Syndemic Synergy of HPV, HIV, and HSV-2 for Oncogenic HPV Replication in Female Sex Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Recruitment
2.2. Study Procedure and Sample Collection
2.3. Genital HPV Sampling and Transport
2.4. DNA Extraction, HPV Genotyping, and Quantitation by Multiplex PCR
2.5. HSV-2 DNA and Prostatic-Specific Antigen (PSA) Detection
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Study Population
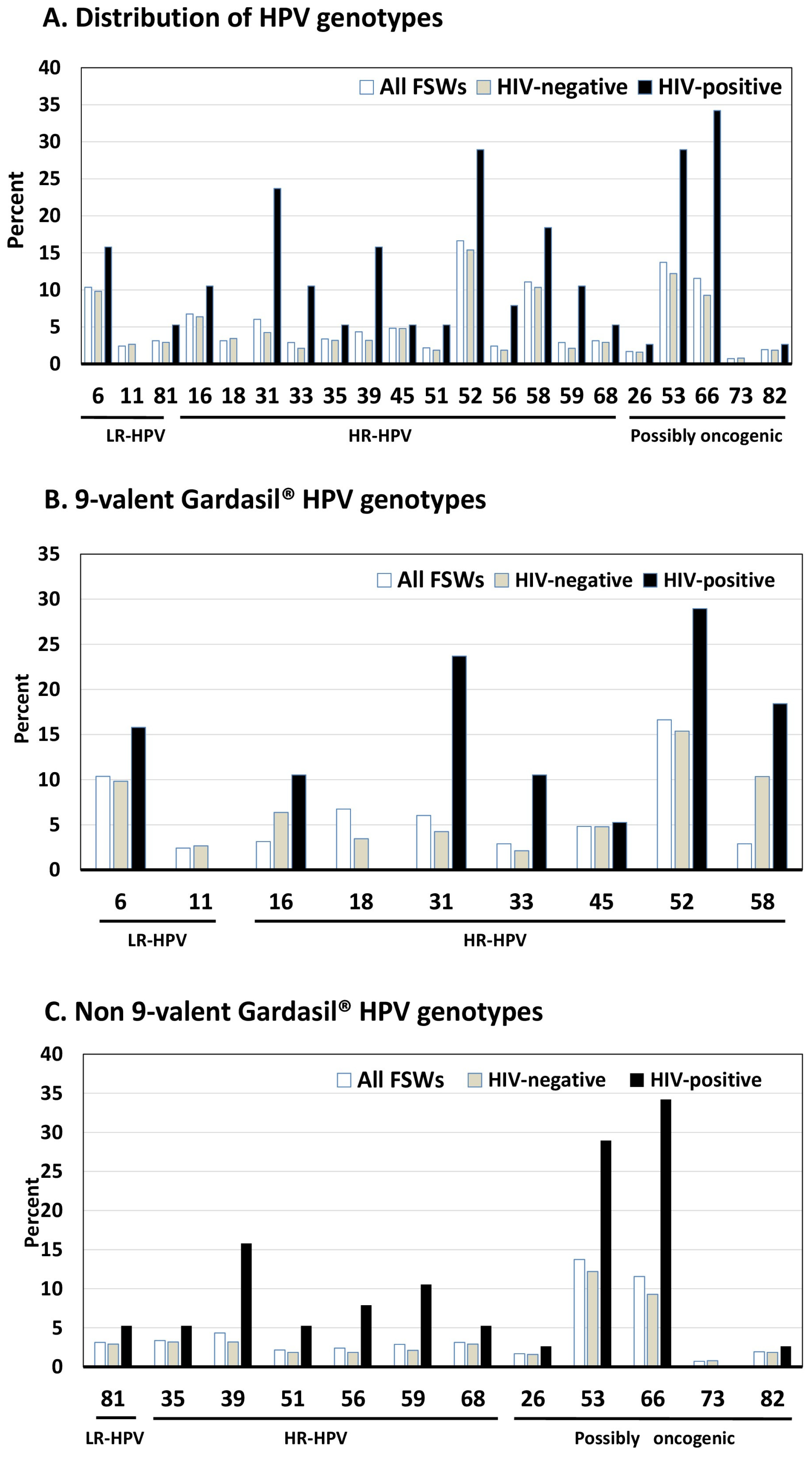
3.2. HPV Prevalence and Genotypes Distribution
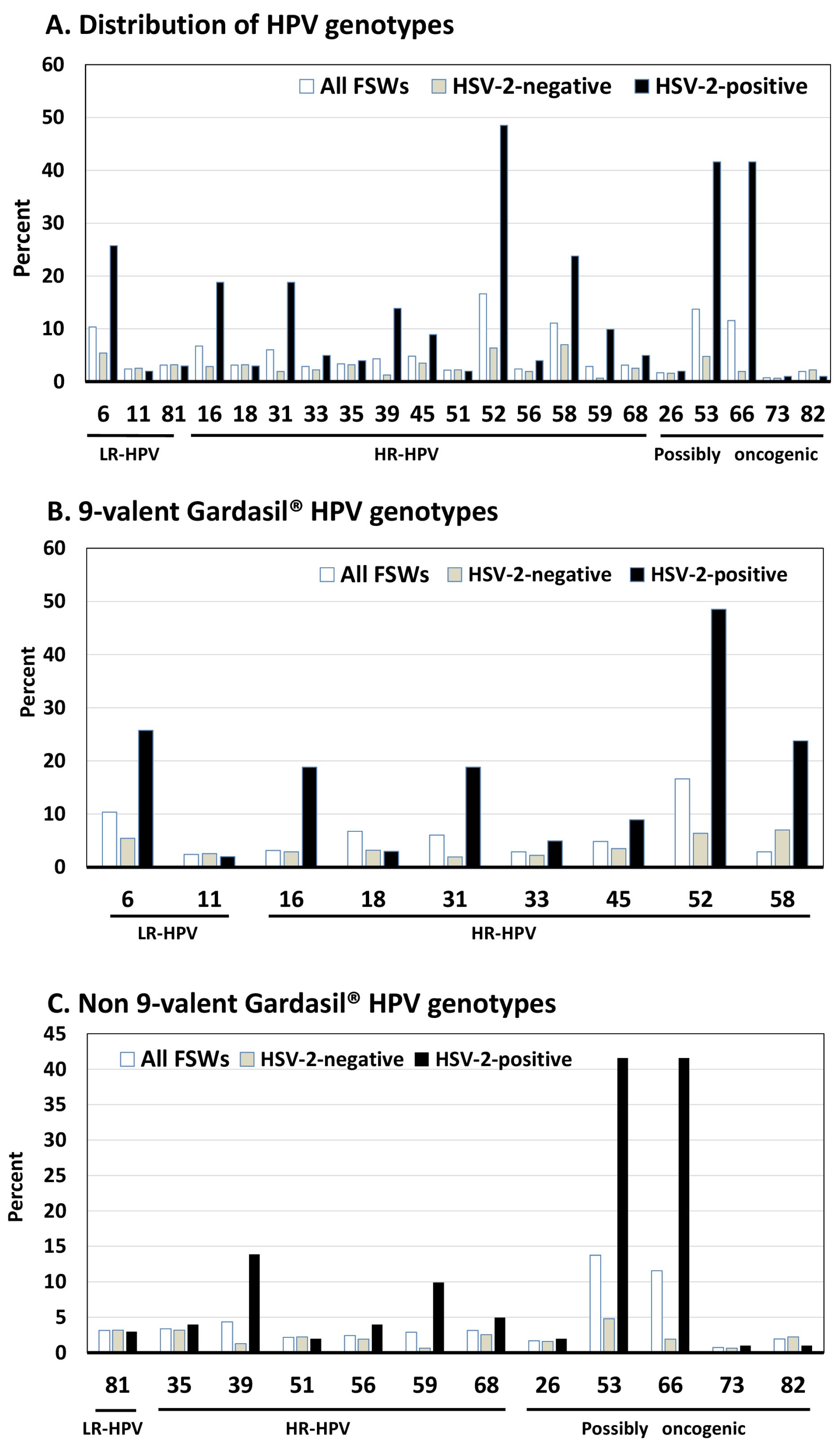
3.3. Quantitative HPV Viral Load by HIV and HSV-2 Status
3.4. Factors of HR-HPV Replication Using Multiple Linear Regression Analysis
3.5. Summary of the Main Results
- ✓
- The seroprevalence of HIV in the study FSWs reached 9.1%, around 8.3-fold higher than the general adult female population in the DRC, reinforcing that FSWs are a key population for HIV in a country of generalized HIV epidemic;
- ✓
- The novel Vaginal Veil Collector V-Veil UP2™ device especially conceived for female genital self-sampling demonstrated high acceptability (~96%) and effectiveness for molecular biology (100.0%);
- ✓
- The distribution of HPV in genital samples appeared atypical and unique, with a mixture of HR-HPVs, PO-HPVs, and LR-HPsV, HPV-52, HPV-58, HPV-16, and HPV-31 and HPV-68 being the predominant HR-HPVs, HPV-53 and HPV-66 the predominant PO-HPVs, and HPV-6 the predominant LR-HPV;
- ✓
- The HR-HPV genotypes in FSWs matched generally with the HPV types targeted by the prophylactic 9-valent Gardasil-9® HPV vaccine, reinforcing the widely use of this prophylactic vaccine for primary prevention of HPV infection and cervical cancer in the FSW key population;
- ✓
- HIV and HSV-2 infections were associated with higher prevalences of genital shedding of any HR-HPV as well as multiple HR-HPVs, and with higher HPV viral load for several genotypes;
- ✓
- By multiple linear regression analysis, the only strong predictor for significantly higher HR-HPV viral load, including Gardasil-9® and non-vaccine HR-HPV, was genital co-infection by HSV-2;
- ✓
- Prophylactic 9-valent Gardasil-9® HPV vaccine in association with diagnosis and treatment of genital HSV-2 co-infection could be useful to prevent HPV-associated cervical lesions.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laga, I.; Niu, X.; Rucinski, K.; Baral, S.; Rao, A.; Chen, D.; Viswasam, N.; Phaswana-Mafuya, N.R.; Diouf, D.; Sabin, K.; et al. Mapping the number of female sex workers in countries across sub-Saharan Africa. Proc. Natl. Acad. Sci. USA 2023, 120, e2200633120. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Beyrer, C.; Muessig, K.; Poteat, T.; Wirtz, A.L.; Decker, M.R.; Sherman, S.G.; Kerrigan, D. Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Papworth, E.; Ceesay, N.; An, L.; Thiam-Niangoin, M.; Ky-Zerbo, O.; Holland, C.; Dramé, F.M.; Grosso, A.; Diouf, D.; Baral, S.D. Epidemiology of HIV among female sex workers, their clients, men who have sex with men and people who inject drugs in West and Central Africa. J. Int. AIDS Soc. 2013, 16 (Suppl. S3), 18751. [Google Scholar] [CrossRef] [PubMed]
- Mbita, G.; Mwanamsangu, A.; Komba, A.N.; Casalini, C.; Luponya, M.; Curran, K.; Christensen, A.; Kim, Y.M.; Reed, J.; Makyao, N.; et al. HIV seroconversion among female sex workers: Retrospective cohort study from a large-scale HIV prevention and sexual and reproductive health program in Tanzania. Front. Reprod. Health 2024, 6, 1332236. [Google Scholar] [CrossRef]
- Jones, H.S.; Anderson, R.L.; Cust, H.; McClelland, R.S.; Richardson, B.A.; Thirumurthy, H.; Malama, K.; Hensen, B.; Platt, L.; Rice, B.; et al. HIV incidence among women engaging in sex work in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health. 2024, 12, e1244–e1260. [Google Scholar] [CrossRef]
- Korenromp, E.L.; Sabin, K.; Stover, J.; Brown, T.; Johnson, L.F.; Martin-Hughes, R.; Ten Brink, D.; Teng, Y.; Stevens, O.; Silhol, R.; et al. New HIV infections among key populations and their partners in 2010 and 2022, by world region: A multisources estimation. J. Acquir. Immune. Defic. Syndr. 2024, 95, e34–e45. [Google Scholar] [CrossRef]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Unaids Data 2024. Geneva: Joint United Nations Programme on HIV/AIDS. 2024. Available online: https://www.unaids.org/en/resources/documents/2024/2024_unaids_data (accessed on 25 January 2025).
- Emmanuel, F.; Ejeckam, C.C.; Green, K.; Adesina, A.A.; Aliyu, G.; Ashefor, G.; Aguolu, R.; Isac, S.; Blanchard, J. HIV epidemic among key populations in Nigeria: Results of the integrated biological and behavioural surveillance survey (IBBSS), 2020–2021. Sex. Transm. Infect. 2025, 101, 10–16. [Google Scholar] [CrossRef]
- Mboumba Bouassa, R.S.; Prazuck, T.; Lethu, T.; Jenabian, M.A.; Meye, J.F.; Bélec, L. Cervical cancer in sub-Saharan Africa: A preventable noncommunicable disease. Expert. Rev. Anti Infect. Ther. 2017, 15, 613–627. [Google Scholar] [CrossRef]
- Lekoane, K.M.B.; Kuupiel, D.; Mashamba-Thompson, T.P.; Ginindza, T.G. Evidence on the prevalence, incidence, mortality and trends of human papilloma virus-associated cancers in sub-Saharan Africa: Systematic scoping review. BMC Cancer 2019, 19, 563. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; Sanjosé, S.; Saraiya, M.; Ferlay, J.; Freddie, B. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- World Health Organization. Global Cancer Observatory. Africa. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/903-africa-fact-sheet.pdf (accessed on 9 February 2025).
- Njagi, S.K.; Mugo, N.R.; Reid, A.J.; Satyanarayana, S.; Tayler-Smith, K.; Kizito, W.; Kwatampora, J.; Waweru, W.; Kimani, J.; Smith, J.S. Prevalence and incidence of cervical intra-epithelial neoplasia among female sex workers in Korogocho, Kenya. Public Health Action 2013, 3, 271–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soohoo, M.; Blas, M.; Byraiah, G.; Carcamo, C.; Brown, B. Cervical HPV infection in female sex workers: A global perspective. Open AIDS J. 2013, 7, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.; Moghoofei, M.; Dorost, A.; Abbasi, S.; Monavari, S.H.; Kiani, S.J.; Tavakoli, A. Prevalence and genotype distribution of genital human papillomavirus infection in female sex workers in the world: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1455. [Google Scholar] [CrossRef]
- Vimpere, L.; Sami, J.; Jeannot, E. Cervical cancer screening programs for female sex workers: A scoping review. Front. Public Health 2023, 11, 1226779. [Google Scholar] [CrossRef]
- Williams-Brennan, L.; Gastaldo, D.; Cole, D.C.; Paszat, L. Social determinants of health associated with cervical cancer screening among women living in developing countries: A scoping review. Arch. Gynecol. Obstet. 2012, 286, 1487–1505. [Google Scholar] [CrossRef] [PubMed]
- Vandepitte, J.M.; Malele, F.; Kivuvu, D.M.; Edidi, S.; Muwonga, J.; Lepira, F.; Abdellati, S.; Kabamba, J.; Van Overloop, C.; Buvé, A. HIV and other sexually transmitted infections among female sex workers in Kinshasa, Democratic Republic of Congo, in 2002. Sex. Transm. Dis. 2007, 34, 203–208. [Google Scholar] [CrossRef]
- Vandenhoudt, H.M.; Langat, L.; Menten, J.; Odongo, F.; Oswago, S.; Luttah, G.; Zeh, C.; Crucitti, T.; Laserson, K.; Vulule, J.; et al. Prevalence of HIV and other sexually transmitted infections among female sex workers in Kisumu, Western Kenya, 1997 and 2008. PLoS ONE 2013, 8, e54953. [Google Scholar] [CrossRef]
- Longo, J.D.; Simaleko, M.M.; Diemer, H.S.; Grésenguet, G.; Brücker, G.; Belec, L. Risk factors for HIV infection among female sex workers in Bangui, Central African Republic. PLoS ONE 2017, 12, e0187654. [Google Scholar] [CrossRef][Green Version]
- Vu, L.; Misra, K. High burden of HIV, syphilis and HSV-2 and factors associated with HIV infection among female sex workers in Tanzania: Implications for early treatment of HIV and pre-exposure prophylaxis (PrEP). AIDS Behav. 2018, 22, 1113–1121. [Google Scholar] [CrossRef]
- Van de Perre, P.; Segondy, M.; Foulongne, V.; Ouedraogo, A.; Konate, I.; Huraux, J.M.; Mayaud, P.; Nagot, N. Herpes simplex virus and HIV-1: Deciphering viral synergy. Lancet Infect. Dis. 2008, 8, 490–497. [Google Scholar] [CrossRef]
- Todd, J.; Riedner, G.; Maboko, L.; Hoelscher, M.; Weiss, H.A.; Lyamuya, E.; Mabey, D.; Rusizoka, M.; Belec, L.; Hayes, R. Effect of genital herpes on cervicovaginal HIV shedding in women co-infected with HIV AND HSV-2 in Tanzania. PLoS ONE 2013, 8, e59037. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.T.; Scott, R.S. The interaction between human papillomavirus and other viruses. Virus Res. 2017, 231, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wen, X. Seropositivity to herpes simplex virus type 2, but not type 1 is associated with cervical cancer: NHANES (1999–2014). BMC Cancer 2017, 17, 726. [Google Scholar] [CrossRef]
- Sausen, D.G.; Shechter, O.; Gallo, E.S.; Dahari, H.; Borenstein, R. Herpes Simplex Virus, Human Papillomavirus, and Cervical Cancer: Overview, Relationship, and Treatment Implications. Cancers 2023, 15, 3692. [Google Scholar] [CrossRef]
- Tsai, A.C.; Mendenhall, E.; Trostle, J.A.; Kawachi, I. Co-occurring epidemics, syndemics, and population health. Lancet 2017, 389, 978–982. [Google Scholar] [CrossRef]
- Mbopi-Kéou, F.X.; Grésenguet, G.; Mayaud, P.; Weiss, H.A.; Gopal, R.; Matta, M.; Paul, J.L.; Brown, D.W.; Hayes, R.J.; Mabey, D.C.; et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: Opportunities for intervention. J. Infect. Dis. 2000, 182, 1090–1096. [Google Scholar] [CrossRef]
- Looker, K.J.; Elmes, J.A.R.; Gottlieb, S.L.; Schiffer, J.T.; Vickerman, P.; Turner, K.M.E.; Boily, M.C. Effect of HSV-2 infection on subsequent HIV acquisition: An updated systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 1303–1316. [Google Scholar] [CrossRef]
- Mihimit, A.; Adawaye, C.; Péré, H.; Costiniuk, C.; Koyalta, D.; Mbopi-Keou, F.X.; Mboumba Bouassa, R.S.; Talla, F.; Moussa, S.; Longo, J.D.; et al. HSV-2 Infection as a Potential Cofactor for HIV Disease Progression and Selection of Drug Resistance Mutations in Adults under WHO-Recommended First-Line Antiretroviral Therapy: A Multicentric, Cross-Sectional Study in Cameroon, Central African Republic, Chad, and Gabon. Trop. Med. Infect. Dis. 2020, 5, 136. [Google Scholar]
- Luchters, S.M.; Vanden Broeck, D.; Chersich, M.F.; Nel, A.; Delva, W.; Mandaliya, K.; Depuydt, C.E.; Claeys, P.; Bogers, J.P.; Temmerman, M. Association of HIV infection with distribution and viral load of HPV types in Kenya: A survey with 820 female sex workers. BMC Infect Dis. 2010, 10, 18. [Google Scholar] [CrossRef]
- Menon, S.; Wusiman, A.; Boily, M.C.; Kariisa, M.; Mabeya, H.; Luchters, S.; Forland, F.; Rossi, R.; Callens, S.; Vanden Broeck, D. Epidemiology of HPV Genotypes among HIV Positive Women in Kenya: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0163965. [Google Scholar] [CrossRef]
- Onyango, C.G.; Ogonda, L.; Guyah, B. The role of co-infections and hormonal contraceptives in cervical intraepithelial neoplasia prevalence among women referred to a tertiary hospital in Western Kenya. Infect. Agent Cancer 2025, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Zhang, D.; Xiao, B. Association between human papillomavirus infection and common sexually transmitted infections, and the clinical significance of different Mycoplasma subtypes. Front. Cell. Infect. Microbiol. 2023, 13, 1145215. [Google Scholar]
- Maueia, C.; Murahwa, A.; Manjate, A.; Sacarlal, J.; Kenga, D.; Unemo, M.; Andersson, S.; Mussá, T.; Williamson, A.L. The relationship between selected sexually transmitted pathogens, HPV and HIV infection status in women presenting with gynaecological symptoms in Maputo City, Mozambique. PLoS ONE 2024, 19, e0307781. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, S.; Xia, Y.; Lin, Y.; Zhou, G.; Yu, Y.; Feng, M. Association between human herpesvirus infection and cervical carcinoma: A systematic review and meta-analysis. Virol. J. 2023, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, Y.; Wang, L.; Guo, R.; Lei, D. Association Between Herpes Simplex Virus Type II and High-Risk Human Papillomavirus Infections: A Population Study of the National Health and Nutrition Examination Survey, 2009–2016. J. Infect. Dis. 2025, 231, e650–e658. [Google Scholar] [CrossRef]
- Mutombo, A.B.; Benoy, I.; Tozin, R.; Bogers, J.; Van Geertruyden, J.P.; Jacquemyn, Y. Prevalence and Distribution of Human Papillomavirus Genotypes Among Women in Kinshasa, The Democratic Republic of the Congo. J. Glob. Oncol. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- ICO/IARC Information Centre on HPV and Cancer. DR Congo. Human Papillomavirus and Related Cancers, Fact Sheet 2023 (2023-03-10). Available online: https://hpvcentre.net/statistics/reports/COD_FS.pdf (accessed on 3 March 2025).
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Longo, J.D.; Simaléko, M.M.; Ngbale, R.; Grésenguet, G.; Brücker, G.; Bélec, L. Spectrum of female commercial sex work in Bangui, Central African Republic. SAHARA J. 2017, 14, 171–184. [Google Scholar] [CrossRef][Green Version]
- McCreesh, N.; Tarsh, M.N.; Seeley, J.; Katongole, J.; White, R.G. Community understanding of Respondent-Driven Sampling in a medical research setting in Uganda: Importance for the use of RDS for public health research. Int. J. Soc. Res. Methodol. 2013, 16, 10. [Google Scholar] [CrossRef]
- UNITAID 2024. Screening and Treatment of Precancerous Lesions for Secondary Prevention of Cervical Cancer. Technology Landscape Report. Available online: https://unitaid.org/uploads/Screening-and-treatment-of-precancerous-lesions-for-secondary-prevention-of-cervical-cancer-technology-landscape-report.pdf (accessed on 3 March 2025).
- Tonen-Wolyec, S.; Batina-Agasa, S.; Muwonga, J.; Fwamba N’kulu, F.; Mboumba Bouassa, R.S.; Bélec, L. Evaluation of the practicability and virological performance of finger-stick whole-blood HIV self-testing in French-speaking sub-Saharan Africa. PLoS ONE 2018, 13, e0189475. [Google Scholar] [CrossRef]
- Tonen-Wolyec, S.; Muwonga Masidi, J.; Kamanga Lukusa, L.F.; Nsiku Dikumbwa, G.; Sarassoro, A.; Bélec, L. Analytical Performance of the Exacto Test HIV Self-Test: A Cross-Sectional Field Study in the Democratic Republic of the Congo. Open Forum Infect. Dis. 2020, 7, ofaa554. [Google Scholar] [CrossRef] [PubMed]
- Tonen-Wolyec, S.; Sarassoro, A.; Muwonga Masidi, J.; Twite Banza, E.; Nsiku Dikumbwa, G.; Maseke Matondo, D.M.; Kilundu, A.; Kamanga Lukusa, L.; Batina-Agasa, S.; Bélec, L. Field evaluation of capillary blood and oral-fluid HIV self-tests in the Democratic Republic of the Congo. PLoS ONE 2020, 15, e0239607. [Google Scholar] [CrossRef] [PubMed]
- Tonen-Wolyec, S.; Batina-Agasa, S.; Muwonga, J.; Mboumba Bouassa, R.S.; Kayembe Tshilumba, C.; Bélec, L. Acceptability, feasibility, and individual preferences of blood-based HIV self-testing in a population-based sample of adolescents in Kisangani, Democratic Republic of the Congo. PLoS ONE 2019, 14, e0218795. [Google Scholar] [CrossRef] [PubMed]
- Nodjikouambaye, Z.A.; Sadjoli, D.; Mboumba Bouassa, R.S.; Péré, H.; Veyer, D.; Adawaye, C.; Robin, L.; Tonen-Wolyec, S.; Tcheguena, M.M.; Moussa, A.M.; et al. Acceptability and Accuracy of Cervical cancer screening using a self-collected veil for HPV DNA testing by multiplex real-time PCR among adult women in sub-Saharan Africa. J. Clin. Res. Med. 2019, 1, 1–15. [Google Scholar]
- Sun, Z.; Zhang, R.; Liu, Z.; Liu, C.; Li, X.; Zhou, W.; Yang, L.; Ruan, Q.; Zhang, X. Development of a fluorescence-based multiplex genotyping method for simultaneous determination of human papillomavirus infections and viral loads. BMC Cancer 2015, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- LeGoff, J.; Péré, H.; Bélec, L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol. J. 2014, 11, 83. [Google Scholar] [CrossRef]
- Tevi-Bénissan, C.; Bélec, L.; Lévy, M.; Schneider-Fauveau, V.; Si Mohamed, A.; Hallouin, M.C.; Matta, M.; Grésenguet, G. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 1997, 4, 367–374. [Google Scholar] [CrossRef]
- Snead, M.C.; Black, C.M.; Kourtis, A.P. The use of biomarkers of semen exposure in sexual and reproductive health studies. J. Womens Health (Larchmt) 2014, 23, 787–791. [Google Scholar] [CrossRef]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of 362 seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Hanai, R.; Caron, P.R.; Wang, J.C. Human TOP3: A single-copy gene encoding DNA topoisomerase III. Proc. Natl. Acad. Sci. USA 1996, 93, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Stahlman, S.; Hargreaves, J.; Weir, S.; Edwards, J.; Rice, B.; Kochelani, D.; Mavimbela, M.; Baral, S. Sampling Key Populations for HIV Surveillance: Results From Eight Cross-Sectional Studies Using Respondent-Driven Sampling and Venue-Based Snowball Sampling. JMIR Public Health Surveill. 2017, 3, e72. [Google Scholar] [CrossRef] [PubMed]
- MacAllister, J.; Sherwood, J.; Galjour, J.; Robbins, S.; Zhao, J.; Dam, K.; Grosso, A.; Baral, S.D. A comprehensive review of available epidemiologic and HIV service data for female sex workers, men who have sex with men, and people who inject drugs in select West and Central African countries. J. Acquir. Immune. Defic.Syndr. 2015, 68 (Suppl. S2), S83–S90. [Google Scholar] [CrossRef]
- Okoye, J.O.; Ofodile, C.A.; Adeleke, O.K.; Obioma, O. Prevalence of high-risk HPV genotypes in sub-Saharan Africa according to HIV status: A 20-year systematic review. Epidemiol. Health 2021, 43, e2021039. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, A.; Assefa, N.; Gure, T.; Seyoum, B.; Mulu, A.; Mihret, A. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection Among Sub-Saharan African Women: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 890880. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Melendy, A.; Rana, R.K.; Pimenta, J.M. Age-specific prevalence of infection with human papillomavirus in females: A global review. J. Adolesc. Health 2008, 43, S5.E1–S5.E62. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Ogembo, R.K.; Gona, P.N.; Seymour, A.J.; Park, H.S.; Bain, P.A.; Maranda, L.; Ogembo, J.G. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0122488. [Google Scholar] [CrossRef]
- Vinodhini, K.; Shanmughapriya, S.; Das, B.C.; Natarajaseenivasan, K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch. Gynecol. Obstet. 2012, 285, 771–777. [Google Scholar] [CrossRef]
- Icenogle, J.P.; Laga, M.; Miller, D.; Manoka, A.T.; Tucker, R.A.; Reeves, W.C. Genotypes and sequence variants of human papillomavirus DNAs from human immunodeficiency virus type 1-infected women with cervical intraepithelial neoplasia. J. Infect. Dis. 1992, 166, 1210–1216. [Google Scholar] [CrossRef]
- Ali-Risasi, C.; Praet, M.; Van Renterghem, L.; Zinga-Ilunga, B.; Sengeyi, D.; Lokomba, V.; Mukamina, L.; Ndarabu, A.; Kayembe, N.N.; Tshilolo, L.; et al. Human papillomavirus genotype profile in Kinshasa, Democratic Republic of the Congo: Implications for vaccination. Med. Trop. 2008, 68, 617–620. [Google Scholar]
- Sangwa-Lugoma, G.; Ramanakumar, A.V.; Mahmud, S.; Liaras, J.; Kayembe, P.K.; Tozin, R.R.; Lorincz, A.; Franco, E.L. Prevalence and determinants of high-risk human papillomavirus infection in women from a sub-Saharan African community. Sex. Transm. Dis. 2011, 38, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Nyakio, O.; Kibukila, F.; Chasinga, T.; Kasongo, B.; Gad, M.; Tambwe, A.; Kakudji, P.; Kalenga, P.; Kakoma, J.-B. Molecular Genotyping of Human Papillomavirus in Women in Sexual Activity in the Province of South Kivu. J. Med. Res. 2020, 6, 74–78. [Google Scholar]
- Iglesias, P.; Tendobi, C.; Carlos, S.; Lozano, M.D.; Barquín, D.; Chiva, L.; Reina, G. Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)-Implications for Pathogenicity and Vaccine Effectiveness. Microorganisms 2022, 10, 2492. [Google Scholar] [CrossRef]
- Padalko, E.; Ali-Risasi, C.; Van Renterghem, L.; Bamelis, M.; De Mey, A.; Sturtewagen, Y.; Vastenavond, H.; Vanden Broeck, D.; Weyers, S.; Praet, M. Evaluation of the clinical significance of human papillomavirus (HPV) 53. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 7–9. [Google Scholar] [CrossRef]
- Denny, L.; Adewole, I.; Anorlu, R.; Dreyer, G.; Moodley, M.; Smith, T.; Snyman, L.; Wiredu, E.; Molijn, A.; Quint, W.; et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int. J. Cancer 2014, 134, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Vaccarella, S.; Franceschi, S. Multiple human papillomavirus infections: The exception or the rule? J. Infect. Dis. 2011, 203, 891–893. [Google Scholar] [CrossRef]
- Okoye, J.O.; Ngokere, A.A.; Erinle, C.; Mbamalu, C. Co-existence of Herpes simplex virus type 2 and two other oncoviruses is associated with cervical lesions in women living with HIV in South-Western Nigeria. Afr. Health Sci. 2020, 20, 1015–1023. [Google Scholar] [CrossRef]
- Diop-Ndiaye, H.; Beiter, K.; Gheit, T.; Sow Ndoye, A.; Dramé, A.; McKay-Chopin, S.; Tommasino, M.; Bouh Boye, C.S.; Sylla, B.; Kane, C.T. Human Papillomavirus infection in senegalese female sex workers. Papillomavirus Res. 2019, 7, 97–101. [Google Scholar] [CrossRef]
- Adler, D.H.; Wallace, M.; Bennie, T.; Abar, B.; Meiring, T.L.; Williamson, A.I.; Bekker, L.G. Cumulative impact of HIV and multiple concurrent human Papillomavirus infections on the risk of cervical dysplasia. Adv. Virol. 2016, 2016, 7310894. [Google Scholar] [CrossRef]
- Suehiro, T.T.; Gimenes, F.; Souza, R.P.; Taura, S.K.I.; Cestari, R.C.C.; Irie, M.M.T.; Boer, C.G.; Consolaro, M.E.L.; Silva, V.R.S.D. High molecular prevalence of HPV and other sexually transmitted infections in a population of asymptomatic women who work or study at a Brazilian university. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e1. [Google Scholar] [CrossRef] [PubMed]
- Bello, B.D.; Spinillo, A.; Alberizzi, P.; Cesari, S.; Gardella, B.; D’Ambrosio, G.; Roccio, M.; Silini, E.M. Cervical infections by multiple human papillomavirus (HPV) genotypes: Prevalence and impact on the risk of precancerous epithelial lesions. J. Med. Virol. 2009, 81, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Dias Genta, M.L.; Martins, T.R.; Mendoza Lopez, R.V.; Sadalla, J.C.; de Carvalho, J.P.M.; Baracat, E.C.; Levi, J.E.; Carvalho, J.P. Multiple HPV genotype infection impact on invasive cervical cancer presentation and survival. PLoS ONE 2017, 12, e0182854. [Google Scholar] [CrossRef] [PubMed]
- De Brot, L.; Pellegrini, B.; Moretti, S.T.; Carraro, D.M.; Soares, F.A.; Rocha, R.M.; Baiocchi, G.; da Cunha, I.W.; de Andrade, V.P. Infections with multiple high-risk HPV types are associated with high-grade and persistent low-grade intraepithelial lesions of the cervix. Cancer Cytopathol. 2017, 125, 138–143. [Google Scholar] [CrossRef]
- Kim, M.; Park, N.J.; Jeong, J.Y.; Park, J.Y. Multiple Human Papilloma Virus (HPV) Infections Are Associated with HSIL and Persistent HPV Infection Status in Korean Patients. Viruses 2021, 13, 1342. [Google Scholar] [CrossRef]
- Adejo, D.S.; Aminu, M.; Oguntayo, O.A.; Ella, E.E.; Kolawole, A.O.; Bature, S.B.; Murtala, A.; Ameh, E.R. Co-infection of human papillomavirus and herpes simplex virus-2 with cervical dysplasia among women in Kaduna State, Nigeria. Afr. J. Clin. Exper. Microbiol. 2024, 25, 381–392. [Google Scholar] [CrossRef]
- Song, D.; Li, H.; Li, H.; Dai, J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol. Lett. 2015, 10, 600–606. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Obiri-Yeboah, D.; Akakpo, P.K.; Mutocheluh, M.; Adjei-Danso, E.; Allornuvor, G.; Amoako-Sakyi, D.; Adu-Sarkodie, Y.; Mayaud, P. Epidemiology of cervical human papillomavirus (HPV) infection and squamous intraepithelial lesions (SIL) among a cohort of HIV-infected and uninfected Ghanaian women. BMC Cancer 2017, 17, 688. [Google Scholar] [CrossRef]
- Camargo, M.; Del Río-Ospina, L.; Soto-De León, S.C.; Sánchez, R.; Pineda-Peña, A.C.; Sussmann, O.; Patarroyo, M.E.; Patarroyo, M.A. Association of HIV status with infection by multiple HPV types. Trop. Med. Int. Health 2018, 23, 1259–1268. [Google Scholar] [CrossRef]
- Lissouba, P.; Van de Perre, P.; Auvert, B. Association of genital human papillomavirus infection with HIV acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2013, 89, 350–356. [Google Scholar] [CrossRef]
- Auvert, B.; Marais, D.; Lissouba, P.; Zarca, K.; Ramjee, G.; Williamson, A.L. High-risk human papillomavirus is associated with HIV acquisition among South African female sex workers. Infect. Dis. Obstet. Gynecol. 2011, 2011, 692012. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240030824 (accessed on 1 December 2024).
- Finocchario-Kessler, S.; Wexler, C.; Maloba, M.; Mabachi, N.; Ndikum-Moffor, F.; Bukusi, E. Cervical cancer prevention and treatment research in Africa: A systematic review from a public health perspective. BMC Womens Health 2016, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, A.M. Elimination of cervical cancer: Challenges for developing countries. Ecancermedicalscience 2019, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Madzima, T.R.; Vahabi, M.; Lofters, A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: Focused literature review. Can. Fam. Physician 2017, 63, 597–601. [Google Scholar]
- Nodjikouambaye, Z.A.; Adawaye, C.; Mboumba Bouassa, R.S.; Sadjoli, D.; Bélec, L. A systematic review of self-sampling for HPV testing in Africa. Int. J. Gynaecol. Obstet. 2020, 149, 123–129. [Google Scholar] [CrossRef]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef]
- Tesfahunei, H.A.; Ghebreyesus, M.S.; Assefa, D.G.; Zeleke, E.D.; Acam, J.; Joseph, M.; Getachew, E.; Kajogoo, V.D.; Bekele, D.; Manyazewal, T. Human papillomavirus self-sampling versus standard clinician-sampling for cervical cancer screening in sub-Saharan Africa: A systematic review and meta-analysis of randomized controlled trials. Infect. Agent. Cancer. 2021, 16, 43. [Google Scholar] [CrossRef]
- Dzobo, M.; Dzinamarira, T.; Jaya, Z.; Kgarosi, K.; Mashamba-Thompson, T. Experiences and perspectives regarding human papillomavirus self-sampling in sub-Saharan Africa: A systematic review of qualitative evidence. Heliyon 2024, 10, e32926. [Google Scholar] [CrossRef]
- Lopez Castro, R.; Escudero Rivas, R.; Ángeles Calderón, M.; Iglesias Linares, L.; Dolores Hurtado González, M.; Méndez Gómez, N.; de la Rosa Martos, B.; Esther Hidalgo Carmona, M.; Luis López Hidalgo, J. Performance of a vaginal self-collection device versus clinician collected cervical samples for the detection of high-risk human papillomavirus. Prev. Med. Rep. 2024, 41, 102705. [Google Scholar] [CrossRef]
- Das, M. WHO launches strategy to accelerate elimination of cervical cancer. Lancet Oncol. 2021, 22, 20–21. [Google Scholar] [CrossRef]
- Ho, G.Y.F.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: A meta-analysis from HPV infection to cervical cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef]
- Strickler, H.D.; Burk, R.D.; Fazzari, M.; Anastos, K.; Minkoff, H.; Massad, L.S.; Hall, C.; Bacon, M.; Levine, A.M.; Watts, D.H.; et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J. Natl. Cancer Inst. 2005, 97, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Gonçalves, M.A.; Franceschi, S.; HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: A meta-analysis. Aids 2006, 20, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Theiler, R.N.; Farr, S.L.; Karon, J.M.; Paramsothy, P.; Viscidi, R.; Duerr, A.; Cu-Uvin, S.; Sobel, J.; Shah, K.; Klein, R.S.; et al. High-risk human papillomavirus reactivation in human immunodeficiency virus-infected women: Risk factors for cervical viral shedding. Obstet. Gynecol. 2010, 115, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Rowhani-Rahbar, A.; Hawes, S.E.; Sow, P.S.; Toure, P.; Feng, Q.; Dem, A.; Dembele, B.; Critchlow, C.W.; N’Doye, I.; Kiviat, N.B. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J. Infect. Dis. 2007, 196, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.G.; Strickler, H.D.; D’Souza, G. Invasive cervical cancer risk among HIV-infected women is a function of CD4 count and screening. J. Acquir. Immune. Defic. Syndr. 2013, 63, e163. [Google Scholar] [CrossRef]
- Massad, L.S.; Xie, X.; Burk, R.; Keller, M.J.; Minkoff, H.; D’Souza, G.; Watts, D.H.; Palefsky, J.; Young, M.; Levine, A.M.; et al. Long-term cumulative detection of human papillomavirus among HIV seropositive women. AIDS 2014, 28, 2601–2608. [Google Scholar] [CrossRef]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Looker, K.J.; Rönn, M.M.; Brock, P.M.; Brisson, M.; Drolet, M.; Mayaud, P.; Boily, M.C. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): Systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J. Int. AIDS Soc. 2018, 21, e25110. [Google Scholar] [CrossRef] [PubMed]
- Konopnicki, D.; Wit, S.D.; Clumeck, N. HPV and HIV coinfection: A complex interaction resulting in epidemiological, clinical and therapeutic implications. Future Med. 2013, 8, 903–915. [Google Scholar] [CrossRef]
- Belglaiaa, E.; Elannaz, H.; Mouaouya, B.; Aksim, M.; Mercier, M.; Prétet, J.L.; Chouham, S.; Mougin, C. Human papillomavirus genotypes among women with or without HIV infection: An epidemiological study of Moroccan women from the Souss area. Infect. Agent Cancer 2015, 10, 44. [Google Scholar] [CrossRef]
- De Vuyst, H.; Mugo, N.R.; Chung, M.H.; McKenzie, K.P.; Nyongesa-Malava, E.; Tenet, V.; Njoroge, J.W.; Sakr, S.R.; Meijer, C.M.; Snijders, P.J.; et al. Prevalence and determinants of human papillomavirus infection and cervical lesions in HIV-positive women in Kenya. Br. J. Cancer 2012, 107, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Sosso, S.M.; Tchouaket, M.C.T.; Fokam, J.; Simo, R.K.; Torimiro, J.; Tiga, A.; Lobe, E.E.; Ambada, G.; Nange, A.; Semengue, E.N.J.; et al. Human immunodeficiency virus is a driven factor of human papilloma virus among women: Evidence from a cross-sectional analysis in Yaoundé, Cameroon. Virol. J. 2020, 17, 69. [Google Scholar] [CrossRef]
- Hanisch, R.A.; Sow, P.S.; Toure, M.; Dem, A.; Dembele, B.; Toure, P.; Winer, R.L.; Hughes, J.P.; Gottlieb, G.S.; Feng, Q.; et al. Influence of HIV-1 and/or HIV-2 infection and CD4 count on cervical HPV DNA detection in women from Senegal, West Africa. J. Clin. Virol. 2013, 58, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Dartell, M.; Rasch, V.; Kahesa, C.; Mwaiselage, J.; Ngoma, T.; Junge, J.; Gernow, A.; Ejlersen, S.F.; Munk, C.; Iftner, T.; et al. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: The PROTECT study. Sex. Transm. Dis. 2012, 39, 201–208. [Google Scholar] [CrossRef]
- Taku, O.; Mbulawa, Z.Z.A.; Phohlo, K.; Garcia-Jardon, M.; Businge, C.B.; Williamson, A.L. Distribution of Human Papillomavirus (HPV) Genotypes in HIV-Negative and HIV-Positive Women with Cervical Intraepithelial Lesions in the Eastern Cape Province, South Africa. Viruses 2021, 13, 280. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, X.; Liu, J.; Zhang, L. Correlation between human papillomavirus viral load and cervical lesions classification: A review of current research. Front. Med. 2023, 10, 1111269. [Google Scholar] [CrossRef]
- Fobian, S.F.; Mei, X.; Crezee, J.; Snoek, B.C.; Steenbergen, R.D.M.; Hu, J.; Ten Hagen, T.L.M.; Vermeulen, L.; Stalpers, L.J.A.; Oei, A.L. Increased human papillomavirus viral load is correlated to higher severity of cervical disease and poorer clinical outcome: A systematic review. J. Med. Virol. 2024, 96, e29741. [Google Scholar] [CrossRef]
- Wu, Z.; Qin, Y.; Yu, L.; Lin, C.; Wang, H.; Cui, J.; Liu, B.; Liao, Y.; Warren, D.; Zhang, X.; et al. Association between human papillomavirus (HPV) 16, HPV18, and other HR-HPV viral load and the histological classification of cervical lesions: Results from a large-scale cross-sectional study. J. Med. Virol. 2017, 89, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Segondy, M.; Ngou, J.; Kelly, H.; Omar, T.; Goumbri-Lompo, O.; Doutre, S.; Mayaud, P.; Didelot, M.N. Diagnostic value of human papillomavirus (HPV) 16 and HPV18 viral loads for the detection of high-grade cervical intraepithelial neoplasia (CIN2+) in a cohort of African women living with HIV. J. Clin.Virol. 2018, 99–100, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Mombo-Maganga, C.; Mangala, C.; Mabika-Obanda, A.K.F.; Maulot-Bangola, D.; Ambounda-Ledaga, N.; Matsomo-Kombet, G.E.; Moukanda-Ifoundou, R.; Boukandou-Bina, J.A.; Obame-N’na, L.; Tommo, M.; et al. Prevalence of high-risk human papillomavirus genotypes and viral load correlated with squamous cell inflammation among women in Gabon. BMC Womens Health 2024, 24, 561. [Google Scholar] [CrossRef]
- Rousseau, M.N.; Costes, V.; Konate, I.; Nagot, N.; Foulongne, V.; Ouedraogo, A.; Van de Perre, P.; Mayaud, P.; Segondy, M.; Yerelon Study Group. Viral load and genomic integration of HPV 16 in cervical samples from HIV-1-infected and uninfected women in Burkina Faso. J. Med. Virol. 2007, 79, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Damay, A.; Didelot-Rousseau, M.N.; Costes, V.; Konate, I.; Ouedraogo, A.; Nagot, N.; Foulongne, V.; Van de Perre, P.; Mayaud, P.; Segondy, M. Viral load and physical status of human papillomavirus (HPV) 18 in cervical samples from female sex workers infected with HPV 18 in Burkina Faso. J. Med. Virol. 2009, 81, 1786–1791. [Google Scholar] [CrossRef]
- Menon, S.; Luchters, S.; Rossi, R.; Callens, S.; Kishor, M.; Bogers, J.; Vanden Broeck, D. Human papilloma virus correlates of high grade cervical dysplasia in HIV-infected women in Mombasa, Kenya: A cross-sectional analysis. Virol. J. 2018, 15, 54. [Google Scholar] [CrossRef]
- Hanisch, R.A.; Cherne, S.L.; Sow, P.S.; Winer, R.L.; Hughes, J.P.; Feng, Q.; Gottlieb, G.S.; Toure, M.; Dem, A.; Kiviat, N.B.; et al. Human papillomavirus type 16 viral load in relation to HIV infection, cervical neoplasia and cancer in Senegal. Cancer Epidemiol. 2014, 38, 369–375. [Google Scholar] [CrossRef]
- Moodley, J.R.; Constant, D.; Hoffman, M.; Salimo, A.; Allan, B.; Rybicki, E.; Hitzeroth, I.; Williamson, A.L. Human papillomavirus prevalence, viral load and pre-cancerous lesions of the cervix in women initiating highly active antiretroviral therapy in South Africa: A cross-sectional study. BMC Cancer 2009, 9, 275. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.; Johnson, L.F.; Marais, D.J.; Gustavsson, I.; Moodley, J.R.; Coetzee, D.; Gyllensten, U.; Williamson, A.L. Increased alpha-9 human papillomavirus species viral load in human immunodeficiency virus positive women. BMC Infect Dis. 2014, 14, 51. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull World Health Organ 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Harfouche, M.; Abu-Hijleh, F.M.; James, C.; Looker, K.J.; Abu-Raddad, L.J. Epidemiology of herpes simplex virus type 2 in sub-Saharan Africa: Systematic review, meta-analyses, and meta-regressions. EClinicalMedicine 2021, 35, 100876. [Google Scholar] [CrossRef] [PubMed]
- Mertz, G.J.; Benedetti, J.; Ashley, R.; Selke, S.A.; Corey, L. Risk factors for the sexual transmission of genital herpes. Ann. Intern. Med. 1992, 116, 197–202. [Google Scholar] [CrossRef]
- Rajagopal, S.; Magaret, A.; Mugo, N.; Wald, A. Incidence of herpes simplex virus type 2 infections in Africa: A systematic review. Open Forum Infect. Dis. 2014, 1, ofu043. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.G.; Liska, T.A.; Bentzen, S.M.; Kim, E.; Chipato, T.; Salata, R.A.; Celentano, D.D.; Morrison, C.S.; Gravitt, P.E. Brief Report: Herpes Simplex Virus Type-2 Shedding and Genital Ulcers During Early HIV in Zimbabwean Women. J. Acquir. Immune Defic. Syndr. 2021, 87, 789–793. [Google Scholar] [CrossRef]
- Bélec, L.; Grésenguet, G.; Mbopi Kéou, F.X.; Mayaud, P. High frequency of asymptomatic shedding of herpes simplex virus type 2 in African women. Clin. Microbiol. Infect. 2000, 6, 56–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phipps, W.; Nakku-Joloba, E.; Krantz, E.M.; Selke, S.; Huang, M.L.; Kambugu, F.; Orem, J.; Casper, C.; Corey, L.; Wald, A. Genital Herpes Simplex Virus Type 2 Shedding Among Adults With and Without HIV Infection in Uganda. J. Infect. Dis. 2016, 213, 439–447. [Google Scholar] [CrossRef]
- Francis, A.-Y.; Oksana, D.; Timmy, D.E.; Richard, A.H.; Mohammed, S.M. Co-infection prevalence of herpes simplex virus types 1 and 2 with human papillomavirus and associated risk factors among asymptomatic women in Ghana. Int. J. Infect. Dis. Ther. 2018, 3, 45–51. [Google Scholar] [CrossRef]
- Wald, A.; Zeh, J.; Selke, S.; Ashley, R.L.; Corey, L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 1995, 333, 770–775. [Google Scholar] [CrossRef]
- Wald, A.; Corey, L.; Cone, R.; Hobson, A.; Davis, G.; Zeh, J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Investig. 1997, 99, 1092–1097. [Google Scholar] [CrossRef]
- Koanga Mogtomo, M.L.; Ngono Ngane, A.; Djiakam Nganwa, G.; Wankam, M.; Brulet Epaka, C.; Amvam Zollo, P.H. Association of cervical inflammation and cervical abnormalities in women infected with herpes simplex virus type 2. Int. J. Trop. Med. Public Health 2014, 1, 1–4. [Google Scholar]
- Smith, J.S.; Herrero, R.; Bosetti, C.; Muñoz, N.; Bosch, F.X.; Eluf-Neto, J.; Castellsagué, X.; Meijer, C.J.; Van den Brule, A.J.; Franceschi, S.; et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 2002, 94, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.H.; Venkatesh, K.K. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am. J. Reprod. Immunol. 2011, 65, 308–316. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, X.; Zheng, Y.; Tang, J.; Cai, W.; Wang, H.; Gao, Y.; Wang, Y. Relationship between cervical disease and infection with human papillomavirus types 16 and 18, and herpes simplex virus 1 and 2. J. Med. Virol. 2012, 84, 1920–1927. [Google Scholar] [CrossRef]
- Omondi, M.A.; Kamassa, E.H.; Katawa, G.; Tchopba, C.N.; Vogelbusch, C.; Parcina, M.; Tchadié, E.P.; Amessoudji, O.M.; Arndts, K.; Karou, S.D.; et al. Hookworm infection associates with a vaginal Type 1/Type 2 immune signature and increased HPV load. Front. Immunol. 2022, 13, 1009968. [Google Scholar] [CrossRef]
- Pillay, R.; Naidoo, P.; Mkhize-Kwitshana, Z.L. Herpes simplex virus type 2 in sub-Saharan Africa and the potential impact of helminth immune modulation. Front. Cell. Infect. Microbiol. 2024, 14, 1471411. [Google Scholar] [CrossRef]
- Daiane de Peder, L.; Mesquita da Silva, C.; Nascimento, B.L.; Malizan, J.A.; Madeira, H.S.; Horvath, J.D.; Silva, E.S.; Vieira Teixeira, J.J. Prevalence of Sexually Transmitted Infections and Risk Factors Among Young People in a Public Health Center in Brazil: A Cross-Sectional Study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 354–362. [Google Scholar] [CrossRef]
| Variable | All FSWs (n = 415) | HIV-Negative (n = 377) | HIV-Positive (n = 38) | p-Value µ |
|---|---|---|---|---|
| Socio-demographics | ||||
| Age (years) | ||||
| All ages [mean (SD)] | 28.2 (8.1) [18–60] * | 27.9 (7.8) [18–60] | 30.7 (10.5) [19–60] | NS |
| Ranges [n (%)] | ||||
| 18–24 | 152 (36.6) | 139 (36.9) | 13 (34.2) | NS |
| 25–29 | 125 (30.1) | 115 (30.5) | 10 (26.3) | NS |
| 30–39 | 98 (23.6) | 91 (24.1) | 7 (18.4) | NS |
| ≥40 | 40 (9.7) | 32 (8.5) | 8 (21.1) | 0.012 |
| Residency [n (%)] | ||||
| Makiso | 97 (23.4) | 83 (22.0) | 14 (36.8) | 0.039 |
| Tshopo | 66 (15.9) | 65 (17.2) | 1 (2.6) | 0.019 |
| Mangobo | 88 (21.2) | 86 (22.8) | 2 (5.3) | 0.011 |
| Kisangani | 84 (20.2) | 80 (21.2) | 4 (10.5) | NS |
| Kabondo | 80 (19.3) | 63 (16.8) | 17 (44.8) | <0.0001 |
| Religion [n (%)] | ||||
| Christian | 231 (55.7) | 222 (58.9) | 9 (23.7) | <0.0001 |
| Muslim | 61 (14.7) | 52 (13.8) | 9 (23.7) | NS |
| Kimbanguist | 60 (14.4) | 47 (12.5) | 13 (34.2) | <0.0003 |
| Other | 63 (15.2) | 56 (14.8) | 7 (18.4) | NS |
| Education level [n (%)] | ||||
| Never attended school | 82 (19.7) | 73 (19.4) | 9 (23.7) | NS |
| Primary school | 172 (41.4) | 150 (39.8) | 22 (57.9) | 0.031 |
| Secondary school | 129 (31.1) | 123 (32.6) | 6 (15.8) | 0.032 |
| University | 32 (7.8) | 31 (8.2) | 1 (2.6) | NS |
| Marital status [n (%)] | ||||
| Unmarried | 333 (80.2) | 301 (79.8) | 32 (84.2) | NS |
| Married | 82 (19.8) | 76 (20.2) | 6 (15.8) | NS |
| Occupation [n (%)] | ||||
| Professional FSWs | 137 (33.0) | 119 (31.6) | 18 (47.4) | 0.048 |
| Professional FSWs (bar waitress and hotel girl) | 62 (14.9) | 54 (14.3) | 8 (21.1) | NS |
| Nonprofessional FSWs (occasional) | 21 (5.1) | 20 (5.3) | 1 (2.6) | NS |
| Nonprofessional FSWs (regular) | 195 (47.0) | 184 (48.8) | 11 (28.9) | 0.019 |
| Income [USD per day; n (%)] | ||||
| 0–5 | 179 (43.1) | 167 (44.3) | 12 (31.6) | NS |
| 6–10 | 138 (33.2) | 132 (35.0) | 6 (15.8) | 0.020 |
| >10 | 98 (23.7) | 78 (20.7) | 20 (52.6) | <0.0001 |
| Risk factors | ||||
| Number of sexual partner(s) [in past 3 days; n (%)] | ||||
| 1 | 223 (53.7) | 210 (56.0) | 13 (31.6) | 0.011 |
| [2–5] | 146 (35.2) | 128 (33.9) | 18 (47.4) | NS |
| >5 | 46 (11.1) | 38 (10.1) | 8 (21.0) | 0.040 |
| Recent unprotected sexual intercourse [n (%)] | 126 (30.4) | 118 (31.3) | 8 (21.0) | NS |
| Previous HIV testing [n (%)] | 237 (57.1) | 221 (58.6) | 16 (42.1) | 0.049 |
| Genital HSV-2 DNA positivity [n (%)] | 101 (24.3) | 76 (20.1) | 25 (65.8) | <0.0001 |
| Variable | All FSWs (n = 415) | HIV-Negative (n = 377) | HIV-Positive (n = 38) | p-Value µ | HSV-2-Negative (n = 314) | HSV-2-Positive (n = 101) | p-Value µ |
|---|---|---|---|---|---|---|---|
| Any HPV [n (%)] | 197 (47.5) | 168 (44.6) | 29 (76.3) | <0.0002 | 96 (30.6) | 101 (100.0) | <0.0006 |
| Any LR-HPV [n (%)] | 66 (15.9) | 58 (15.4) | 8 (21.0) | NS | 35 (11.1) | 31 (30.7) | <0.0001 |
| HPV-6 | 43 (10.4) | 37 (9.8) | 6 (15.8) | NS | 17 (5.4) | 26 (25.7) | <0.0001 |
| HPV-11 | 10 (2.4) | 10 (2.7) | 0 (0.0) | NS | 8 (2.5) | 2 (1.9) | NS |
| HPV-81 | 13 (3.1) | 11 (2.9) | 2 (5.3) | NS | 10 (3.2) | 3 (2.9) | NS |
| Any HR-HPV [n (%)] | 153 (36.9) | 129 (34.2) | 24 (63.1) | <0.0005 | 62 (19.7) | 91 (90.1) | <0.0001 |
| HPV-16 | 28 (6.7) | 24 (6.4) | 4 (10.5) | NS | 9 (2.9) | 19 (18.8) | <0.0001 |
| HPV-18 | 13 (3.1) | 13 (3.4) | 0 (0.0) | NS | 10 (3.2) | 3 (2.9) | NS |
| HPV-31 | 25 (6.0) | 16 (4.2) | 9 (23.7) | <0.0002 | 6 (1.9) | 19 (18.8) | <0.0001 |
| HPV-33 | 12 (2.9) | 8 (2.1) | 4 (10.5) | 0.017 | 7 (2.2) | 5 (4.9) | NS |
| HPV-35 | 14 (3.4) | 12 (3.2) | 2 (5.3) | NS | 10 (3.2) | 4 (3.9) | NS |
| HPV-39 | 18 (4.4) | 12 (3.2) | 6 (15.8) | 0.003 | 4 (1.3) | 14 (13.9) | <0.0001 |
| HPV-45 | 20 (4.8) | 18 (4.8) | 2 (5.3) | NS | 11 (3.5) | 9 (8.9) | 0.034 |
| HPV-51 | 9 (2.2) | 7 (1.8) | 2 (5.3) | NS | 7 (2.2) | 2 (1.9) | NS |
| HPV-52 | 69 (16.6) | 58 (15.4) | 11 (28.9) | 0.040 | 20 (6.4) | 49 (48.5) | <0.0001 |
| HPV-56 | 10 (2.4) | 7 (1.8) | 3 (7.9) | 0.020 | 6 (1.9) | 4 (3.9) | NS |
| HPV-58 | 46 (11.1) | 39 (10.3) | 7 (18.4) | NS | 22 (7.0) | 24 (23.8) | <0.0001 |
| HPV-59 | 12 (2.9) | 8 (2.1) | 4 (10.5) | 0.017 | 2 (0.6) | 10 (9.9) | <0.0001 |
| HPV-68 | 13 (3.1) | 11 (2.9) | 2 (5.3) | NS | 8 (2.5) | 5 (4.9) | NS |
| Any PO-HPV [n (%)] | 112 (26.9) | 88 (23.3) | 24 (63.2) | <0.0001 | 34 (10.8) | 78 (77.2) | <0.0001 |
| HPV-26 | 7 (1.7) | 6 (1.6) | 1 (2.6) | NS | 5 (1.6) | 2 (1.9) | NS |
| HPV-53 | 57 (14.7) | 50 (13.3) | 11 (28.9) | NS | 15 (4.8) | 42 (41.6) | <0.0001 |
| HPV-66 | 48 (11.6) | 35 (9.3) | 13 (34.2) | <0.001 | 6 (1.9) | 42 (41.6) | <0.0001 |
| HPV-73 | 3 (0.7) | 3 (7.9) | 0 (0.0) | NS | 2 (0.6) | 1 (0.9) | NS |
| HPV-82 | 8 (1.9) | 7 (1.8) | 1 (2.6) | NS | 7 (2.2) | 1 (0.9) | NS |
| Multiple types of any HPV | 130 (31.3) | 107 (28.4) | 23 (60.5) | <0.0005 | 46 (14.6) | 85 (84.1) | <0.0001 |
| Multiple types of HR-HPV | 70 (16.9) | 57 (15.1) | 13 (34.2) | <0.003 | 26 (8.3) | 44 (43.6) | <0.0001 |
| Multiple types of PO-HPV | 11 (2.7) | 9 (2.4) | 2 (5.3) | NS | 1 (0.3) | 10 (9.9) | <0.0001 |
| Vaccine targeted HPV [n (%)] | |||||||
| Any 4-valent vaccine types * | 87 (20.9) | 77 (20.4) | 10 (26.3) | NS | 41 (13.0) | 46 (45.6) | <0.0001 |
| Multiple 4-valent vaccine types | 5 (1.2) | 5 (1.3) | 0 (0.0) | NS | 2 (0.6) | 3 (2.9) | NS |
| Any 9-valent vaccine types ** | 164 (39.5) | 140 (37.1) | 24 (63.2) | <0.002 | 73 (23.2) | 91 (90.1) | <0.0001 |
| Multiple 9-valent vaccine types | 63 (15.2) | 52 (13.8) | 11 (28.9) | 0.013 | 25 (7.9) | 38 (37.6) | <0.0001 |
| Variable [mean ± SD log Copies/104 Cells] | All FSWs (n = 415) | HIV-Negative (n = 377) | HIV-Positive (n = 38) | P µ | HSV-2-Negative (n = 314) | HSV-2-Positive (n = 101) | P µ |
|---|---|---|---|---|---|---|---|
| LR-HPV load | |||||||
| HPV-6 | 0.58 ± 1.71 | 0.55 ± 1.68 | 0.86 ± 2.04 | NS | 0.35 ± 1.28 | 1.42 ± 2.31 | <0.0001 |
| HPV-11 | 0.14 ± 0.89 | 0.15 ± 0.93 | 0.00 ± 0.00 | NA | 0.15 ± 0.93 | 0.15 ± 0.93 | NS |
| HPV-81 | 0.18 ± 1.00 | 0.16 ± 0.94 | 0.35 ± 1.49 | NS | 0.17 ± 0.97 | 0.19 ± 0.19 | NS |
| HR-HPV load | |||||||
| HPV-16 | 0.37 ± 1.41 | 0.35 ± 1.36 | 0.64 ± 1.89 | NS | 0.15 ± 0.91 | 1.07 ± 2.18 | <0.0001 |
| HPV-18 | 0.17 ± 0.94 | 0.18 ± 0.98 | 0.00 ± 0.00 | NA | 0.17 ± 0.94 | 0.15 ± 0.91 | NS |
| HPV-31 | 0.31 ± 1.27 | 0.22 ± 1.04 | 1.31 ± 2.39 | <0.0001 | 0.09 ± 0.69 | 1.00 ± 2.15 | <0.0001 |
| HPV-33 | 0.15 ± 0.87 | 0.11 ± 0.73 | 0.57 ± 1.69 | <0.003 | 0.11 ± 0.75 | 0.26 ± 0.83 | NS |
| HPV-35 | 0.18 ± 0.97 | 0.17 ± 0.94 | 0.30 ± 1.28 | NS | 0.17 ± 0.97 | 0.19 ± 1.47 | NS |
| HPV-39 | 0.24 ± 1.16 | 0.18 ± 1.01 | 0.87 ± 2.07 | <0.0002 | 0.06 ± 0.60 | 0.79 ± 1.93 | <0.0001 |
| HPV-45 | 0.27 ± 1.21 | 0.27 ± 1.22 | 0.28 ± 1.21 | NS | 0.19 ± 1.06 | 0.49 ± 1.14 | 0.028 |
| HPV-51 | 0.12 ± 0.81 | 0.10 ± 0.74 | 0.31 ± 1.34 | NS | 0.12 ± 0.80 | 0.11 ± 0.97 | NS |
| HPV-52 | 0.92 ± 2.08 | 0.84 ± 1.99 | 1.69 ± 2.70 | 0.023 | 0.33 ± 1.30 | 2.74 ± 2.75 | <0.0001 |
| HPV-56 | 0.13 ± 0.83 | 0.10 ± 0.72 | 0.45 ± 1.55 | NS | 0.10 ± 0.74 | 0.21 ± 1.26 | NS |
| HPV-58 | 0.60 ± 1.72 | 0.56 ± 1.66 | 1.04 ± 2.24 | NS | 0.38 ± 1.39 | 1.29 ± 2.04 | <0.0001 |
| HPV-59 | 0.16 ± 0.95 | 0.12 ± 0.85 | 0.55 ± 1.64 | <0.004 | 0.04 ± 0.49 | 0.54 ± 1.35 | <0.0001 |
| HPV-68 | 0.16 ± 0.94 | 0.16 ± 0.92 | 0.28 ± 1.20 | NS | 0.14 ± 0.85 | 0.27 ± 1.21 | NS |
| PO-HPV load | |||||||
| HPV-26 | 0.09 ± 0.71 | 0.08 ± 0.68 | 0.15 ± 0.95 | NS | 0.17 ± 0.94 | 0.09 ± 0.82 | NS |
| HPV-53 | 0.81 ± 1.98 | 0.74 ± 1.90 | 1.58 ± 2.55 | <0.0001 | 0.31 ± 1.27 | 2.37 ± 2.23 | <0.0001 |
| HPV-66 | 0.66 ± 1.77 | 0.54 ± 1.62 | 1.86 ± 2.64 | <0.0001 | 0.09 ± 0.66 | 2.42 ± 2.74 | <0.0001 |
| HPV-73 | 0.04 ± 0.47 | 0.04 ± 0.49 | 0.00 ± 0.00 | NA | 0.04 ± 0.45 | 0.05 ± 0.84 | NS |
| HPV-82 | 0.10 ± 0.75 | 0.09 ± 0.72 | 0.16 ± 0.99 | NS | 0.18 ± 0.79 | 0.06 ± 0.00 | NS |
| Cumulative HPV load | |||||||
| Any HPV | 6.41 ± 9.18 | 5.72 ± 8.47 | 13.26 ± 12.69 | <0.0001 | 3.36 ± 6.92 | 15.89 ± 8.07 | <0.0001 |
| LR-HPV | 0.89 ± 2.08 | 0.86 ± 2.05 | 1.21 ± 2.41 | NS | 0.63 ± 1.79 | 1.72 ± 2.60 | <0.0001 |
| HR-HPV | 3.81 ± 6.90 | 3.36 ± 6.25 | 8.28 ± 10.60 | <0.0001 | 2.08 ± 5.70 | 9.16 ± 8.05 | <0.0001 |
| PO-HPV | 1.71 ± 2.83 | 1.50 ± 2.71 | 3.77 ± 3.28 | <0.0001 | 0.65 ± 1.81 | 5.00 ± 3.16 | <0.0001 |
| Gardasil-4® HR-HPV $ | 0.54 ± 1.81 | 0.53 ± 1.80 | 0.63 ± 1.89 | NS | 0.32 ± 1.44 | 1.23 ± 2.06 | <0.0001 |
| Gardasil-9® HR-HPV £ | 2.80 ± 4.88 | 2.53 ± 4.55 | 5.52 ± 6.82 | <0.0003 | 1.44 ± 3.79 | 7.02 ± 5.68 | <0.0001 |
| Non-vaccine HR-HPV & | 1.00 ± 2.92 | 0.83 ± 2.66 | 2.75 ± 4.52 | <0.0001 | 0.64 ± 2.52 | 2.14 ± 3.67 | <0.0001 |
| HR-HPV Cumulative Viral Load (log Copies/10,000 Cells) | Gardasil-9® £ HR-HPV Cumulative Viral Load (log Copies/10,000 Cells) | Non-Vaccine & HR-HPV Cumulative Viral Load (log Copies/10,000 Cells) | ||||
|---|---|---|---|---|---|---|
| Coefficient β [95% CI] | p-Value $ | Coefficient β [95% CI] | p-Value | Coefficient β [95% CI] | p-Value | |
| Age | 0.017 [−0.06–0.10] | 0.686 | −0.009 [−0.06–0.05] | 0.761 | 0.03 [−0.01–0.06] | 0.179 |
| Residency | ||||||
| Kabondo | Ref. | – | Ref. | – | Ref. | – |
| Mangobo | 1.47 [−2.45–1.81] | 0.208 | 1.17 [−0.41–2.75] | 0.145 | 0.29 [−0.75–1.34] | 0.579 |
| Kisangani | 1.15 [−1.15–3.45] | 0.326 | 0.41 [−1.18–1.99] | 0.614 | 0.74 [−0.31–1.79] | 0.164 |
| Tshopo | 1.89 [−0.43–4.22] | 0.110 | 1.12 [−0.48–2.72] | 0.172 | 0.78 [−0.28–1.84] | 0.150 |
| Makiso | 1.91 [−0.12–3.93] | 0.065 | 1.31 [−0.08–2.71] | 0.066 | 0.59 [−0.33–1.52] | 0.205 |
| Religion | ||||||
| Christian | Ref. | – | Ref. | – | Ref. | – |
| Muslim | −0.37 [−2.26–1.51] | 0.697 | −0.03 [−1.33–1.27] | 0.961 | −0.34 [−1.2–0.52] | 0.435 |
| Kimbanguist | 0.76 [−1.19–2.71] | 0.439 | 0.09 [−1.26–1.44] | 0.894 | 0.67 [−0.22–1.56] | 0.135 |
| Other religion | 0.32 [−1.62–2.26] | 0.742 | 0.45 [−0.89–1.79] | 0.504 | −0.13 [−1.02–0.76] | 0.771 |
| Education level | ||||||
| Never schooled | Ref. | – | Ref. | – | Ref. | – |
| Primary | 0.68 [−1.12–2.47] | 0.458 | 0.68 [−0.56–1.92] | 0.276 | −0.01 [−0.83–0.81] | 0.984 |
| Secondary | −0.04 [−1.97–1.9] | 0.968 | −0.12 [−1.46–1.21] | 0.856 | 0.08 [−0.8–0.97] | 0.852 |
| University | −0.6 [−3.4–2.19] | 0.67 | 0.06 [−1.87–1.99] | 0.953 | −0.66 [−1.93–0.61] | 0.307 |
| Marital status | ||||||
| Married | Ref. | – | Ref. | – | Ref. | – |
| Unmarried | 1.39 [−0.31–3.09] | 0.107 | 0.72 [−0.46–1.89] | 0.227 | 0.67 [−0.1–1.45] | 0.088 |
| Occupation | ||||||
| Occasional non-FSW | Ref. | – | Ref. | – | Ref. | – |
| Regular non-FSW | 0.14 [−3.27–3.54] | 0.936 | −0.008 [−2.36–2.34] | 0.994 | 0.14 [−1.41–1.70] | 0.852 |
| Professional FSW | 1.54 [−2.75–5.84] | 0.481 | 0.57 [−2.39–3.54] | 0.705 | 0.97 [−0.99–2.93] | 0.331 |
| Professional FSW in bars | −2.89 [−6.35–0.56] | 0.101 | −1.46 [−3.85–0.92] | 0.229 | −1.43 [−3.01–0.15] | 0.076 |
| Daily income in dollars (USD) | ||||||
| [0–5] | Ref. | – | Ref. | – | Ref. | – |
| [6–10] | −0.02 [−1.77–1.74] | 0.983 | −0.002 [−1.21–1.21] | 0.997 | −0.02 [−0.82–0.78] | 0.967 |
| >10 | 1.98 [−0.93–4.89] | 0.18 | 0.75 [−1.26–2.76] | 0.464 | 1.23 [−0.09–2.56] | 0.067 |
| Number of sexual partners in past 3 days | ||||||
| 1 | Ref. | – | Ref. | – | Ref. | – |
| [2–5] | −0.17 [−1.66–1.33] | 0.825 | −0.24 [−1.27–0.8] | 0.653 | 0.07 [−0.61–0.75] | 0.845 |
| >5 | −2.45 [−9.87–4.97] | 0.514 | −0.56 [−5.68–4.57] | 0.83 | −1.89 [−5.28–1.49] | 0.269 |
| Recent unprotected sexual intercourse | 1.71 [−0.39–3.8] | 0.109 | 1.3 [−0.15–2.75] | 0.078 | 0.41 [−0.55–1.37] | 0.398 |
| Previous HIV test | −0.58 [−1.86–0.7] | 0.37 | −0.17 [−1.06–0.71] | 0.698 | −0.41 [−0.99–0.18] | 0.168 |
| Genital HSV-2 positivity | 5.32 [3.54–7.1] | <0.001 | 4.59 [3.37–5.82] | <0.001 | 0.84 [0.12–1.55] | 0.021 |
| HIV status | 2.01 [−0.47–4.48] | 0.11 | 0.91 [−0.8–2.62] | 0.295 | 1.1 [−0.03–2.23] | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muwonga Tukisadila, J.; Mboumba Bouassa, R.-S.; Tonen-Wolyec, S.; Loemba, H.; Muwonga, J.; Belec, L. Syndemic Synergy of HPV, HIV, and HSV-2 for Oncogenic HPV Replication in Female Sex Workers. Trop. Med. Infect. Dis. 2025, 10, 157. https://doi.org/10.3390/tropicalmed10060157
Muwonga Tukisadila J, Mboumba Bouassa R-S, Tonen-Wolyec S, Loemba H, Muwonga J, Belec L. Syndemic Synergy of HPV, HIV, and HSV-2 for Oncogenic HPV Replication in Female Sex Workers. Tropical Medicine and Infectious Disease. 2025; 10(6):157. https://doi.org/10.3390/tropicalmed10060157
Chicago/Turabian StyleMuwonga Tukisadila, Jonathan, Ralph-Sydney Mboumba Bouassa, Serge Tonen-Wolyec, Hugues Loemba, Jeremie Muwonga, and Laurent Belec. 2025. "Syndemic Synergy of HPV, HIV, and HSV-2 for Oncogenic HPV Replication in Female Sex Workers" Tropical Medicine and Infectious Disease 10, no. 6: 157. https://doi.org/10.3390/tropicalmed10060157
APA StyleMuwonga Tukisadila, J., Mboumba Bouassa, R.-S., Tonen-Wolyec, S., Loemba, H., Muwonga, J., & Belec, L. (2025). Syndemic Synergy of HPV, HIV, and HSV-2 for Oncogenic HPV Replication in Female Sex Workers. Tropical Medicine and Infectious Disease, 10(6), 157. https://doi.org/10.3390/tropicalmed10060157







