRef]
- Luo, T.; Wang, J.; Wang, Q.; Wang, X.; Zhao, P.; Zeng, D.D.; Zhang, Q.; Cao, Z. Reconstruction of the Transmission Chain of COVID-19 Outbreak in Beijing’s Xinfadi Market, China. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 116, 411–417. [Google Scholar] [CrossRef]
- Son, W.-S. RISEWIDs Team Individual-based simulation model for COVID-19 transmission in Daegu, Korea. Epidemiol. Health 2020, 42, e2020042. [Google Scholar] [CrossRef]
- Medley, A.M.; Marston, B.J.; Toda, M.; Kobayashi, M.; Weinberg, M.; Moriarty, L.F.; Jungerman, M.R.; Surpris, A.C.A.; Knust, B.; Acosta, A.M.; et al. Use of US Public Health Travel Restrictions during COVID-19 Outbreak on Diamond Princess Ship, Japan, February-April 2020. Emerg. Infect. Dis. 2021, 27, 710–718. [Google Scholar] [CrossRef]
- US Food Processing Plants Become COVID-19 Hot Spots|CIDRAP. 27 April 2020. Available online: https://www.cidrap.umn.edu/covid-19/us-food-processing-plants-become-covid-19-hot-spots (accessed on 6 May 2025).
- Takano, T.; Nakatani, Y.; Nagai, A.; Izumoto, N.; Ono, Y.; Inoue, A.; Takemura, H.; Kunishima, H. Analysis of COVID-19 nosocomial clusters in an Omicron strain epidemic: Importance of patient education on infection control measures. Infect. Prev. Pract. 2024, 6, 100410. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Papaefthymiou, A.; Georgilas, N.; Doulberis, M.; Kountouras, J. The Potential Role of Super Spread Events in SARS-COV-2 Pandemic; a Narrative Review. Arch. Acad. Emerg. Med. 2020, 8, e74. [Google Scholar]
- Wegehaupt, O.; Endo, A.; Vassall, A. Superspreading, overdispersion and their implications in the SARS-CoV-2 (COVID-19) pandemic: A systematic review and meta-analysis of the literature. BMC Public Health 2023, 23, 1003. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Luo, M.; Jia, T.; Zhang, X.; Hou, Z.; Gao, F.; Wang, X.; Wu, X.; Cheng, W.; Li, G.; et al. COVID-19 Super Spreading Event Amongst Elderly Individuals—Jilin Province, China, January 2021. China CDC Wkly. 2021, 3, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Identification of Superspreading Environment Under COVID-19 Through Human Mobility Data|Scientific Reports. Available online: https://www.nature.com/articles/s41598-021-84089-w (accessed on 6 May 2025).
- Ao, D.; He, X.; Liu, J.; Xu, L. Strategies for the development and approval of COVID-19 vaccines and therapeutics in the post-pandemic period. Signal Transduct. Target. Ther. 2023, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.U.; Hartigan-Go, K.Y.; Brillantes, A.B.; Ruiz, K.E.V.; Baysic, I.S.; Valenzuela, S.A. Public policy (not the coronavirus) should shape what endemic means. J. Glob. Health 2022, 12, 03050. [Google Scholar] [CrossRef]
- Infection Prevention and Control in the Context of COVID-19: A Guideline. 21 December 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-guideline-2023.4 (accessed on 7 May 2025).
- Che Mat, N.F.; Edinur, H.A.; Abdul Razab, M.K.A.; Safuan, S. A Single Mass Gathering Resulted in Massive Transmission of COVID-19 Infections in Malaysia with Further International Spread. J. Travel Med. 2020, 27, taaa059. [Google Scholar] [CrossRef]
- Notifiable Infectious Diseases in Shanghai (2020)—Shanghai Municipal Health Commission. Available online: https://wsjkw.sh.gov.cn/yqxx/20210406/1c1deb9ff839447185255d63f7b953e4.html (accessed on 5 June 2025).
- Notifiable Infectious Diseases in Shanghai (2021)—Shanghai Municipal Health Commission. Available online: https://wsjkw.sh.gov.cn/yqxx/20220426/fba512e3bf7e4eaaa32bd82ef723c4d0.html (accessed on 5 June 2025).
- See the Latest Data in Your Region. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/region (accessed on 5 June 2025).
- Fekedulegn, D.; Andrew, M.; Violanti, J.; Hartley, T.; Charles, L.; Burchfiel, C. Comparison of statistical approaches to evaluate factors associated with metabolic syndrome. J. Clin. Hypertens. Greenwich Conn 2010, 12, 365–373. [Google Scholar] [CrossRef]
- Karpowicz, J.; O’Rourke, S.; Clyne, A.; Silvia, J.; Cooper, T.; Comella, J.; Rajotte, J. Characteristics of COVID-19 Workplace Clusters in Rhode Island. R. I. Med. J. 2021, 104, 42–45. [Google Scholar]
- Hsu, C.-Y.; Chen, Y.-M.; Su, C.-W.; Ku, M.-S.; Kim, Y.; Jensen, T.; Luh, D.-L. Preparedness for containing COVID-19 outbreak in mass religious gathering with non-pharmaceutical interventions (NPIs). J. Formos. Med. Assoc. Taiwan Yi Zhi 2021, 120 (Suppl. S1), S57–S68. [Google Scholar] [CrossRef]
- Alsayyad, A.; Chlif, S.; Mohamed, A.; Habbash, F.; Ayoob, Z.; Almarabheh, A.; Al Sayed, K.; Alsaleh, A.; Alhajeri, M.; Alzayani, S.; et al. Super-spreading social events for COVID-19 transmission: Evidence from the investigation of six early clusters in Bahrain. Front. Public Health 2023, 11, 1216113. [Google Scholar] [CrossRef]
- Kochańczyk, M.; Grabowski, F.; Lipniacki, T. Super-spreading events initiated the exponential growth phase of COVID-19 with 0 higher than initially estimated. R. Soc. Open Sci. 2020, 7, 200786. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Li, M.; Tian, R.; Zhao, Y.; Cao, B.; Yao, L.; Sheng, X.; Yu, Y. Epidemiological clustered characteristics of coronavirus disease 2019 (COVID-19) in three phases of transmission in Jilin Province, China. PLoS ONE 2023, 18, e0279879. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Chen, X.; Zheng, C.; Liu, H.; Wang, G.; Zhang, B.; Li, Z.; Zhang, W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: A literature review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.Y.; Maslamani, Y.A.; Muaddi, M.A.; Alameer, A.A.; Alqassim, A.Y.; Doweri, A.A.; Zaylaee, M.M.; Rayani, H.Y.; Darraj, A.Y.; Hejri, Y.M.; et al. Real-world effectiveness of COVID-19 vaccines: A retrospective cohort study of vaccinated individuals in Jazan, Saudi Arabia. J. Infect. Public Health 2023, 16, 1512–1517. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Bravo, L.; Marks, F.; Aziz, A.B.; You, Y.A.; Sugimoto, J.; Li, P.; Garcia, J.; Rockhold, F.; Clemens, R.; et al. Impact of Vaccination With the SCB-2019 Coronavirus Disease 2019 Vaccine on Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Household Contact Study in the Philippines. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, 1180–1187. [Google Scholar] [CrossRef]
- Braeye, T.; Catteau, L.; Brondeel, R.; van Loenhout, J.A.F.; Proesmans, K.; Cornelissen, L.; Van Oyen, H.; Stouten, V.; Hubin, P.; Billuart, M.; et al. Vaccine effectiveness against transmission of alpha, delta and omicron SARS-COV-2-infection, Belgian contact tracing, 2021–2022. Vaccine 2023, 41, 3292–3300. [Google Scholar] [CrossRef]
- GOV.UK. COVID-19 Vaccine Surveillance Report: Week 48. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1134074/vaccine-surveillance-report-week-48-2022.pdf (accessed on 5 June 2025).
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef]
- Martignoni, M.M.; Mohammadi, Z.; Loredo-Osti, J.C.; Hurford, A. Extensive SARS-CoV-2 testing reveals BA.1/BA.2 asymptomatic rates and underreporting in school children. Can. Commun. Dis. Rep. Releve Mal. Transm. Au Can. 2023, 49, 155–165. [Google Scholar] [CrossRef]
- Ibrahim, N.K. Epidemiologic surveillance for controlling COVID-19 pandemic: Types, challenges and implications. J. Infect. Public Health 2020, 13, 1630–1638. [Google Scholar] [CrossRef]
- Binnicker, M.J. Challenges and Controversies to Testing for COVID-19. J. Clin. Microbiol. 2020, 58, e01695-20. [Google Scholar] [CrossRef]
- Böhm, R.; Sprengholz, P.; Betsch, C.; Partheymüller, J. Filter Questions in Symptom Assessment Affect the Prevalence of (A)Symptomatic COVID-19 Cases. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2023, 43, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Lima, A.R.J.; Ribeiro, G.; de Lima, L.P.O.; Barros, C.R.D.S.; Marqueze, E.C.; Martins, A.J.; Martininghi, M.; Palmieri, M.; Caldeira, L.A.V.; et al. Epidemiological and Genomic Analysis of Asymptomatic SARS-CoV-2 Infections during the Delta and Omicron Epidemic Waves in São Paulo City, Brazil. Viruses 2023, 15, 2210. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, X.; Wang, L.; Yan, R.; Pan, Y.; Wang, J.; Chen, Z. Clinical and virological features of asymptomatic and mild symptomatic patients with SARS-CoV-2 Omicron infection at Shanghai Fangcang shelter hospital. Immun. Inflamm. Dis. 2023, 11, e1033. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef]
- Arashiro, T.; Miwa, M.; Nakagawa, H.; Takamatsu, J.; Oba, K.; Fujimi, S.; Kikuchi, H.; Iwasawa, T.; Kanbe, F.; Oyama, K.; et al. COVID-19 vaccine effectiveness against severe COVID-19 requiring oxygen therapy, invasive mechanical ventilation, and death in Japan: A multicenter case-control study (MOTIVATE study). Vaccine 2024, 42, 677–688. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, Y.; Zhu, H.; Ling, Y.; Zou, Y.; Zhang, Z.; Guo, H.; Liu, Y.; Cheng, X.; Liu, M.; et al. Observations about symptomatic and asymptomatic infections of 494 patients with COVID-19 in Shanghai, China. Am. J. Infect. Control 2020, 48, 1045–1050. [Google Scholar] [CrossRef]
- Gupta, S.; Mitra, A. Challenge of post-COVID era: Management of cardiovascular complications in asymptomatic carriers of SARS-CoV-2. Heart Fail. Rev. 2022, 27, 239–249. [Google Scholar] [CrossRef]
- Zhang, K.; Zhong, X.; Fan, X.; Yu, D.; Chen, Z.; Zhao, C.; Zhang, X.; Guan, Z.; Wei, X.; Wan, S.; et al. Asymptomatic infection and disappearance of clinical symptoms of COVID-19 infectors in China 2022-2023: A cross-sectional study. Sci. Rep. 2024, 14, 18232. [Google Scholar] [CrossRef]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109229118. [Google Scholar] [CrossRef]
- Lim, C.; Nam, Y.; Oh, W.S.; Ham, S.; Kim, E.; Kim, M.; Kim, S.; Kim, Y.; Jeong, S. Characteristics of transmission routes of COVID-19 cluster infections in Gangwon Province, Korea. Epidemiol. Infect. 2022, 150, e19. [Google Scholar] [CrossRef] [PubMed]
- Deslandes, A.; Calba, C.; Mahdjoub, S.; Zhu-Soubise, A.; Mathey, D.; Ardoin, A. Superspreading events of SARS-CoV-2 in Paris: A retrospective analysis of data from the first wave of COVID-19 in 2020. J. Infect. Public Health 2021, 14, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Saliba, V.; Lopez Bernal, J.; Ramsay, M.E.; Ladhani, S.N. SARS-CoV-2 infection and transmission in educational settings: A prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect. Dis. 2021, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2031756. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Das, A.; Pan, C.Q.; Xie, W. Transmission dynamics of COVID-19 among index case family clusters in Beijing, China. Epidemiol. Infect. 2020, 149, e74. [Google Scholar] [CrossRef]
- Gunadi; Wibawa, H.; Hakim, M.S.; Marcellus; Trisnawati, I.; Khair, R.E.; Triasih, R.; Irene; Afiahayati; Iskandar, K.; et al. Molecular epidemiology of SARS-CoV-2 isolated from COVID-19 family clusters. BMC Med. Genomics 2021, 14, 144. [Google Scholar] [CrossRef]
- Song, W.-L.; Zou, N.; Guan, W.-H.; Pan, J.-L.; Xu, W. Clinical characteristics of COVID-19 in family clusters: A systematic review. World J. Pediatr. WJP 2021, 17, 355–363. [Google Scholar] [CrossRef]
- Novelli, S.; Opatowski, L.; Manto, C.; Rahib, D.; de Lamballerie, X.; Warszawski, J.; Meyer, L.; On Behalf of The EpiCoV Study Group. Risk Factors for Community and Intrahousehold Transmission of SARS-CoV-2: Modeling in a Nationwide French Population-Based Cohort Study, the EpiCoV Study. Am. J. Epidemiol. 2024, 193, 134–148. [Google Scholar] [CrossRef]
- Derqui, N.; Koycheva, A.; Zhou, J.; Pillay, T.D.; Crone, M.A.; Hakki, S.; Fenn, J.; Kundu, R.; Varro, R.; Conibear, E.; et al. Risk factors and vectors for SARS-CoV-2 household transmission: A prospective, longitudinal cohort study. Lancet Microbe 2023, 4, e397–e408. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.-Y.; Liu, M.-J.; Fang, L.-Q.; Dean, N.E.; Wong, G.W.K.; Yang, X.-B.; Longini, I.; Halloran, M.E.; Wang, H.-J.; et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: A retrospective observational study. Lancet Infect. Dis. 2021, 21, 617–628. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Factors Associated With Household Transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2122240. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Lessler, J.; Eckerle, I.; Lauer, S.A.; Kaiser, L.; Vuilleumier, N.; Cummings, D.A.T.; Flahault, A.; Petrovic, D.; Guessous, I.; et al. Insights into household transmission of SARS-CoV-2 from a population-based serological survey. Nat. Commun. 2021, 12, 3643. [Google Scholar] [CrossRef] [PubMed]
- Klee, B.; Diexer, S.; Xu, C.; Gottschick, C.; Hartmann, C.; Meyer-Schlinkmann, K.M.; Kuhlmann, A.; Rosendahl, J.; Binder, M.; Gekle, M.; et al. Household transmission of Omicron variant of SARS-CoV-2 under conditions of hybrid immunity—A prospective study in Germany. Infection 2025, 53, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shen, P.; Yan, J.; Huang, Y.; Ba, X.; Lin, W.; Wang, H.; Huang, Y.; Qin, K.; Wang, Y.; et al. Exploring the Clinical Characteristics of COVID-19 Clusters Identified Using Factor Analysis of Mixed Data-Based Cluster Analysis. Front. Med. 2021, 8, 644724. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, Y.; Liu, F.; Zhang, H.; Zhang, F.; Wang, L. Epidemiological characteristics of COVID-19 clusters in Hainan, China. Medicine 2021, 100, e27512. [Google Scholar] [CrossRef]
- Liao, J.; Liu, X.F.; Xu, X.-K.; Zhou, T. COVID-19 spreading patterns in family clusters reveal gender roles in China. J. R. Soc. Interface 2023, 20, 20230336. [Google Scholar] [CrossRef]
- Diao, K.-Y.; Zhang, X.-C.; Huang, S.; Wang, H.-L.; Gang, Y.-D.; Deng, Y.-P.; Han, P.-L.; Pang, T.; Yu, J.-L.; Guo, Y.-K.; et al. Features of family clusters of COVID-19 patients: A retrospective study. Travel Med. Infect. Dis. 2021, 39, 101950. [Google Scholar] [CrossRef]
- Li, W.; Gong, J.; Zhou, J.; Fan, H.; Qin, C.; Gong, Y.; Hu, W. The Analysis of Patterns of Two COVID-19 Outbreak Clusters in China. Int. J. Environ. Res. Public. Health 2022, 19, 4876. [Google Scholar] [CrossRef]
- Song, R.; Han, B.; Song, M.; Wang, L.; Conlon, C.P.; Dong, T.; Tian, D.; Zhang, W.; Chen, Z.; Zhang, F.; et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing, China. J. Infect. 2020, 81, e26–e30. [Google Scholar] [CrossRef]
- Lendacki, F.R.; Forst, L.; Weber, E.; Mehta, S.D.; Kerins, J.L. COVID-19 Clusters and Outbreaks Among Non-Health Care, Noncongregate Workers in Chicago, Illinois: Surveillance Through the First Omicron Wave. J. Occup. Environ. Med. 2023, 65, e211–e218. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, Z.; Zeng, T.; Sun, S.; Lu, Y.; Teng, Z.; Tian, M.; Wang, J.; Li, S.; Fan, X.; et al. Case clustering, contact stratification, and transmission heterogeneity of SARS-CoV-2 Omicron BA.5 variants in Urumqi, China: An observational study. J. Glob. Health 2023, 13, 06018. [Google Scholar] [CrossRef] [PubMed]
- Dequeker, S.; Callies, M.; Catteau, L.; Int Panis, L.; Islamaj, E.; Klamer, S.; Latour, K.; Pauwels, M.; Vernemmen, C.; Mahieu, R.; et al. COVID-19 Clusters in Belgian Nursing Homes: Impact of Facility Characteristics and Vaccination on Cluster Occurrence, Duration and Severity. Viruses 2023, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Bias in observational study designs: Cross sectional studies. BMJ 2015, 350, h1286. [Google Scholar] [CrossRef] [PubMed]
- Common Biases in Observational Studies and How to Mitigate Them. Pro Pharma. 26 August 2024. Available online: https://propharmaresearch.com/en/resources/diffusion/common-biases-observational-studies-and-how-mitigate-them (accessed on 5 June 2025).
- Grimes, D.A.; Schulz, K.F. Bias and causal associations in observational research. Lancet 2002, 359, 248–252. [Google Scholar] [CrossRef]
- Ikeue, K.; Kato, H.; Tanaka, M.; Yamakage, H.; Kato, S.; Iwasa, M.; Oishi, K.; Yamamoto, Y.; Kanasaki, M.; Masuda, I.; et al. Phase Angle Is a Potential Novel Early Marker for Sarcopenia and Cognitive Impairment in the General Population. J. Cachexia Sarcopenia Muscle 2025, 16, e13820. [Google Scholar] [CrossRef]
- Yaw, L.K.; Burrell, M.; Ho, K.M. Long-Term Outcomes and Determinants of New-Onset Mental Health Conditions After Trauma. JAMA Netw. Open 2025, 8, e250349. [Google Scholar] [CrossRef]


| Count (col. %) | Contagious Case a (row %) | Risk Ratio b (95% CI) | p-Value | |
|---|---|---|---|---|
| Age | ||||
| 0–19 years | 15 (4%) | 0 (0%) | ||
| 20–39 years | 122 (32%) | 14 (11%) | ||
| 40–59 years | 132 (35%) | 11 (8%) | ||
| 60–88 years | 112 (29%) | 11 (10%) | ||
| Gender | ||||
| Male | 203 (53%) | 16 (8%) | ||
| Female | 178 (47%) | 20 (11%) | ||
| Initial symptoms | ||||
| Fever | ||||
| No | 146 (38%) | 11 (8%) | 1.0 (reference) | |
| Yes | 235 (62%) | 25 (11%) | 1.0 (0.46–2.30) | 0.95 |
| Dry cough | ||||
| No | 259 (68%) | 25 (10%) | 1.0 (reference) | |
| Yes | 122 (32%) | 11 (9%) | 1.3 (0.56–2.85) | 0.58 |
| Sore throat | ||||
| No | 347 (91%) | 29 (8%) | 1.0 (reference) | |
| Yes | 34 (9%) | 7 (21%) | 2.9 (1.00–8.48) | 0.05 |
| Runny nose | ||||
| No | 359 (94%) | 30 (8%) | 1.0 (reference) | |
| Yes | 22 (6%) | 6 (27%) | 4.8 (1.40–16.44) | 0.01 |
| Weakness | ||||
| No | 338 (89%) | 31 (9%) | 1.0 (reference) | |
| Yes | 43 (11%) | 5 (12%) | 0.9 (0.29–2.53) | 0.79 |
| BMI c | ||||
| Underweight | 41 (11%) | 2 (5%) | 1.23 (0.23, 6.66) | 0.81 |
| Normal | 170 (45%) | 14 (8%) | 1.00 (reference) | |
| Overweight | 170 (45%) | 20 (12%) | 1.94 (0.85,4.42) | 0.12 |
| Comorbid condition | ||||
| Diabetes | ||||
| No | 352 (92%) | 30 (9%) | 1.0 (reference) | |
| Yes | 29 (8%) | 6 (21%) | 3.8 (1.01–14.60) | 0.05 |
| High blood pressure | ||||
| No | 311 (82%) | 27 (9%) | 1.0 (reference) | |
| Yes | 70 (19%) | 9 (13%) | 1.9 (0.64–5.60) | 0.25 |
| Heart disease | ||||
| No | 357 (94%) | 32 (9%) | 1.0 (reference) | |
| Yes | 24 (7%) | 4 (17%) | 2.0 (0.46–8.37) | 0.36 |
| Clinical manifestation | ||||
| Mild (non-pneumonia) | 24 (6%) | 2 (8%) | 1.00 (reference) | |
| Mild (pneumonia) | 332 (87%) | 28 (8%) | 0.63 (0.12, 3.37) | 0.59 |
| Severe | 9 (2%) | 2 (22%) | 1.22 (0.12, 12.45) | 0.87 |
| Critically severe | 16 (4%) | 4 (25%) | 12.82 (0.81, 203.79) | 0.07 |
| Seeking medical help | ||||
| Diagnosed at first medical visit | 257 (67%) | 16 (6%) | 1.0 (reference) | |
| ≥2 medical visits before diagnosis d | 124 (33%) | 20 (16%) | 2.1 (1.00–4.58) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Fang, Q.; Chen, J.; Hu, L.; Lu, Y.; Zheng, Y.; Zhu, Y.; Jin, B.; Xiao, W.; Mao, S.; et al. Epidemiological Analysis of the COVID-19 Clusters in the Early Stages of the Epidemic in Shanghai, China: Pandemic-to-Epidemic Response Shift. Trop. Med. Infect. Dis. 2025, 10, 170. https://doi.org/10.3390/tropicalmed10060170
Kong D, Fang Q, Chen J, Hu L, Lu Y, Zheng Y, Zhu Y, Jin B, Xiao W, Mao S, et al. Epidemiological Analysis of the COVID-19 Clusters in the Early Stages of the Epidemic in Shanghai, China: Pandemic-to-Epidemic Response Shift. Tropical Medicine and Infectious Disease. 2025; 10(6):170. https://doi.org/10.3390/tropicalmed10060170
Chicago/Turabian StyleKong, Dechuan, Qiwen Fang, Jian Chen, Linjie Hu, Yihan Lu, Yaxu Zheng, Yiyi Zhu, Bihong Jin, Wenjia Xiao, Shenghua Mao, and et al. 2025. "Epidemiological Analysis of the COVID-19 Clusters in the Early Stages of the Epidemic in Shanghai, China: Pandemic-to-Epidemic Response Shift" Tropical Medicine and Infectious Disease 10, no. 6: 170. https://doi.org/10.3390/tropicalmed10060170
APA StyleKong, D., Fang, Q., Chen, J., Hu, L., Lu, Y., Zheng, Y., Zhu, Y., Jin, B., Xiao, W., Mao, S., Jiang, C., Gong, X., Lin, S., Han, R., Yu, X., Qiu, Q., Sun, X., Pan, H., & Wu, H. (2025). Epidemiological Analysis of the COVID-19 Clusters in the Early Stages of the Epidemic in Shanghai, China: Pandemic-to-Epidemic Response Shift. Tropical Medicine and Infectious Disease, 10(6), 170. https://doi.org/10.3390/tropicalmed10060170







