Abstract
Following the emergence of COVID-19, breakthrough SARS-CoV-2 infections have demonstrated substantial heterogeneity in both occurrence and clinical severity. This case–control study aimed to elucidate the factors associated with the incidence, duration, and severity of SARS-CoV-2 symptoms among Chinese adults during the Omicron wave. The analysis was based on data from a national COVID-19 surveillance program encompassing six provinces—Jiangsu, Chongqing, Shandong, Hunan, Anhui, and Yunnan—and included both laboratory-confirmed and clinically diagnosed cases. Data were systematically collected between February and April 2023. For each confirmed case, a matched control was selected through simple random sampling, matched on sex, age (±5 years), and province of residence. Multivariate logistic regression analyses were employed to assess a range of potential determinants, including demographic characteristics, lifestyle behaviors, and pre-existing medical conditions, in relation to the risk of infection, as well as the persistence and severity of symptoms following SARS-CoV-2 breakthrough infection. A total of 10,426 cases and 10,426 matched controls were included in the final analysis. Among the infected individuals, 963 (9.24%) reported persistent symptoms, while 773 (7.41%) experienced moderate-to-severe clinical manifestations. Occasional alcohol consumption, presence of comorbidities, tea and coffee intake, overweight status, and a longer interval since the last vaccination dose were all significantly associated with increased odds of infection (OR > 1, FDR < 0.05). Conversely, weekly alcohol consumption and smoking were associated with a decreased risk (OR < 1, FDR < 0.05). Female sex was significantly associated with both persistent and moderate-to-severe symptoms. Additional risk factors for prolonged or severe symptoms included older age, being underweight or overweight, a history of immunotherapy, coffee consumption, and the presence of comorbidities. These findings underscore the multifactorial nature of SARS-CoV-2 infection outcomes and highlight the interplay between host characteristics and behavioral factors. The results support the development of personalized prevention strategies aimed at reducing the clinical burden and long-term impact of COVID-19.
1. Introduction
The COVID-19 pandemic, as a major public health crisis, has had profound adverse impacts on human health, the global economy, and societal functioning. During 2020–2021, approximately 16 million deaths were attributed to COVID-19, and global life expectancy declined by 1.6 years [1]. While most individuals recover within days of infection, a subset experience symptoms that persist for weeks or longer—commonly referred to as “post-acute COVID-19 symptoms” or “long COVID” [2]. The risk and severity of symptoms vary among populations with different immunization statuses. Complete COVID-19 vaccination has been shown to reduce the risk of both infection and the development of long COVID symptoms [3,4]. Several studies have identified risk factors for long COVID. For example, large cohort studies conducted in China and the United Kingdom have demonstrated that female sex, older age, and pre-existing comorbidities are associated with an increased risk of long COVID [5,6]. A systematic review and meta-analysis of 41 studies further found that female sex, advanced age, higher body mass index (BMI), and smoking were significantly associated with symptoms persisting for three months or longer following the acute phase of COVID-19 infection [7].
In China, the COVID-19 vaccine coverage rate has exceeded 90% [8]. However, due to ongoing viral evolution, the threat of SARS-CoV-2 has not been fully mitigated. Notably, following the lifting of strict public health measures in late 2022, a widespread outbreak dominated by the Omicron subvariants BA.5.2 and BF.7 occurred, leading to a large wave of infections across the country. To investigate the determinants of breakthrough infection, symptom persistence, and moderate-to-severe clinical manifestations, we conducted a case–control study. We collected data on disease onset, clinical symptoms, lifestyle behaviors, and demographic characteristics from affected individuals during this period.
2. Method
2.1. Data Sources
This retrospective analysis utilized data from an established COVID-19 epidemiological survey conducted between 4 February and 10 April 2023. The study protocol was approved by the Ethics Review Committee of the Chinese Center for Disease Control and Prevention. The participants included adult permanent residents (≥18 years) recruited from six provinces in China: Jiangsu, Chongqing, Shandong, Hunan, Anhui, and Yunnan. COVID-19 vaccination records were obtained from the National Integrated Vaccination Service Management Information System. Eligible participants were enrolled using a previously described protocol. Briefly, individuals from heterologous vaccine trial cohorts (ClinicalTrials.gov identifiers: NCT04892459, NCT04952727, NCT05043259, NCT05303584, NCT05204589) were matched 1:4 with community-based controls [9]. All participants were invited to participate in a telephone survey. Prior to the commencement of the survey, oral informed consent was obtained from each individual. To meet the research investigation requirements of this study, we designed a standardized questionnaire (Appendix B Method 1). The participants completed a standardized questionnaire through a structured question-and-answer format.
The standardized questionnaire focused on four aspects: (1) Basic demographic information, including residence, height, weight, age, and sex. (2) Lifestyle factors, such as alcohol consumption, smoking, tea consumption, and coffee consumption. Drinking habits were defined as the consumption of beer, liquor, or other alcoholic beverages. Smoking habits were defined as tobacco use, including cigarettes and traditional pipes. A lifestyle habit was considered present if the individual engaged in it at least once every six months. (3) Previous medical history, including comorbidities, allergies, and immunotherapy. The chronic diseases recorded included hypertension, hyperlipidemia, diabetes, stroke, coronary heart disease, and others. (4) COVID-19-related information, including infection status and date, diagnostic method, symptoms, symptom duration, and severity (Appendix B Method 1).
2.2. Study Participants
COVID-19 cases were defined as either (1) etiology-confirmed cases, based on positive nucleic acid or rapid antigen test results, or (2) clinical cases presenting at least two typical symptoms (e.g., fever, cough, weakness, fatigue, headache, myalgia, sore throat, rhinitis, dyspnea, nausea, diarrhea, or anorexia) in conjunction with a known COVID-19 exposure. When the case–control cohort was established, one-to-one individual matching was performed, with each case matched to a control participant based on sex, province of residence, and age (±5 years). A sequential matching procedure was employed as follows: for each control, all eligible cases meeting the matching criteria were identified, and one was randomly selected using simple random sampling to minimize potential selection bias. Once matched, the selected case was removed from the case pool to avoid duplicate assignment. The entire matching process was implemented using R software (version 4.4.0), with the sample function employed for random selection. A fixed random seed was set to ensure reproducibility of the results. The cohort selection flow is presented in Figure 1.
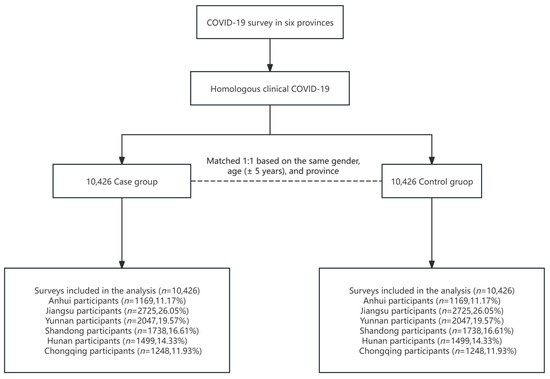
Figure 1.
Selection of cohorts with three doses of an inactivated vaccine from different regions of China prior to the pandemic.
2.3. Study Design
A case–control design was used in this study. The COVID-19 cases in our study included etiology-confirmed cases based on positive nucleic acid or rapid antigen test results and clinical cases presenting with at least two typical symptoms in combination with a COVID-19 exposure. We further analyzed the factors influencing incidence, persistence, and severity symptoms of SARS-CoV-2 breakthrough infection, including demographic characteristics, lifestyle habits, and medical history.
For the purposes of this study, the frequency of these habits was categorized into three groups: “never”, “occasionally”, and “weekly”. The “never” category was defined as abstaining from the habit for six months or more. The responses “Occasionally”, “Only during specific times”, and “1–3 times a month” were grouped into the “occasionally” category, defined as a frequency greater than once every six months but less than three times per month. The “weekly” category was defined as engaging in the habit at least once per week.
The severity and duration of symptoms were systematically documented. A prior study comprehensively categorized long-term COVID-19 symptoms into nine non-neurological organ systems and nine nervous system-related manifestations [10]. Building upon this framework, the symptoms in our study were classified into five categories: respiratory, gastrointestinal, constitutional, neurological/sensory, and musculoskeletal symptoms. Symptom severity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) standards (Appendix A Table A1), with moderate-to-severe symptoms defined as grade 2 or higher. For symptom persistence, we adopted the definition from a primary care study on long-term COVID-19 sequelae [2], where post-acute COVID-19 was characterized by symptom persistence exceeding 21 days following disease onset. Accordingly, persistent symptoms in this study were defined as those lasting ≥ 21 days.
2.4. Statistical Analyses
To enhance data quality, we excluded samples with missing key variables (including sex, age, height, weight, COVID-19 infection status, post-infection symptoms, lifestyle habits, and self-reported illness status) and identified duplicate entries.
For descriptive statistics of baseline features, continuous variables, such as age and BMI, were summarized as the mean (standard deviation) or the median (interquartile range, IQR), and categorical variables were summarized as frequency (percentage). Independent-samples t-tests, chi-square tests, or Fisher’s exact tests were used to assess differences between groups.
Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated using multivariate logistic regression analyses to identify factors associated with COVID-19 incidence. Subsequently, multivariate logistic regression models were employed to examine the relationships between COVID-19 outcomes (including moderate-to-severe symptoms and persistent symptoms) and various factors, such as demographic characteristics, lifestyle factors, and comorbidities. Statistical significance was determined at p < 0.05, with p-values adjusted using the False Discovery Rate (FDR) method. The analysis included patients with varying clinical presentations of COVID-19, and ORs with CIs were used to quantify the strength of associations between predictors and COVID-19-related outcomes.
A Venn diagram was used to illustrate the overlap among the five symptom categories in relation to moderate-to-severe and persistent symptoms. Two-sided p-values were reported for all statistical tests, and a p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.4.0).
3. Results
3.1. Description of Study Population
We included a total of 10,462 patients infected with COVID-19 and 10,462 uninfected controls. As shown in the baseline characteristics (Table 1), the two groups were balanced in terms of sex and age. The mean BMI was 23.88 ± 3.39 in the case group and 23.72 ± 3.52 in the control group. Among the participants, 3278 (31.44%) in the case group and 4002 (38.38%) in the control group were smokers. Regarding alcohol consumption, 3740 participants (35.75%) in the case group and 3904 (37.32%) in the control group reported alcohol use. In terms of tea consumption, 4538 participants (43.38%) in the case group and 4099 (39.18%) in the control group reported habitual tea drinking. Additionally, 700 (6.69%) in the case group and 552 (5.28%) in the control group reported habitual coffee consumption. Regarding comorbidities, 3281 participants (31.47%) in the case group and 2613 (25.06%) in the control group had at least one comorbidity. In the case group, 2333 (22.38%) had a single comorbidity, 697 (6.69%) had two, and 251 (2.41%) had three or more. In the control group, 1985 (19.04%) had a single comorbidity, 484 (4.64%) had two, and 144 (1.38%) had three or more. The five most common comorbidities across both groups were hypertension (2807 [13.46%]), stroke (498 [2.39%]), hyperlipidemia (173 [0.83%]), myocardial infarction (131 [0.63%]), and diabetes (125 [0.60%]).

Table 1.
Baseline characteristics of the participants.
3.2. COVID-19 Symptoms
According to the surveillance data from the Chinese Center for Disease Control and Prevention, BA.5.2 and BF.7 were the predominant variants circulating in China at the end of 2022 [11]. In this study, we investigated 19 symptoms associated with COVID-19 during this period (Table 2), including constitutional symptoms (9284 [88.74%]), respiratory symptoms (7470 [71.40%]), musculoskeletal symptoms (4674 [44.68%]), neurological and sensory symptoms (4218 [40.32%]), and gastrointestinal symptoms (2148 [20.53%]). Among individual symptoms, the highest correlation was found between hypogeusia and hyposmia (Pearson correlation = 0.57, p < 0.0001). After clustering, the Pearson correlation between symptoms was less than 0.24 (Appendix C Figure A1). There were 963 (9.21%) participants who experienced persistent symptoms. The most common persistent symptom categories were respiratory symptoms, affecting 600 individuals; followed by constitutional symptoms, affecting 282 individuals; neurological or sensory symptoms, affecting 176 individuals; musculoskeletal symptoms, affecting 82 individuals; and gastrointestinal symptoms, affecting 64 individuals. There were 773 (7.39%) individuals with moderate-to-severe symptoms. The most common symptoms were respiratory symptoms in 416 individuals, musculoskeletal symptoms in 218 participants, followed by constitutional symptoms in 198 participants, neurological or sensory symptoms in 155 participants, and gastrointestinal symptoms in 52 participants (Figure 2 and Table 2).

Table 2.
COVID-19 symptoms.
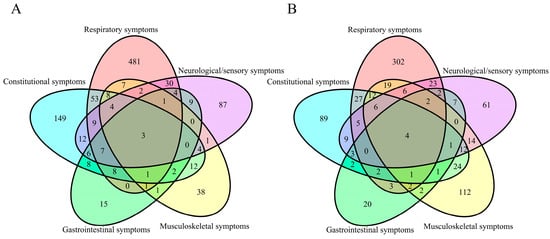
Figure 2.
Coexistence of symptom categories: (A) symptoms persisting for ≥21 days; (B) symptoms with severity ≥ grade 2.
3.3. Factors Associated with the Incidence of COVID-19
In the multivariable regression analysis of factors associated with COVID-19 incidence (Table 3), smoking (OR: 0.69, FDR < 0.001) and weekly alcohol consumption (OR: 0.90, FDR = 0.030) were associated with a lower risk compared to never smoking or alcohol consumption. Conversely, factors associated with an increased COVID-19 incidence included a higher BMI (OR: 1.08, FDR = 0.010), occasional alcohol consumption (OR: 1.15, FDR < 0.001), occasional coffee consumption (OR: 1.19, FDR = 0.010), weekly coffee consumption (OR: 1.41, FDR = 0.040), occasional tea consumption (OR: 1.34, FDR < 0.001), weekly tea consumption (OR: 1.29, FDR < 0.001), and history of allergies (OR: 1.37, FDR < 0.001) compared to their respective reference groups. Additionally, individuals with chronic conditions, such as hypertension only (OR: 1.27, FDR < 0.001), hyperlipemia only (OR: 1.70, FDR = 0.009), having one chronic disease (OR: 1.29, FDR < 0.001), having two chronic diseases (OR: 1.57, FDR < 0.001), or having three or more chronic diseases (OR: 1.85, FDR < 0.001), had a higher likelihood of infection compared to those without chronic diseases.

Table 3.
Multivariate analysis of the incidence of COVID-19.
3.4. Factors Associated with Symptom Categories
Multivariate analysis was conducted to examine factors influencing five categories of symptoms, focusing on persistent symptoms (lasting ≥ 21 days) or moderate-to-severe symptoms (severity of grade 2 or higher) (Figure 3).
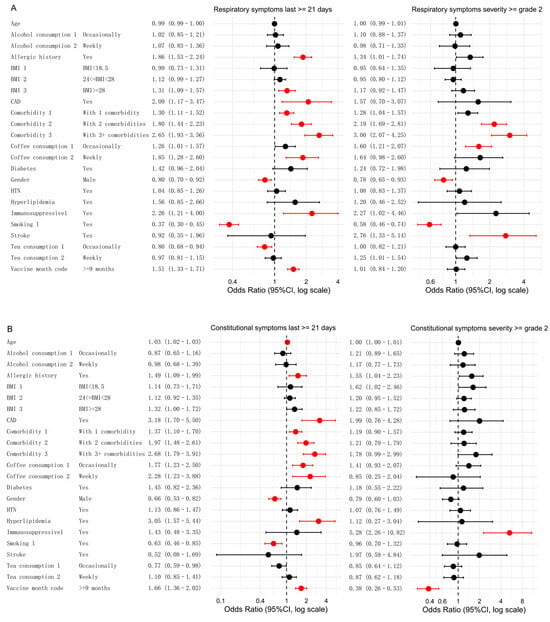
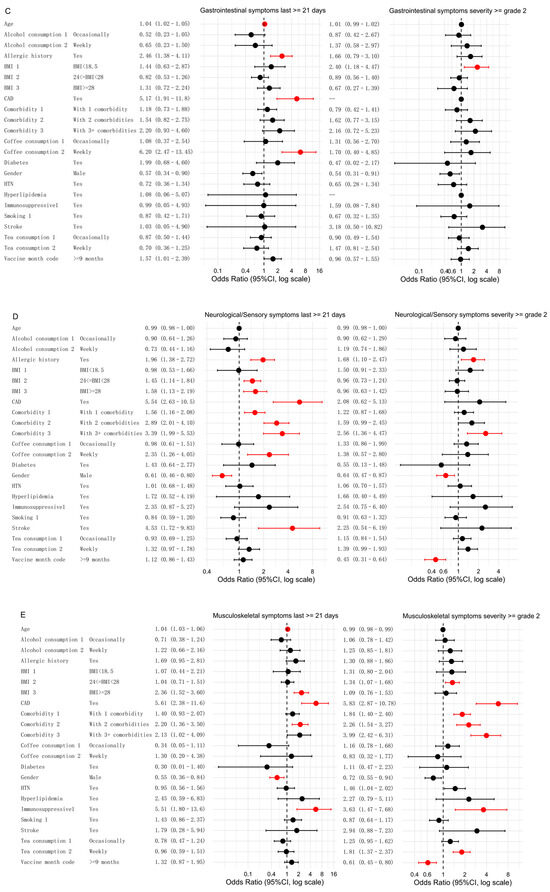
Figure 3.
Associations between different influencing factors and both persistent and moderate-to-severe symptoms. Multivariate analyses were conducted to assess the relationships for five symptom categories: respiratory symptoms (A), constitutional symptoms (B), gastrointestinal symptoms (C), neurological or sensory symptoms (D), and musculoskeletal symptoms (E). Significant factors (FDR < 0.05) are highlighted in red, while non-significant factors are shown in black. Note: For gastrointestinal symptoms of grade ≥2, the number of participants with coronary artery disease (CAD) and hyperlipidemia was 142 and 143, respectively. Because of insufficient sample size, the statistical results (p = 0.98 for both) were not meaningful and would compromise the clarity of the forest plot; therefore, these variables are indicated as “—”.
In the analysis of respiratory symptoms, smoking and male sex were significantly associated with a lower likelihood of persistent symptoms. Smokers had a lower risk compared to non-smokers (OR: 0.37; FDR < 0.001), and males had a lower risk than females (OR: 0.80, FDR = 0.009). A similar trend emerged for more severe respiratory symptoms (grade 2 or higher). Smokers had a lower risk (OR: 0.58, FDR < 0.001), and males had a lower risk than females (OR: 0.78, FDR = 0.045). Conversely, two or more comorbidities were significantly associated with an increased risk of both symptom categories compared to those without these factors. Additional factors specifically associated with respiratory persistent symptoms included BMI ≥ 28 (OR: 1.31, FDR = 0.009), weekly coffee consumption (OR: 1.85, FDR = 0.002), coronary artery disease (CAD) (OR: 2.09, FDR = 0.020), and the presence of any comorbidity (OR: 1.30, FDR = 0.003). A history of allergies (OR: 1.86, FDR < 0.001) and immunotherapy (OR: 2.26, FDR = 0.020) were also associated with an increased risk. Occasional tea consumption was negatively associated with persistent symptoms (OR: 0.80, FDR = 0.020). The significant risk factors for moderate-to-severe respiratory symptoms included occasional coffee consumption (OR: 1.60, FDR = 0.004) and a history of stroke (OR: 2.76, FDR = 0.020) (Figure 3A).
For constitutional symptoms (Figure 3B), age was identified as a risk factor, with older individuals having a higher likelihood of experiencing persistent symptoms (OR: 1.03, FDR < 0.001). A history of allergies was associated with an increased risk of persistent symptoms (OR: 1.49, FDR = 0.020) compared to those without a history of allergies. In contrast, male sex (OR: 0.66, FDR < 0.001) and smoking (OR: 0.63, FDR = 0.009) were negatively associated with persistent symptoms. The risk factors for persistent symptoms included multiple chronic diseases, CAD (OR: 3.18, FDR < 0.001), hyperlipidemia (OR: 3.05, FDR = 0.002), coffee consumption (occasional vs. never: OR: 1.77, FDR = 0.005; weekly vs. never: OR: 2.28, FDR = 0.010). For moderate-to-severe symptoms, the risk factors included immunosuppressive therapy (OR: 5.28, FDR < 0.001).
For gastrointestinal symptoms, the risk factors for symptoms lasting ≥21 days included older age (OR: 1.04, FDR < 0.001), a history of allergies (OR: 2.46, FDR = 0.004), CAD (OR: 5.17, FDR = 0.001), and weekly coffee consumption (OR: 6.20, FDR < 0.001). The only significant risk factor for moderate-to-severe gastrointestinal symptoms was underweight (BMI < 18.5) (OR: 2.40, FDR = 0.047) (Figure 3C).
In the multivariate analysis of neurological and sensory symptoms, male sex was found to be a protective factor. Compared to females, males had a lower likelihood of experiencing persistent symptoms (OR: 0.61, FDR = 0.002) and a lower likelihood of more severe symptoms (grade 2 or higher) (OR: 0.64, FDR = 0.030). A history of allergies was associated with an increased risk of both persistent symptoms (OR: 1.96, FDR < 0.001) and moderate-to-severe symptoms (OR: 1.68, FDR = 0.049) compared to individuals without a history of allergies. The risk factors for persistent symptoms included a history of allergies, overweight/obesity (BMI ≥ 24), CAD, stroke, weekly coffee consumption, and having one or more comorbidities. Additionally, having three or more comorbidities was associated with an increased risk of moderate-to-severe symptoms (Figure 3D).
For musculoskeletal symptoms, male sex was again a protective factor. Compared to females, males had a lower likelihood of experiencing persistent symptoms (OR: 0.55, FDR = 0.020). The risk factors for both symptom categories included CAD, immunosuppressive therapy, and chronic disease with two comorbidities compared to those without these factors. Chronic disease with one or more comorbidities was a significant risk factor, specifically for moderate-to-severe musculoskeletal symptoms. Obesity (BMI ≥ 28) was positively correlated with persistent symptoms, while overweight status (24 ≤ BMI < 28) was associated with an increased risk of moderate-to-severe symptoms. Weekly tea consumption was also associated with an increased risk of moderate-to-severe musculoskeletal symptoms.
4. Discussion
In this study, we conducted a multi-regional, large-scale retrospective case–control analysis to explore the factors associated with COVID-19 infection and symptoms in China. Our findings demonstrated that during the predominance of the BA.5.2 and BF.7 variants at the end of 2022, 9% of the participants with infection experienced persistent symptoms, while 7% experienced moderate-to-severe symptoms. Among the persistent symptoms, the most frequently reported were cough, fatigue, hypogeusia, myalgia, and anorexia. Similarly, the most common moderate-to-severe symptoms included cough, fatigue, myalgia, pharyngalgia, and headache. These findings are consistent with observations reported in previous studies [10,12,13].
In our analysis of factors influencing susceptibility to COVID-19, both smoking and weekly alcohol consumption emerged as protective factors. However, these findings do not imply that smoking or excessive alcohol consumption should be encouraged during the pandemic, as such behaviors are associated with numerous chronic diseases. Further mechanistic studies are required to validate these observations. Conversely, pre-existing chronic conditions, including hypertension and hyperlipidemia, were associated with an elevated risk of COVID-19 infection. This aligns with prior research indicating that comorbidities increase the likelihood of developing long COVID [14,15]. Notably, a dose–response relationship was observed, with higher numbers of comorbidities corresponding to greater infection risk. Additionally, individuals with a history of allergies demonstrated increased susceptibility to COVID-19, potentially attributable to underlying immune dysregulation [16,17]. Tea consumption was associated with an increased risk of breakthrough infection. While some studies suggest green tea may confer protection against the Omicron variant [18], others report no correlation [19]. The observed risk in our study may be partially explained by the social interactions typically accompanying tea consumption in China, which could enhance interpersonal contact and transmission opportunities. Similarly, coffee consumption was identified as a risk factor for SARS-CoV-2 infection, consistent with findings from a Japanese cohort study where higher coffee intake correlated with increased Omicron infection risk among triple-vaccinated healthcare workers [19].
In analyzing symptom-specific associations, most trends mirrored those observed for infection risk. However, certain factors exhibited divergent effects across symptom categories. Male sex consistently served as a protective factor against both persistent and moderate-to-severe symptoms across nearly all categories. This may reflect sex-based differences in immune responses: females exhibit stronger adaptive immunity and heightened proinflammatory cytokine production [20], potentially exacerbating symptom severity and duration during breakthrough infections. Advanced age emerged as a risk factor for persistent constitutional, gastrointestinal, and musculoskeletal symptoms, likely due to age-related immune decline impairing viral clearance and prolonging recovery. Comorbidities were risk factors for most symptom categories except gastrointestinal manifestations, with risk escalating alongside comorbidity burden. These findings are corroborated by a meta-analysis of 41 studies [6]. Contrary to most published findings, smoking was not associated with symptom persistence or severity in our analysis and even appeared protective against respiratory symptoms. While preliminary data suggest nicotine receptor interactions might underlie this observation [21], such hypotheses remain unconfirmed and do not justify smoking initiation or continuation. Coffee consumption, particularly weekly intake, was associated with the most persistent symptoms. Mechanistically, daily consumption exceeding 200 mL correlates with elevated proinflammatory markers (e.g., CRP, IL-6, TNF-α) [22,23], which may impair antiviral immunity. The last inactivated vaccine dose exceeding 9 months was identified as a risk factor for persistent symptoms but served as a protective factor against symptom severity. This is because neutralizing antibody titers decline to pre-vaccination levels 8 months post-booster [24]. Studies on immunity persistence after original COVID-19 vaccination indicate that memory B and T cells decrease six months post-vaccination, particularly against the Omicron variant [25,26]. Consequently, during breakthrough infections, the immune response is weaker, resulting in reduced inflammatory factor secretion and milder symptoms compared to those vaccinated within 9 months, though symptoms may persist longer. Although BMI was not consistently associated with infection or general symptom risk, both low (<18.5) and high BMI (≥28) were linked to higher risks of persistent and severe symptoms in breakthrough cases, relative to individuals with BMI values of 18.5–28.
Existing research indicates that certain risk factors demonstrate consistent effects across multiple COVID-19 variants. Studies tracking the viral evolution from Alpha to Beta to Gamma variants in 2020 have identified several persistent risk factors for long COVID, including advanced age [27,28], female sex [29,30], and pre-existing comorbidities [31,32,33], such as hypertension, hyperlipidemia, and diabetes. These findings suggest that these demographic and clinical characteristics maintain their predictive value regardless of the specific viral variant, highlighting consistent patterns in COVID-19 susceptibility throughout the pandemic’s progression.
Our study has several limitations. First, the retrospective design may introduce recall bias, as participants might inaccurately recall details regarding their lifestyle, medical history, and symptoms. Second, symptom data were self-reported, which could lead to variability in reporting accuracy and consistency across individuals. Third, because of the limited availability of rapid antigen tests or nucleic acid tests during outbreaks, only a small percentage of reported COVID-19 cases were confirmed by etiology. Clinical cases of COVID-19 confirmed by symptoms and exposure history may be misdiagnosed, and our study may have missed some asymptomatic cases. Fourth, some influencing factors were not included in the questionnaire, such as dietary pattern, occupation, ethnicity, and education level. Despite these limitations, our study provides valuable insights into the common factors influencing COVID-19 infection and its associated symptoms among Chinese adults.
In conclusion, our findings indicate that coffee consumption is associated with an elevated risk of infection and more persistent symptoms. Additionally, comorbidities and female sex were identified as significant risk factors. While abnormal BMI was not consistently associated with COVID-19 infection or symptoms, it was not found to be a protective factor. Although the COVID-19 epidemic has subsided, SARS-CoV-2 continues to evolve and mutate. To better prepare for future health emergencies, further research is needed to investigate the factors influencing SARS-CoV-2 outcomes in individuals with diverse immune statuses.
Author Contributions
J.L. and W.W. designed the study protocol. W.W. and R.Q. drafted this manuscript. J.L., W.W. and R.Q. contributed to the critical review and revision of this manuscript. S.J. contributed to the data interpretation and revision of this manuscript. W.W., R.Q. and S.L. were responsible for statistical analysis. H.P., M.X., Y.L., X.L., Q.W., L.Z., J.T., H.Y. and P.J. participated in the site work. W.W. and R.Q. contributed to the literature search. Z.P. contributed to linking the databases of the trials to the Vaccination Integrated Service Management Information System. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grant numbers 82341031, 82173584, and 82222062) and the Science Fund for Distinguished Young Scholars of Jiangsu Province (grant number BK20220064).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Chinese Center for Disease Control and Prevention (protocol code 202303 and date of approval March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
According to the national policies of the People’s Republic of China, the data of this research will not be made public.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Criteria for grading symptoms.
Table A1.
Criteria for grading symptoms.
| Grade Classification |
|---|
| COVID-19 symptoms |
| Cough |
| Grade 1: Mild symptoms; require over-the-counter medication |
| Grade 2: Require prescription medication; affect instrumental activities of daily living * |
| Grade 3: Severe symptoms; limited self-care ability |
| Fatigue |
| Grade 1: Fatigue, relieved after resting |
| Grade 2: Fatigue, not relieved after resting |
| Grade 3: Fatigue, not relieved after resting; affects personal daily activities |
| Headache |
| Grade 1: Mild headache |
| Grade 2: Moderate headache; affects instrumental activities of daily living * |
| Grade 3: Severe headache; limited self-care ability |
| Myalgia |
| Grade 1: Mild pain |
| Grade 2: Moderate pain; affects instrumental activities of daily living * |
| Grade 3: Severe pain; affects personal daily activities |
| Pharyngalgia |
| Grade 1: Mild throat pain; rough sound |
| Grade 2: Moderate throat pain; requires analgesics |
| Grade 3: Moderate throat pain; requires analgesics |
| Rhinitis (nasal obstruction/itching/runny nose/sneezing) |
| Grade 1: Mild symptoms; no intervention needed |
| Grade 2: Moderate symptoms; medical intervention required |
| Grade 3: With bloody nasal secretions or nosebleeds |
| Dyspnea |
| Grade 1: Shortness of breath with moderate activities |
| Grade 2: Shortness of breath with minimal activity |
| Grade 3: Shortness of breath at rest; limited self-care ability |
| Grade 4: Life-threatening; requires urgent intervention |
| Grade 5: Death |
| Nausea |
| Grade 1: Decreased appetite without changes in diet habits |
| Grade 2: Reduced oral intake without significant weight loss, dehydration, or malnutrition |
| Grade 3: Inadequate oral intake of energy and fluids; requires nasogastric, parenteral nutrition, or hospitalization |
| Vomiting |
| Grade 1: 1–2 episodes within 24 h (5 min intervals) |
| Grade 2: 3–5 episodes within 24 h (5 min intervals) |
| Grade 3: ≥6 episodes within 24 h (5 min intervals) |
| Grade 4: Life-threatening; requires urgent treatment |
| Grade 5: Death |
| Diarrhea |
| Grade 1: Less than four bowel movements per day compared to baseline; slight increase in stoma output |
| Grade 2: 4–6 bowel movements per day compared to baseline; intravenous hydration <24 h; moderate increase in stoma output |
| Grade 3: ≥7 bowel movements per day compared to baseline; fecal incontinence; requires inpatient treatment; severe increase in stoma output; affects instrumental activities of daily living |
| Grade 4: Life-threatening; requires urgent treatment |
| Grade 5: Death |
| Anorexia |
| Grade 1: Reduced appetite without changes in diet habits |
| Grade 2: Altered diet but without weight loss or malnutrition; oral nutritional supplementation required |
| Grade 3: Altered diet but without weight loss or malnutrition; oral nutritional supplementation required |
| Grade 4: Life-threatening; requires urgent treatment |
| Grade 5: Death |
| Ophthalmalgia/Redness and Swelling/Conjunctivitis/Eye discomfort |
| Grade 1: Mild pain |
| Grade 2: Moderate pain; affects instrumental activities of daily living * |
| Grade 3: Severe pain; affects personal daily activities |
| Ostalgia |
| Grade 1: Mild pain |
| Grade 2: Moderate pain; affects instrumental activities of daily living * |
| Grade 3: Severe pain; affects personal daily activities |
| Hyposmia |
| Grade 1: Altered sense of smell but does not affect normal diet habits |
| Grade 2: Altered taste affecting normal diet habits (e.g., oral supplements); toxic or uncomfortable taste; loss of taste |
| Chills |
| Grade 1: Mild sensation of cold; shivering; teeth chattering |
| Grade 2: Moderate full-body shivering; requires anesthesia |
| Grade 3: Severe or delayed response or lack of response to anesthesia. |
| Attention disorders |
| Grade 1: Mild inability to concentrate or decreased level of concentration |
| Grade 2: Moderate impairment of concentration; affects instrumental activities of daily living |
| Grade 3: Severe impairment of concentration or severely reduced level of concentration; inability to perform self-care |
| Alopecia |
| Grade 1: Less than 50% hair loss, no difference in appearance from a distance, but noticeable up close. Requires a change in hairstyle to conceal hair loss but does not require a wig or hairpiece |
| Grade 2: Greater than 50% hair loss, significantly noticeable symptoms, requires a wig or hairpiece, psychological impact |
| Others |
| Grade 1: Mild; asymptomatic or mild symptoms; only clinically or diagnostically discovered; no treatment required |
| Grade 2: Moderate; minimal, localized, or non-invasive treatment indications; impact on age-related instrumental activities of daily living |
| Grade 3: Severe or of significant medical importance but not immediately life-threatening; hospitalization or extension of hospitalization indications; disability; impact on self-care activities ** |
Note: * Instrumental activities of daily living refer to tasks such as cooking, grocery or clothing shopping, using the telephone, managing finances, and more; ** Self-care activities of daily living refer to bathing, dressing, and undressing, eating, using the toilet, taking medications, and not being bedridden.
Appendix B. Method 1
SARS-CoV-2 Infection Survey Questionnaire Based on Clinical Trial Cohorts and Community Control Cohort
Opening remarks: Hello, is this XXX? I’m an investigator from the XX center for disease control and prevention. (For clinical cohorts only: You have participated in our previous clinical trial and received the COVID-19 vaccines before.) We are conducting a COVID-19 incidence survey, and we would like to collect information about the COVID-19 infection status, which may take about ten minutes of your time. Are you willing to participate in our survey? (If yes, continue; if not, stop). Are you available for the survey now? (If not, we can reschedule a time for the telephone survey).
- Demographic Characteristics
- Study Number: _____
- Place of Residence (Specify to the Village/Town):
- _______Province_______City_______District/County_______Town/Village/Street
- Height (cm)______
- Weight (kg)______
- Lifestyle
- Have you consumed alcohol in the past year__(1) Never/Rarely (2) Occasionally (3) Only during specific times (e.g., summer) (4) 1–3 times a month (5) ≥1 time a week [if choose 5, proceed to question 1.1; otherwise, skip to question 2]1.1 How many days a week have you consumed alcohol in the past year__(1) 1–2 days a week (2) 3–5 days a week (3) daily or nearly daily
- Have you smoked in the past year__(1) Never/Rarely (2) Occasionally (3) Only during specific times (e.g., holidays) (4) 1–3 times a month (5) ≥1 time a week [if choose 5, proceed to question 2.1; otherwise, skip to question 3]2.1 How many days a week have you smoked in the past year__(1) 1–2 days a week (2) 3–5 days a week (3) daily or nearly daily
- Have you consumed tea regularly in the past year__(1) Never/Rarely (2) Occasionally (3) Only during specific times (e.g., summer) (4) 1–3 times a month (5) ≥1 time a week [if choose 5, proceed to question 3.1; otherwise, skip to question 4]3.1 How many days a week have you consumed tea in the past year__(1) 1–2 days a week (2) 3–5 days a week (3) daily or nearly daily
- Have you consumed coffee regularly in the past year__(1) Never/Rarely (2) Occasionally (3) Only during specific times (e.g., summer) (4) 1–3 times a month (5) ≥1 time a week [if choose 5, proceed to question 4.1; otherwise, skip to question 5]4.1 How many days a week have you consumed coffee in the past year__(1) 1–2 days a week (2) 3–5 days a week (3) daily or nearly daily
- Medical History
- Do you have any of the following chronic diseases? (Select all that apply)(1) hypertension (2) diabetes (3) hyperlipidemia (4) coronary atherosclerotic heart disease (5) stroke (including ischemic and hemorrhagic) (6) tumors (7) chronic bronchitis (8) chronic kidney disease (9) HIV/AIDS (10) others__ (11) none
- Do you have a history of allergies or allergic reactions in the past__(1) Yes (2) No
- Have you received immunosuppressive treatment in the past six months (including allergy treatment, cytotoxic therapy, or the use of corticosteroid medications) __(1) Yes, the name of the medication is__ (2) No
- COVID-19 infection information
- Did you have a COVID-19 infection before 1 November 2022?(1) Yes (2) No
- Have you had a positive nucleic acid test result since 1 November 2022?(1) Yes, the first positive sample date was__ (2) No (3) Not done
- Have you had a positive antigen rapid test result since 1 November 2022?(1) Yes, the first positive sample date was __ (2) No (3) Not done
- Do you have any typical symptoms of COVID-19 infection? [If choose ‘Do not have typical symptoms,’ end the questionnaire.](1) Have typical symptoms (2) Do not have typical symptoms
- Since November 2022, the date of COVID-19 onset (first appearance of typical symptoms of COVID-19, such as fever, cough, fatigue, headache, muscle pain, sore throat, rhinitis, dyspnea, nausea/diarrhea/anorexia) was __
- What symptoms of infection have you experienced? (Multiple choices, if any, please fill in the highest grade)
- (1)
- Fever, or the highest body temperature is __ (degrees) (please fill in a value between 37.3–45.0 or “unknown”), duration (days) __
- (2)
- Cough, highest grade__, duration (days) __Grade classification: Grade 1: Mild symptoms; require over-the-counter medication; Grade 2: Require prescription medication; affect instrumental activities of daily living *;Grade 3: Severe symptoms; limited self-care ability.
- (3)
- Fatigue, highest grade__, duration (days) __Grade classification: Grade 1: Fatigue, relieved after resting; Grade 2: Fatigue, not relieved after resting; Grade 3: Fatigue, not relieved after resting; affects personal daily activities.
- (4)
- Headache, highest grade__, duration (days)__Grade classification: Grade 1: Mild headache; Grade 2: Moderate headache; affects instrumental activities of daily living*; Grade 3: Severe headache; limited self-care ability.
- (5)
- Muscle pain, highest grade__, duration (days)__Grade classification: Grade 1: Mild pain; Grade 2: Moderate pain; affects instrumental activities of daily living *; Grade 3: Severe pain; affects personal daily activities.
- (6)
- Sore throat, highest grade__, duration (days)__Grade classification: Grade 1: Mild throat pain; hoarseness; Grade 2: Moderate throat pain; requires analgesics; Grade 3: Severe throat pain; requires endoscopy.
- (7)
- Rhinitis (nasal obstruction/itching/runny nose/sneezing), highest grade__, duration (days)__Grade classification: Grade 1: Mild symptoms; no intervention needed; Grade 2: Moderate symptoms; medical intervention required; Grade 3: With bloody nasal secretions or nosebleeds.
- (8)
- Dyspnea, highest grade__, duration (days)__Grade classification: Grade 1: Shortness of breath with moderate activities; Grade 2: Shortness of breath with minimal activity; affects instrumental activities of daily living *; Grade 3: Shortness of breath at rest; limited self-care ability; Grade 4: Life-threatening; requires urgent intervention; Grade 5: Death.
- (9)
- Nausea, highest grade__, duration (days)__Grade classification: Grade 1: Decreased appetite without changes in diet habits; Grade 2: Reduced oral intake without significant weight loss, dehydration, or malnutrition; Grade 3: Inadequate oral intake of energy and fluids; requires nasogastric, parenteral nutrition, or hospitalization.
- (10)
- Vomiting, highest grade__, duration (days)__Grade classification: Grade 1: 1–2 episodes within 24 h (5-min intervals); Grade 2: 3–5 episodes within 24 h (5-min intervals); Grade 3: ≥6 episodes within 24 h (5-min intervals); Grade 4: Life-threatening; requires urgent treatment; Grade 5: Death.
- (11)
- Diarrhea, highest grade__, duration (days)__Grade classification: Grade 1: Less than 4 bowel movements per day compared to baseline; slight increase in stoma output; Grade 2: 4–6 bowel movements per day compared to baseline; intravenous hydration <24 h; moderate increase in stoma output; Grade 3: ≥7 bowel movements per day compared to baseline; fecal incontinence; requires inpatient treatment; severe increase in stoma output; affects instrumental activities of daily living; Grade 4: Life-threatening; requires urgent treatment; Grade 5: Death.
- (12)
- Anorexia, highest grade__, duration (days)__Grade classification: Grade 1: Reduced appetite without changes in diet habits; Grade 2: Altered diet but without weight loss or malnutrition; oral nutritional supplementation required; Grade 3: Marked weight loss or malnutrition symptoms (e.g., insufficient oral calorie intake); requires nasogastric or parenteral nutrition; Grade 4: Life-threatening; requires urgent treatment; Grade 5: Death.
- (13)
- Ophthalmalgia/Redness and Swelling/Conjunctivitis/Eye discomfort, highest grade__, duration (days)__Grade classification: Grade 1: Mild pain; Grade 2: Moderate pain; affects instrumental activities of daily living *; Grade 3: Severe pain; affects personal daily activities.
- (14)
- Bone pain, highest grade__, duration (days)__Grade classification: Grade 1: Mild pain; Grade 2: Moderate pain; affects instrumental activities of daily living *; Grade 3: Severe pain; affects personal daily activities.
- (15)
- Hyposmia, highest grade__, duration (days)__Grade classification: Grade 1: Altered sense of smell but does not affect normal diet habits; Grade 2: Altered sense of smell affecting normal diet habits (e.g., oral supplements); toxic or uncomfortable odor; loss of smell.
- (16)
- Hypogeusia, highest grade__, duration (days)__Grade classification: Grade 1: Altered taste but does not affect normal diet habits; Grade 2: Altered taste affecting normal diet habits (e.g., oral supplements); toxic or uncomfortable taste; loss of taste.
- (17)
- Chills, highest grade__, duration (days)__Grade classification: Grade 1: Mild sensation of cold; shivering; teeth chattering; Grade 2: Moderate full-body shivering; requires anesthesia; Grade 3: Severe or delayed response or lack of response to anesthesia.
- (18)
- Concentration disorders, highest grade__, duration (days)__Grade classification: Grade 1: Mild inability to concentrate or decreased level of concentration; Grade 2: Moderate impairment of concentration; affects instrumental activities of daily living; Grade 3: Severe impairment of concentration or severely reduced level of concentration; inability to perform self-care.
- (19)
- Hair loss, highest grade__, duration (days)__Grade classification: Grade 1: Less than 50% hair loss, no difference in appearance from a distance, but noticeable up close. Requires a change in hairstyle to conceal hair loss but does not require a wig or hairpiece; Grade 2: Greater than 50% hair loss, significantly noticeable symptoms, requires a wig or hairpiece, psychological impact.
- (20)
- Others__, highest grade__, duration (days)__Grade classification: Grade 1: Mild; asymptomatic or mild symptoms; only clinically or diagnostically discovered; no treatment required; Grade 2: Moderate; minimal, localized, or non-invasive treatment indications; impact on age-related instrumental activities of daily living; Grade 3: Severe or of significant medical importance but not immediately life-threatening; hospitalization or extension of hospitalization indications; disability; impact on self-care activities; Grade 4: Life-threatening, requiring urgent treatment; Grade 5: Death.
(Note: In all the Grade classifications mentioned above, *Instrumental Activities of Daily Living refer to tasks such as cooking, grocery or clothing shopping, using the telephone, managing finances, and more; **Activities of Daily Living (ADL) refer to bathing, dressing, and undressing, eating, using the toilet, taking medications, and not being bedridden. If the symptoms have not concluded at the time of filling out the questionnaire, please use “+” to indicate, for example, 10+.)
- 7.
- Has the symptoms of COVID-19 completely disappeared? [if no, proceed to question 9](1) Yes (2) No
- 8.
- Date when COVID-19 symptoms completely disappeared or tested negative: ____
- 9.
- Did you have any possible exposure to a confirmed or suspected COVID-19 case or a history of being in a location with potential exposure before the onset of typical symptoms (e.g., staying in a hospital or visiting crowded places)?(1) Yes (2) No
- 10.
- Have you sought medical care at an outpatient clinic due to a COVID-19 infection?(1) Yes (2) No
- 11.
- Have you been hospitalized due to a COVID-19 infection? [if no, proceed to question 13)(1) Yes (2) No
- 12.
- Did you receive any of the following treatments during the hospitalization?(1) Hospital treatment without oxygen supplementation(2) Hospital treatment with oxygen supplementation(3) Hospital treatment with high-flow oxygen therapy(4) Hospital treatment involving intubation and mechanical ventilation(5) Hospital treatment involving mechanical ventilation and external organ support, such as ECMO therapy
- 13.
- Did you take medication for the treatment of COVID-19__ [if no, proceed to question 15](1) Yes (2) No
- 14.
- Do you know the name of the medications you took?(1) Name of medications taken:Medication for COVID-19—Name 1: _____Duration of medication (days): _____Medication for COVID-19—Name 2: _____Duration of medication (days): _____Medication for COVID-19—Name 3: _____Duration of medication (days): _____Medication for COVID-19—Name 4: _____Duration of medication (days): _____Medication for COVID-19—Name 5: _____Duration of medication (days): _____Medication for COVID-19—Name 6: _____Duration of medication (days): _____Medication for COVID-19—Name 7: _____Duration of medication (days): _____Medication for COVID-19—Name 8: _____Duration of medication (days): _____Medication for COVID-19—Name 9: _____Duration of medication (days): _____Medication for COVID-19—Name 10: _____Duration of medication (days): _____
- 15.
- Severity of COVID-19 infection: __(1) Mild: Symptomatic patients meeting the case definition for COVID-19 without evidence of viral pneumonia or hypoxia.(2) Moderate: Adolescent or adult with clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) but no signs of severe pneumonia (including SpO2 ≥ 90% on room air).(3) Severe: Adolescent or adult with clinical signs of pneumonia (fever, cough, dyspnoea) plus one of the following: respiratory rate > 30 breaths/min, severe respiratory distress, or SpO2 < 90% on room air.(4) Critical: Acute respiratory distress syndrome (ARDS); or Sepsis; or Septic shock; or Acute thrombosis.
- 16.
- Did you experience a recurrence of COVID-19?(1) Yes, recurrence started on _____ and ended on ______.(2) No
- 17.
- Did you have any housemate (family members or door roommates, etc.) when infected with COVID-19 [if no, proceed to end](1) Yes (2) No
- 18.
- Infections status among housemates (family members or dorm roommates, etc.)
- Housemate 1
- ① Age (in years) ____
- ② Gender____ (1) Male (2) Female
- ③ COVID-19 vaccination status____ (1) Yes (2) No
- ④ Did they infect with COVID-19 (with typical symptoms, or positive nucleic acid test, or positive antigen rapid test) ____ (1) Yes (2) No
- Housemate 2
- ① Age (in years) ____
- ② Gender____ (1) Male (2) Female
- ③ COVID-19 vaccination status____ (1) Yes (2) No
- ④ Did they infect with COVID-19 (with typical symptoms, or positive nucleic acid test, or positive antigen rapid test) ____ (1) Yes (2) No
- Housemate 3
- ① Age (in years) ____
- ② Gender____ (1) Male (2) Female
- ③ COVID-19 vaccination status____ (1) Yes (2) No
- ④ Did they infect with COVID-19 (with typical symptoms, or positive nucleic acid test, or positive antigen rapid test) ____ (1) Yes (2) No
- Housemate 4
- ① Age (in years) ____
- ② Gender____ (1) Male (2) Female
- ③ COVID-19 vaccination status____ (1) Yes (2) No
- ④ Did they infect with COVID-19 (with typical symptoms, or positive nucleic acid test, or positive antigen rapid test) ____ (1) Yes (2) No
- Housemate 5
- ① Age (in years) ____
- ② Gender____ (1) Male (2) Female
- ③ COVID-19 vaccination status____ (1) Yes (2) No
- ④ Did they infect with COVID-19 (with typical symptoms, or positive nucleic acid test, or positive antigen rapid test) ____ (1) Yes (2) No
Closing remarks: You have answered all the questions. You can contact me through this phone number if you have any additional information to provide or any related questions to ask. Thank you for your contribution, goodbye!
Investigator: Investigation Organization: Investigation Date:
Appendix C
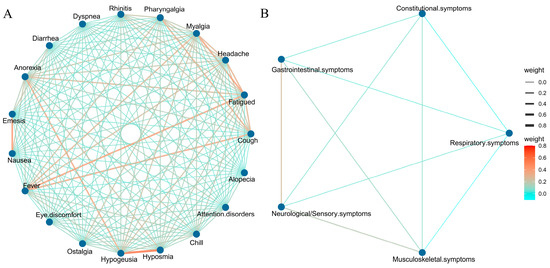
Figure A1.
Pearson correlation analysis between symptoms. (A) Correlations between each individual symptom. (B) Associations between symptoms after classification.
References
- Schumacher, A.E.; Kyu, H.H.; Aali, A.; Abbafati, C.; Abbas, J.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; ElHafeez, S.A.; Abdelmasseh, M.; et al. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 1989–2056. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’cOurt, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.R.; Kobayashi, T.; Callado, G.Y.; Pardo, I.; Gutfreund, M.C.; Hsieh, M.K.; Lin, V.; Alsuhaibani, M.; Hasegawa, S.; Tholany, J.; et al. The effectiveness of COVID-19 vaccine in the prevention of post-COVID conditions: A systematic literature review and meta-analysis of the latest research. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e168. [Google Scholar] [CrossRef] [PubMed]
- de Gier, B.; Huiberts, A.J.; Hoeve, C.E.; Hartog, G.D.; van Werkhoven, H.; van Binnendijk, R.; Hahné, S.J.M.; de Melker, H.E.; Hof, S.v.D.; Knol, M.J. Effects of COVID-19 vaccination and previous infection on Omicron SARS-CoV-2 infection and relation with serology. Nat. Commun. 2023, 14, 4793. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Y.; Li, Y.; Huang, L.; Yang, T.; Si, J.; Wang, L.; Zhao, X.; Ma, X.; Gao, G.F. Long COVID facts and findings: A large-scale online survey in 74,075 Chinese participants. Lancet Reg. Health-West. Pac. 2024, 52, 101218. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; et al. Coronavirus (COVID-19) Vaccinations. 2020. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 6 March 2025).
- Jia, S.; Yin, Z.; Pan, H.; Wang, F.; Liu, X.; Wang, Q.; Zhang, L.; Tang, J.; Yang, H.; Du, J.; et al. Relative effectiveness of a heterologous booster dose with adenovirus type 5 vectored COVID-19 vaccine versus three doses of inactivated COVID-19 vaccine in adults during a nationwide outbreak of omicron predominance, in China: A retrospective, individually matched cohort-control study. Emerg. Microbes Infect. 2024, 13, 2332660. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Hartman, T.C.O.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- COVID-19 Clinical and Surveillance Data-9 December 2022 to 23 January 2023, China. Chinese Center for Disease Control and Prevention. Available online: https://en.chinacdc.cn/news/latest/202301/W020230126558725888448.pdf (accessed on 13 March 2025).
- Richard, S.A.; Pollett, S.D.; Fries, A.C.; Berjohn, C.M.; Maves, R.C.; Lalani, T.; Smith, A.G.; Mody, R.M.; Ganesan, A.; Colombo, R.E.; et al. Persistent COVID-19 Symptoms at 6 Months After Onset and the Role of Vaccination Before or After SARS-CoV-2 Infection. JAMA Netw. Open 2023, 6, e2251360. [Google Scholar] [CrossRef]
- Arnold, D.T.; Milne, A.; Samms, E.; Stadon, L.; Maskell, N.A.; Hamilton, F.W. Symptoms After COVID-19 Vaccination in Patients with Persistent Symptoms After Acute Infection: A Case Series. Ann. Intern. Med. 2021, 174, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.D.; Escobar, G.J.; Lu, Y.; Schlessinger, D.; Steinman, J.B.; Steinman, L.; Lee, C.; Liu, V.X. Risk of severe COVID-19 infection among adults with prior exposure to children. Proc. Natl. Acad. Sci. USA 2022, 119, e2204141119. [Google Scholar] [CrossRef]
- Azambuja, P.; Bastos, L.S.; Batista-Da-Silva, A.A.; Ramos, G.V.; Kurtz, P.; Dias, C.M.; da Silva, E.P.; Arouca, L.E.; Soares, J.; Sejvar, J.J.; et al. Prevalence, risk factors, and impact of long COVID in a socially vulnerable community in Brazil: A prospective cohort study. Lancet Reg. Health Am. 2024, 37, 100839. [Google Scholar] [CrossRef]
- Ren, J.; Pang, W.; Luo, Y.; Cheng, D.; Qiu, K.; Rao, Y.; Zheng, Y.; Dong, Y.; Peng, J.; Hu, Y.; et al. Impact of Allergic Rhinitis and Asthma on COVID-19 Infection, Hospitalization, and Mortality. J. Allergy Clin. Immunol. Pract. 2022, 10, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Huang, Z.; Lan, M.; Ye, J.; Chen, J.; Guo, H.; Xiao, J.; Zhuang, S.; Wu, J.; Yang, C.; et al. The duration and breadth of antibody responses to 3-dose of inactivated COVID-19 vaccinations in healthy blood donors: An observational study. Front. Immunol. 2022, 13, 1027924. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, M.; Lee, S.; Lee, J.I.; Park, J. Green Tea Consumption and the COVID-19 Omicron Pandemic Era: Pharmacology and Epidemiology. Life 2023, 13, 852. [Google Scholar] [CrossRef]
- Islam, Z.; Yamamoto, S.; Mizoue, T.; Konishi, M.; Ohmagari, N. Coffee and Green Tea Consumption With the Risk of COVID-19 Among the Vaccine Recipients in Japan: A Prospective Study. J. Epidemiol. 2024, 34, 444–452. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- van Zyl-Smit, R.N.; Richards, G.; Leone, F.T. Tobacco smoking and COVID-19 infection. Lancet Respir. Med. 2020, 8, 664–665. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C. Associations between coffee consumption and inflammatory markers in healthy persons: The ATTICA study. Am. J. Clin. Nutr. 2004, 80, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Sezer, Z.; Pavel, S.T.I.; Inal, A.; Yetiskin, H.; Kaplan, B.; Uygut, M.A.; Aslan, A.F.; Bayram, A.; Mazicioglu, M.; Unuvar, G.K.; et al. Long-Term Immunogenicity and Safety of a Homologous Third Dose Booster Vaccination with TURKOVAC: Phase 2 Clinical Study Findings with 32-Week Post-Booster Follow-Up. Vaccines 2024, 12, 140. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Baraff, A.; Fox, A.; Shahoumian, T.; Hickok, A.; O’hAre, A.M.; Bohnert, A.S.B.; Boyko, E.J.; Maciejewski, M.L.; Bowling, C.B.; et al. Rates and factors associated with documentation of diagnostic codes for long COVID in the National Veterans Affairs Health Care System. JAMA Netw. Open 2022, 5, e2224359. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Smith, L.; Koyanagi, A.; Jacob, L. Prevalence of and factors associated with post-coronavirus disease 2019 (COVID-19) condition in the 12 months after the diagnosis of COVID-19 in adults followed in general practices in Germany. Open Forum Infect. Dis. 2022, 9, ofac333. [Google Scholar] [CrossRef]
- Pazukhina, E.; Andreeva, M.; Spiridonova, E.; Bobkova, P.; Shikhaleva, A.; El-Taravi, Y.; Rumyantsev, M.; Gamirova, A.; Bairashevskaia, A.; Petrova, P.; et al. Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: A prospective, cohort study in Moscow (StopCOVID). BMC Med. 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Baruch, J.; Zahra, C.; Cardona, T.; Melillo, T. National long COVID impact and risk factors. Public Health 2022, 213, 177–180. [Google Scholar] [CrossRef]
- Dias, M.B.; Medeiros, A.P.V.; de Melo, S.S.; Fonseca, C.S.; Jacob-Filho, W.; Avelino-Silva, T.J.; Aliberti, M.J.R.; for the CO-FRAIL Study Group. The long and winding road of COVID-19 in survivors of hospitalisation: Symptoms trajectory and predictors of long COVID. J. Intern. Med. 2023, 293, 264–268. [Google Scholar] [CrossRef]
- Chudzik, M.; Babicki, M.; Kapusta, J.; Kałuzińska-Kołat, Ż.; Kołat, D.; Jankowski, P.; Mastalerz-Migas, A. Long-COVID clinical features and risk factors: A retrospective analysis of Patients from the STOP-COVID registry of the PoLoCOV Study. Viruses 2022, 14, 1755. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Guijarro, C.; Torres-Macho, J.; Velasco-Arribas, M.; Plaza-Canteli, S.; Hernández-Barrera, V.; Arias-Navalón, J.A. Diabetes and the risk of long-term post-COVID symptoms. Diabetes 2021, 70, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).