Abstract
Background: The availability of laboratory tests to screen and diagnose migrants and travellers for neglected and tropical parasitic diseases significantly impacts individual and public health. Italian scientific societies for parasitology, tropical diseases, and global health developed a survey to assess number and geographical localisation of laboratories able to carry out adequate diagnostics. Methods: An open-ended and multiple-choice questionnaire was constructed and sent to 752 members working in Italian microbiology laboratories via scientific societies’ mailing lists. Data concerning malaria, cystic echinococcosis, leishmaniasis, schistosomiasis, strongyloidiasis, and Chagas disease were included. Results: Members from 96 laboratories replied. At least one laboratory responded from 18 out of 20 Italian regions. Serological tests for Schistosoma spp., Strongyloides stercoralis, Trypanosoma cruzi, Echinococcus spp., and Leishmania spp. are performed in <50% of responding laboratories. Only 56.6% of labs provide all three recommended tests for malaria diagnosis in the emergency room. Direct identification methods availability varies for Schistosoma eggs (75–95.8%), S. stercoralis larvae (53.1%), trypomastigotes (59.4%), and Leishmania amastigotes (53.1%). Geographical differences (mainly northern versus southern regions) were evident. Conclusions: The survey underlines the need to improve diagnosis for neglected and tropical diseases, to define a network of reference laboratories for testing less prevalent diseases, and to share information, education, and training for both clinicians and microbiologists/parasitologists.
1. Introduction
With the increase in world travel and access to varied populations and geographic areas, we continue to see more tropical diseases and infections outside areas of endemicity due to the rapidity with which people and organisms can move from one place to another. Travel has also become accessible and more affordable for many people worldwide, including those with compromised overall health status [1].
Many European countries are either the final destination or a transit point for migrants from low- and middle-income countries (LMICs). From an epidemiological perspective, this means that many migrants come from areas with different patterns of infectious diseases compared to the countries they are migrating to.
This observation underlines the importance of laboratory test availability to screen migrants for prevalent infectious diseases that significantly impact individual and public health, as recommended by several national and international guidelines [2,3].
According to a survey conducted in nine Italian sentinel centres, 4123 Neglected Tropical Diseases (NTDs) cases were diagnosed within a 7-year period (2011–2017), with schistosomiasis and strongyloidiasis being the most common NTDs, accounting for about one-third each of all the diagnosed cases, followed by Chagas disease caused by Trypanosoma cruzi [4].
Tilli et al. used hospital discharge records to retrieve the number of hospitalisations for some NTDs. Among 7195 hospitalisations, schistosomiasis, strongyloidiasis, and Chagas disease were the most common NTDs after leishmaniasis [5].
Besides migrants, those infections may also involve travellers and European residents who travel to visit friends and relatives (VFR). Malaria is the most diagnosed infection after travel to tropical countries, but schistosomiasis and, less frequently, Chagas and schistosomiasis may be imported infections into Europe—for example, the schistosomiasis outbreak in Corsica, France [6,7,8].
It is important to underline that some southern European countries, like Italy, share a similar disease endemicity with certain LMICs, including infectious diseases such as cystic echinococcosis (CE) [9,10,11] and leishmaniasis [12,13]. Furthermore, due to climate change, vector-borne diseases typical of tropical areas, like malaria or dengue, are at risk of emerging in these regions because of changing vector endemicity [14,15].
Indeed, most guidelines recommend specific population screening for the most prevalent tropical diseases, such as malaria, and some neglected tropical diseases (NTDs), such as schistosomiasis and strongyloidiasis.
In Table 1, a synthesis of screening recommendations is presented.

Table 1.
Synthesis of screening recommendation for migrants (national and European guidelines): schistosomiasis, strongyloidiasis, malaria, Chagas disease, and leishmaniasis.
Screening is not the only activity that needs laboratory diagnostic tools. Diagnostic tests used in screening usually show good sensitivity but poorer specificity, which means that diagnostic confirmation with a second test may be necessary.
Moreover, alongside screening, malaria and NTDs may manifest in individuals displaying specific signs and symptoms. Specific diagnostic tools are needed to confirm the diagnosis and guide case management in this case. Outpatient and inpatient management may be necessary.
The current epidemiological scenario should lead to easier and broader access to diagnosis and treatment in Italy. In fact, diagnostic procedures in medical parasitology require a great interpretative experience with very few exceptions. Currently, very few procedures can be automated for routine laboratory use, and even when antigen, serological, or molecular biology tests are available, they do not always have sufficient sensitivity and specificity. Parasitological diagnosis can today be defined as multiparametric; the organism’s identification relies on morphologic characteristics that can be difficult to differentiate without experience. Serological tests and the recent development of bio-molecular tests can improve the microbiological diagnosis. Although morphology can be “learned” at the microscope, knowledge about the life cycle, epidemiology, infectivity, geographic range, clinical symptoms, range of illness, disease presentation depending on immune status, and recommended therapy is critical to the operation of any laboratory providing diagnostic services in medical parasitology.
Unfortunately, several issues hinder both treatment and laboratory diagnosis. The difficulties in accessing diagnosis outside of referral centres [21] are critical obstacles to following guidelines, providing patient care, testing to evaluate treatment response, and attempting to control the reintroduction of non-endemic diseases (such as malaria, strongyloidiasis, and schistosomiasis) or attempting to control and eliminate diseases like Chagas disease, malaria, and schistosomiasis.
From these premises, there is a need to teach and support human parasitological diagnostics [22]. The AMCLI Study Committee for Parasitology (CoSP) has provided comprehensive updates to intestinal, blood, and reticuloendothelial system parasitosis diagnostic pathways. The CoSP, in collaboration with the Italian Society of Tropical Medicine and Global Health (SIMET) and the Italian Society of Parasitology (SoIPa) contributed to mapping the availability of laboratory tests for the most prevalent and relevant neglected and tropical diseases in Italy.
This investigation aimed to create a network of national reference laboratories for parasitological diagnostics that could be contacted for consultation and/or specialist tests.
This work will focus on assessing access, types of tests used, and geographic distribution of diagnostic tools for malaria, schistosomiasis, strongyloidiasis, Chagas disease, CE, and leishmaniasis. Table 2 provides a literature review of Italian papers reporting the prevalence of the diseases focused on in this survey.

Table 2.
Review of prevalence and incidence data in Italy.
2. Materials and Methods
A questionnaire consisting of open-ended and multiple-choice questions was constructed using online Google Forms and was sent to the members of the three associations working in microbiology laboratories all over Italy via the AMCLI, SoIPa, and SIMET mailing lists (752 members). Not knowing the total number of laboratories in Italy, we decided to reach them through these three of the largest Italian associations that deal with parasitology.
The questionnaire is structured into 8 modules: laboratory details, pre-analytics, microscopic and cultural examinations for faecal parasites, microscopic and cultural examinations for blood parasites and parasites of the reticuloendothelial system and other body areas, antigen research, molecular biology, parasitological serology, and workload. Google Forms creates online Google Excel sheets that can then be downloaded by survey managers and modified for data analysis using the Microsoft Excel© 2021 application.
Overall, the questionnaire covered the diagnosis of faecal, blood, and reticuloendothelial system parasitosis. In this paper, we decided to summarise the survey’s results about malaria, schistosomiasis, strongyloidiasis, and Chagas disease because of their prevalence among the migrant population and CE and leishmaniasis because of an endemic transmission in Italy as well as among countries of origin of migrants.
The results were presented using frequencies, means, and medians.
3. Results
Ninety-six laboratories replied to the questionnaire. At least one laboratory responded from 18 out of 20 Italian regions. Sixteen (16.7%) were laboratories in the private sector, and eighty (83.3%) were in the public sector.
Eighty-one out of ninety-six laboratories (84.4%) belonged to hospitals.
Figure 1 shows the number and the geographical distribution of the responding laboratories.
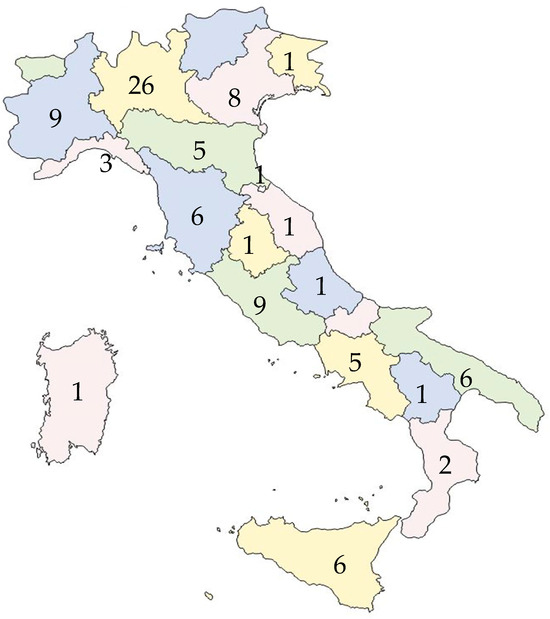
Figure 1.
Number and the geographical distribution of the responding laboratories.
Fifty out of ninety-six laboratories were in northern regions, with only 21 participating laboratories from southern regions (Figure 2).
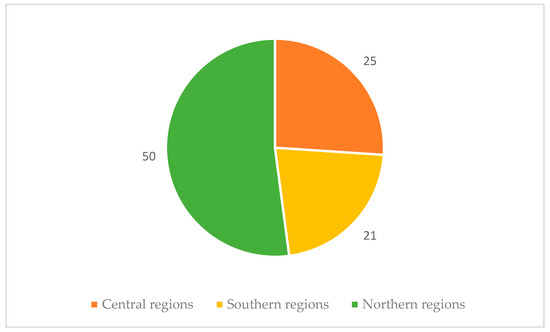
Figure 2.
Distribution of participating laboratories according to geographical area.
Regarding the pre-analytical data, the questionnaire showed that only 36 out of 96 (37.5%) laboratories routinely receive the patient’s case history form that usually accompanies the clinical samples for parasitological investigations. When requesting parasitological tests, the attending physician should share clinical and epidemiological information. This helps the microbiologist choose the best testing techniques and request additional tests if needed.
3.1. Schistosomiasis
Seventy-five percent (72 out of 96) of laboratories can perform egg identification in urine. Among the respondents, 75% (72 out of 96) directly search for S. haematobium in urine, 87.5% of which carry out the centrifugation method. Ninety-two labs (95.8%) are able to look for eggs in stools. Twenty-seven laboratories (28.1%) had access to serology. For 27 laboratories, 10 different commercial kits are used: ELISA (8 laboratories), ICT (8 labs), WB (9 labs), IHA (3 labs), and IFA (1 lab). Two laboratories perform PCR on urine and 15 PCR on stools (four of them using a homemade assay).
3.2. Strongyloidiasis
Fifty-three out of ninety-six (53.1%) laboratories perform direct research for S. stercoralis, and the technique mainly used is agar plate culture examination (45.1%, 23 out of 51). Regarding the directly targeted research of this parasite, 23 laboratories perform only the culture, 10 perform the Berman test, 1 performs the Harada–Mori test. Ten laboratories out of ninety-six use two or more direct tests. Only 15 laboratories declare to use PCR. Among the respondents, 28 laboratories (29.2%) had access to serology. For these 28 laboratories, seven different commercial kits based on the ELISA method are used.
3.3. Chagas Disease
A total of 57 out of 96 laboratories (59.4%) perform direct research for Trypanosoma spp. The technique used is the research of trypomastigotes by microscopy with thin and thick blood smears. One laboratory (1.7%) uses the microhematocrit method. Serology for T. cruzi is available in 19 out of 96 labs (19.8%). In ten labs out of the nineteen that perform serology (52.6%), at least two different serologic tests are provided; in most cases, an ELISA test is associated with a second assay. In 3 labs out of 19 (15.8%), three different serological assays are available. In 6 out of 96 labs (6.3%), nucleic acid amplification tests (NAATs) for T. cruzi are available, and in 4 out of 6 cases, it is a homemade PCR.
3.4. Malaria
Malaria diagnosis is performed by 86% of the laboratories that answered the questionnaire. Most labs (66.3%) perform both thick and thin smears, and 31.3% only use the thin film. Among the laboratories that routinely perform the thick smear, 19 use the May–Grunwald Giemsa stain, which is not suitable for this type of preparation. The colouring diluted in water at pH 7.2 is instead used by 73.2% of the laboratories. A rapid ICT (immunochromatographic test) is widely available (71 laboratories, 73.9%), and a pan-malaria rapid test is used in all but two labs. Serology for Plasmodium spp. is available in 16 out of 96 labs (16.7%), with enzyme immunoassay assay being the most distributed (13/16, 81.2%). A total of 51 labs out of 83 (61.4%) are performing molecular biology for malaria diagnosis; 35 labs (68.6%) use only LAMP (loop-mediated isothermal amplification) method, 10 (19.6%) use both LAMP and PCR, and 5 (11.8%) use only PCR.
Figure 3 sums up the different diagnostic attitudes among the national laboratories.
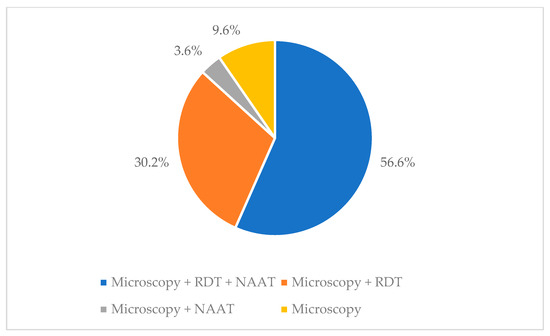
Figure 3.
Different malaria diagnostic approaches among the national laboratories (footnote: RDT = Rapid Diagnostic Test, NAAT = Nucleic Acid Amplification Tests).
3.5. Echinococcosis
Of the 96 laboratories that answered the survey, approximately half (47%) perform serology for this tapeworm, 14/96 perform the ELISA test, 11/96 use two or three combinations of tests to confirm the diagnosis (for example, 5 labs using ELISA tests in combination with the Western blot test). The direct search for parasites on cyst fluid is carried out by 46/96 laboratories, of which 30 declare that they only search for Echinococcus spp.
3.6. Leishmaniasis
Of 96 laboratories, 51 (53.1%) declare that they perform microscopic and/or culture research for Leishmania spp. in bone marrow, spleen, and lymph nodes. The research in 47 laboratories is performed by microscopic examination; only 4 perform both microscopy and culture. Microscopic and/or culture search for Leishmania spp. on the skin and subcutaneous material is performed by 37 laboratories; even in this case, only 4 laboratories perform both microscopy and culture. Serology is performed in 45 laboratories (47%); the most used technique is an ICT assay (9/45), followed by Western blot (6/45); 13 laboratories declare to use two or three tests to reach the diagnosis. PCR is performed in 24 labs (25%); 16/24 use commercially available kits, while the remaining 8 labs use in-house PCR.
3.7. Geographic Distribution of Tests
The distribution of testing for screening and diagnosing the six diseases shows significant inequality across the country.
Figure 4 and Figure 5 illustrate the nationwide availability of the tests throughout the country. Over 50% of laboratories offering screening or diagnostic tests are located in the north, approximately 30% are in central Italy, and less than 20% are in the south.
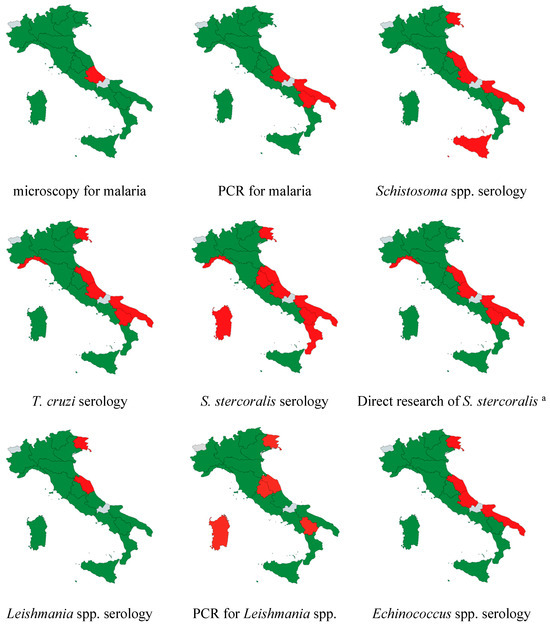
Figure 4.
Regional availability of the different screening and diagnostic tests in the country. a culture, PCR, Baermann test, Harada–Mori test. Legend: Green = available at least in one responding lab, red = not available among the responding labs, grey = no laboratory in the region responded the survey.
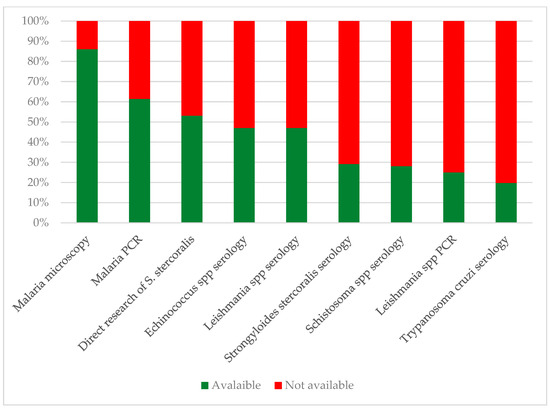
Figure 5.
Availability of selected diagnostic tests for neglected and tropical parasitic diseases in Italian laboratories. Direct research of S. stercoralis includes at least one among culture, PCR, Baermann test, and Harada–Mori test.
Only two laboratories conduct Strongyloides serology in the southern regions, specifically for four Schistosoma spp. and four T. cruzi serologies. Thirteen laboratories provide all three serologies (12 public hospitals and one private lab), with one hospital located in the southern regions.
4. Discussion
Advances in laboratory techniques have revolutionised parasite diagnostics over the past several decades. Although it is important to realise that not all laboratories can perform each procedure in the same way, it is very important to understand the pros and cons of the laboratory procedure and comply with the guidelines. For this reason, our purpose with the questionnaire was to map the availability of parasitological diagnostics tests to facilitate networking and consultation among different laboratories.
Geographical differences were evident either in the number of responding laboratories or in the availability of tests. In Italy, significant disparities in the quality of health services and economic inequalities are well-known [36]. However, we cannot conclude about a larger responding percentage from laboratories in the northern regions because the denominator is unknown. Indeed, the survey was sent to the registered emails of scientific society members rather than to each laboratory. This means that we cannot exclude the fact that a greater number of scientific society members work in the northern regions.
Widespread implementation of rapid antigen detection tests has greatly expanded access to tests for global parasitic threats such as malaria, even if there is still the need for a microscopic diagnosis, which is also useful for measuring parasitaemia.
Recently, the introduction of multiplex molecular panels for human gastrointestinal infections has enhanced the identification of common intestinal protozoa in faeces along with bacterial and viral pathogens. Despite the benefits provided by novel diagnostics in terms of sensitivity, automation, and early results, increased reliance on non-microscopy-based methods may contribute to the progressive, widespread loss of morphology expertise for morphological parasite identification. Such loss might negatively impact patient care, public health, and epidemiology. For example, for malaria diagnosis among the laboratories that routinely perform the thick smear, 19 out of 58 use the May–Grunwald Giemsa stain, which is not suitable for this type of preparation, and the colouring diluted in water at pH 7.2 is instead used by 73.2% of the laboratories.
Inadequate microscopy experience may lead to missed and inaccurate diagnoses and erroneous descriptions of emergent human parasitic diseases [37].
To guarantee correct and effective parasitological diagnostics, it is necessary to use the most reliable and targeted techniques, applied according to national and international guidelines [22]. The first step in complying with national and international guidelines on migrant screening is the availability of laboratory tests. Besides screening, neglected and tropical diseases, such as malaria, leishmaniasis, CE, schistosomiasis, strongyloidiasis, and Chagas disease, are prevalent in Italy, either among migrants, travellers, or current residents [4,5]. Although morphology can be “learned” at the microscope, knowledge about the life cycle, epidemiology, infectivity, geographic range, clinical symptoms, range of illness, disease presentation depending on immune status, and recommended therapy is critical to the operation of any laboratory providing diagnostic services in medical parasitology [1].
Unfortunately, we have observed a progressive decline in courses dedicated to parasitology in medical faculties all over Europe for a long time [38].
Despite the more common migrant routes arriving in the southern Italian regions as the first destination [39] and the recommendation of screening upon arrival [2,3], many laboratories in the south cannot offer the recommended serology assays.
In those areas, migrant screening for schistosomiasis, strongyloidiasis, and Chagas disease is a great challenge.
Moreover, there is a concern regarding the country’s approach to malaria diagnosis. Malaria is one of the few urgent infectious diseases to be diagnosed in the emergency room. Therefore, it is troubling that 14% of labs surveyed do not offer any tests for malaria diagnosis. Moreover, only 56.6% of labs performing at least one test for malaria may provide all three recommended tests (microscopy, rapid test, and NAAT) [22].
The results are not significantly different when considering other NTDs, such as leishmaniasis and CE. Even though these diseases are endemic in Italy, the availability of diagnostic tests is not consistent across the country. Serology for Leishmania spp. is carried out in less than 50% of labs, and only 4 out of 96 labs perform both culture and microscopy. Serology availability is geographically widespread; by contrast, PCR seems not to be available in regions such as Sardinia, which is among the top three regions in terms of the number of diagnoses. For echinococcosis, serology is performed by only half of the responding labs and seems to be lacking in Apulia, which ranks third in terms of reported cases.
Even though most Italian regions seem to perform most of the tests discussed in this paper, it does not mean those exams are easily accessible for patients.
Undocumented migrants in Italy face barriers in accessing tests. These individuals have the right to emergency and essential care through a dedicated healthcare code (STP), which must be obtained from the health authority. An exemption from direct healthcare costs applies in cases of extreme poverty.
The definition of essential care is not clearly defined. Although the prevention of chronic and debilitating diseases such as schistosomiasis, Chagas disease, and strongyloidiasis can be evidently listed as essential care, this lack of clarity could pose a barrier to migrant screening.
In Italy, the Essential Levels of Assistance (LEA) represent the list of lab tests the public health care system must make available to people. In 2017, with a dedicated decree, this list was updated to include Schistosoma spp., Strongyloides spp., and Chagas disease serology [40]. Unfortunately, after seven years, this decree has not been applied at the national level. However, only some regions have already implemented the test, relying on their own fund. This means that in most regions, those tests can be easily prescribed during hospitalisation but not in the outpatient setting or primary health care services, which are the preferred sites for screening.
For Chagas disease, for instance, the Italian law requests the blood donor centres to perform serology tests since 2015 [16,41]. By contrast, our survey showed that most (80.2%) of the responding microbiology laboratories in this survey do not perform Chagas serology.
A national survey published by the Italian National Blood Centre in 2022 involving 81% of Italian blood donor centres showed that blood donor screening seems to be well implemented at national level [42].
The survey showed that 98% of the responding centres performing collection/validation of blood and blood components declared to be able to perform T. cruzi serology for blood donors at risk. Studies that analysed seroprevalence among blood donors reported a positive rate from 0.06 to 0.5% [33,42]. However, the access to serology for diagnostic purposes (i.e., migrants from endemic areas with cardiological or gastrointestinal symptoms) or screening of different populations (i.e., pregnant women) is completely different since a microbiology laboratory should test samples from these subjects while it is not possible to test these samples in a blood donor centre.
For this reason, it can happen that in the same Italian city, an Italian blood donor who returned from a two-week trekking trip in Peru will be subjected to mandatory testing for T. cruzi antibody before blood donation, while it may be challenging to test an asymptomatic Bolivian pregnant woman or a Salvadorian man with arrhythmic disturbance.
An established network of laboratories performing different tests for different diseases may be a solution to overcome this lack of equity and avoid wasting resources on providing diagnostic kits in contexts where the number of requests is expected to be low according to the origin of migrants in the area covered by the laboratory. To ensure the interruption of transmission in non-endemic countries, autochthonous recipients of blood or organs must be protected from transmission, as well as those born in endemic countries or with siblings coming from those geographical areas. There is an urgent need to organise a network of general practitioners, obstetricians, paediatricians, infectious disease experts, and laboratories to ensure effective diagnostics and clinical protocols, preventing an overload of unused kits in inexperienced centres.
To complicate the picture, even when the diagnosis is made, early and appropriate treatment for the above-mentioned diseases is hindered by the lack of access to drugs, most of which are included in the WHO’s essential drug list [43,44].
Our study has some limitations. We cannot precisely estimate the percentage of responding laboratories because the denominator is unknown. Indeed, the survey was sent to the registered email of scientific society members and not to each laboratory. This affects the generalisability of the results even though the significant difference in test availability between northern and southern regions seems to support the likelihood of this finding. Another negative aspect is that we do not know the total number of microbiology laboratories, either public or private, and their distribution in Italy. Finally, we did not explicitly ask the laboratory if they are able to send samples to other laboratories to perform specific tests not available locally. Indeed, it could be another way for people to access good-quality tests. However, in the available free text in the survey sheet, only one laboratory stated that samples could be sent to the Italian National Institute for some tests and, in the survey, the laboratory was considered as an accessible testing point.
5. Conclusions
The results of this survey should support an analysis of the barriers and facilitators that explain this geographic difference. Even though the overall results may merely represent an example of the well-known healthcare access disparities among Italian regions [36], these exacerbate the inequalities in access for the migrant population.
We can pinpoint several unmet needs, as follows: practical education regarding parasite diagnoses; conducting audits and external assessments to ensure that at least the most critical diagnoses, like malaria, are performed; establishing reference laboratories at a regional level for centralising specific and non-urgent tests; and creating a network for sharing information, education, and training for both clinicians and microbiologists/parasitologists.
These activities must be undertaken to enhance diagnosis and treatment, ensuring compliance with international and national recommendations.
Author Contributions
Conceptualization A.C., E.O., L.Z., and A.R.; Data curation E.O., F.B. (Francesco Bernieri), L.P., L.C., F.B. (Fabrizio Bruschi), and A.R.; Formal analysis A.C., E.O., and F.B. (Francesco Bernieri); Methodology A.C., E.O., F.B. (Francesco Bernieri), L.C., L.P., and A.R.; Supervision L.Z., G.C., F.B. (Fabrizio Bruschi), and A.R.; Validation G.C. and F.B. (Fabrizio Bruschi); Writing—original draft: A.C. and E.O.; Writing—review and editing L.Z., F.B. (Fabrizio Bruschi), and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Italian Network for Neglected Tropical Diseases (IN-NTDs) for supporting the survey and the microbiologists of the laboratories who answered the survey and helped acquire all the data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garcia, L.S. Practical Guide to Diagnostic Parasitology, 3rd ed.; Wiley and sons, Ltd.: Washington, DC, USA, 2021. [Google Scholar]
- ECDC, Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-screening-and-vaccination-infectious-diseases-newly (accessed on 11 December 2024).
- Istituto Nazionale per la Promozione Della Salute Delle Popolazioni Migranti e Per il Contrasto Delle Malattie Della Povertà. I Controlli Alla Frontiera. La Frontiera dei Controlli. Controlli Sanitari All’arrivo e Percorsi di Tutela Per i Migranti Ospiti. Available online: https://www.inmp.it/lg/LG_Migranti-integrata.pdf (accessed on 11 December 2024).
- Zammarchi, L.; Gobbi, F.; Angheben, A.; Spinicci, M.; Buonfrate, D.; Calleri, G.; De Paola, M.; Bevilacqua, N.; Carrara, S.; Attard, L.; et al. Schistosomiasis, strongyloidiasis and Chagas disease: The leading imported neglected tropical diseases in Italy. J. Travel. Med. 2020, 27, taz100. [Google Scholar] [CrossRef]
- Tilli, M.; Botta, A.; Bartoloni, A.; Corti, G.; Zammarchi, L. Hospitalization for Chagas disease, dengue, filariasis, leishmaniasis, schistosomiasis, strongyloidiasis, and Taenia solium taeniasis/cysticercosis, Italy, 2011–2016. Infection 2020, 48, 695–713. [Google Scholar] [CrossRef]
- Gautret, P.; Cramer, J.P.; Field, V.; Caumes, E.; Jensenius, M.; Gkrania-Klotsas, E.; de Vries, P.J.; Grobusch, M.P.; Lopez-Velez, R.; Castelli, F.; et al. Infectious diseases among travellers and migrants in Europe, Eurotravnet 2010. Eurosurveillance 2012, 17, 20205. [Google Scholar] [CrossRef] [PubMed]
- Grobusch, M.P.; Weld, L.; Goorhuis, A.; Hamer, D.H.; Schunk, M.; Jordan, S.; Mockenhaupt, F.P.; Chappuis, F.; Asgeirsson, H.; Caumes, E.; et al. Travel-related infections presenting in Europe: A 20-year analysis of EuroTravNet surveillance data. Lancet Reg. Health—Eur. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Grobusch, M.P.; Mühlberger, N.; Jelinek, T.; Bisoffi, Z.; Corachán, M.; Harms, G.; Matteelli, A.; Fry, G.; Hatz, C.; Gjørup, I.; et al. Imported Schistosomiasis in Europe: Sentinel Surveillance Data from TropNetEurop. J. Travel. Med. 2006, 10, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Legnardi, M.; Fittipaldo, A.; Drigo, M.; Cassini, R. Epidemiological distribution of Echinococcus granulosus s.l. infection in human and domestic animal hosts in European Mediterranean and Balkan countries: A systematic review. PLoS Negl. Trop. Dis. 2020, 14, e0008519. [Google Scholar] [CrossRef]
- Casulli, A.; Abela-Ridder, B.; Petrone, D.; Fabiani, M.; Bobić, B.; Carmena, D.; Šoba, B.; Zerem, E.; Gargaté, M.J.; Kuzmanovska, G.; et al. Unveiling the incidences and trends of the neglected zoonosis cystic echinococcosis in Europe: A systematic review from the MEmE project. Lancet Infect. Dis. 2023, 23, e95. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Rossi, P.; Galati, F.; Mariconti, M.; Nicoletti, G.J.; Rinaldi, F.; Casulli, A.; Pozio, E.; Brunetti, E. The Italian registry of cystic echinococcosis (RIEC): The first prospective registry with a European future. Eurosurveillance 2015, 20, 21115. [Google Scholar] [CrossRef]
- Todeschini, R.; Musti, M.A.; Pandolfi, P.; Troncatti, M.; Baldini, M.; Resi, D.; Natalini, S.; Bergamini, F.; Galletti, G.; Santi, A.; et al. Re-emergence of human leishmaniasis in northern Italy, 2004 to 2022: A retrospective analysis. Eurosurveillance 2024, 29, 2300190. [Google Scholar] [CrossRef]
- Berriatua, E.; Jumakanova, Z.; Muñoz, C.; Ortuño, M.; Pérez-Cutillas, P. Surveillance, Prevention and Control of Leishmaniases in the European Union and Its Neighbouring Countries; ECDC: Stockholm Sweden, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/leishmaniasis-surveillance-eu.pdf (accessed on 11 December 2024).
- George, A.M.; Ansumana, R.; de Souza, D.K.; Niyas, V.K.M.; Zumla, A.; Bockarie, M.J. Climate change and the rising incidence of vector-borne diseases globally. Int. J. Infect. Dis. 2024, 139, 143–145. [Google Scholar] [CrossRef]
- Raele, D.A.; Severini, F.; Toma, L.; Menegon, M.; Boccolini, D.; Tortorella, G.; Di Luca, M.; Cafiero, M.A. Anopheles sacharovi in Italy: First record of the historical malaria vector after over 50 years. Parasit. Vectors 2024, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- CTN Centro Nazionale Trapianti. Valutazione Dell’idoneità del Donatore in Relazione a Patologie Infettive. Revisione 2.0 del 15/02/2024. Available online: https://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_620_allegato.pdf (accessed on 11 December 2024).
- Requena-Méndez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Muñoz, J. The Laboratory Diagnosis and Follow Up of Strongyloidiasis: A Systematic Review. PLoS Negl. Trop. Dis. 2013, 7, e2002. [Google Scholar] [CrossRef]
- Ministero Della Salute Direzione Generale Della Prevenzione Sanitaria. Prevenzione e Controllo Della Malaria in Italia. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2016&codLeg=57366&parte=1%20&serie=null (accessed on 11 December 2024).
- Gravidanza Fisiologica 2023, xiii, 248 p. Linea Guida 1/2023 SNLG. Available online: https://www.iss.it/documents/20126/9184367/SNLG+1_2023+Gravidanza-fisiologica+Parte-1.pdf/1b3c23be-4578-48f8-277a-88cf0a98de6c?t=1708700731145 (accessed on 11 December 2024).
- Carlier, Y.; Altcheh, J.; Angheben, A.; Freilij, H.; Luquetti, A.O.; Schijman, A.G.; Segovia, M.; Wagner, N.; Vinas, P.A. Congenital Chagas disease: Updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl. Trop. Dis. 2019, 13, e0007694. [Google Scholar] [CrossRef]
- Zammarchi, L.; Vellere, I.; Stella, L.; Bartalesi, F.; Strohmeyer, M.; Bartoloni, A. The diagnosis of neglected tropical diseases (NTDs) in Italy: Reply. Intern. Emerg. Med. 2017, 12, 725–726. [Google Scholar] [CrossRef] [PubMed]
- AMCLI-CoSP, Comitato di Studio per la Parassitologia dell’Associazione Microbiologi Clinici Italiani. Percorso Diagnostico delle Parassitosi Intestinali. 2022. Available online: https://www.amcli.it/wp-content/uploads/2022/03/Percorso-diagnostico-delle-Parassitosi-Intestinali.pdf (accessed on 11 December 2024).
- Martelli, G.; Di Girolamo, C.; Zammarchi, L.; Angheben, A.; Morandi, M.; Tais, S.; Degani, M.; El Hamad, I.; Caligaris, S.; Ciannameo, A.; et al. Seroprevalence of five neglected parasitic diseases among immigrants accessing five infectious and tropical diseases units in Italy: A cross-sectional study. Clin. Microbiol. Infect. 2017, 23, 335.e1–335.e5. [Google Scholar] [CrossRef]
- Tilli, M.; Botta, A.; Mantella, A.; Nuti, B.; Bartoloni, A.; Boccalini, S.; Zammarchi, L. Community-based seroprevalence survey of schistosomiasis and strongyloidiasis by means of Dried Blood Spot testing on Sub-Saharan migrants resettled in Italy. New Microbiol. 2021, 44, 62–65. [Google Scholar] [PubMed]
- Marrone, R.; Mazzi, C.; Ouattara, H.; Cammilli, M.; Pontillo, D.; Perandin, F.; Bisoffi, Z. Screening for Neglected Tropical Diseases and other infections in African refugees and asylum seekers in Rome and Lazio region, Italy. Travel. Med. Infect. Dis. 2023, 56, 102649. [Google Scholar] [CrossRef]
- Buonfrate, D.; Gobbi, F.; Marchese, V.; Postiglione, C.; Badona Monteiro, G.; Giorli, G.; Napoletano, G.; Bisoffi, Z. Extended screening for infectious diseases among newly arrived asylum seekers from Africa and Asia, Verona province, Italy, April 2014 to June 2015. Eurosurveillance 2018, 23, 17-00527. [Google Scholar] [CrossRef]
- Beltrame, A.; Buonfrate, D.; Gobbi, F.; Angheben, A.; Marchese, V.; Monteiro, G.B.; Bisoffi, Z. The hidden epidemic of schistosomiasis in recent African immigrants and asylum seekers to Italy. Eur. J. Epidemiol. 2017, 32, 733–735. [Google Scholar] [CrossRef]
- Buonfrate, D.; Baldissera, M.; Abrescia, F.; Bassetti, M.; Caramaschi, G.; Giobbia, M.; Mascarello, M.; Rodari, P.; Scattolo, N.; Napoletano, G.; et al. Epidemiology of Strongyloides stercoralis in northern Italy: Results of a multicentre case-control study, February 2013 to July 2014. Eurosurveillance 2016, 21, 30310. [Google Scholar] [CrossRef]
- Buonfrate, D.; Marrone, R.; Silva, R.; Mirisola, C.; Ragusa, A.; Mistretta, M.; Perandin, F.; Bisoffi, Z. Prevalence of Strongyloidiasis in a Cohort of Migrants in Italy and Accuracy of a Novel ELISA Assay for S. stercoralis Infection, a Cross-Sectional Study. Microorganisms 2016, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, C.; Martelli, G.; Ciannameo, A.; Vocale, C.; Fini, M.; Stefanini, A.; Landini, M.P.; Viale, P.; Verucchi, G. Chagas Disease in a Non-endemic Country: A Multidisciplinary Research, Bologna, Italy. J. Immigr. Minor. Health 2016, 18, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Galimberti, L.; Grande, R.; Bianco, R.; Oreni, L.; Traversi, L.; Ricaboni, D.; Bestetti, G.; Lai, A.; Mileto, D.; et al. Chagas disease knocks on our door: A cross-sectional study among Latin American immigrants in Milan, Italy. Clin. Microbiol. Infect. 2018, 24, 1340.e1–1340.e6. [Google Scholar] [CrossRef]
- Pane, S.; Giancola, M.L.; Piselli, P.; Corpolongo, A.; Repetto, E.; Bellagamba, R.; Cimaglia, C.; Carrara, S.; Ghirga, P.; Oliva, A.; et al. Serological evaluation for Chagas disease in migrants from Latin American countries resident in Rome, Italy. BMC Infect. Dis. 2018, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Mangano, V.D.; Prato, M.; Marvelli, A.; Moscato, G.; Bruschi, F. Screening of at-risk blood donors for Chagas disease in non-endemic countries: Lessons from a 2-year experience in Tuscany, Italy. Transfus. Med. 2021, 31, 63–68. [Google Scholar] [CrossRef]
- Dorrucci, M.; Boccolini, D.; Bella, A.; Lucarelli, C.; D’Amato, S.; Caraglia, A.; Maraglino, F.P.; Severini, C.; Gradoni, L.; Pezzotti, P. Malaria surveillance system and Hospital Discharge Records: Assessing differences in Italy, 2011–2017 database analysis. Travel. Med. Infect. Dis. 2022, 48, 102322. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Malaria Annual Epidemiological Report for 2022; ECDC: Solna, Sweden, 2022. [Google Scholar]
- Naghavi, M. GBD 2021 Italy Subnational Burden of Disease Collaborators. State of health and inequalities among Italian regions from 2000 to 2021: A systematic analysis based on the Global Burden of Disease Study 2021. Lancet Public Health 2025, 10, e309–e320. [Google Scholar] [CrossRef]
- Bradbury, R.S.; Sapp, S.G.H.; Potters, I.; Mathison, B.A.; Frean, J.; Mewara, A.; Sheorey, H.; Tamarozzi, F.; Couturier, M.R.; Chiodini, P.; et al. Where Have All the Diagnostic Morphological Parasitologists Gone? J. Clin. Microbiol. 2022, 60, e0098622. [Google Scholar] [CrossRef]
- Bruschi, F. How parasitology is taught in medical faculties in Europe? Parasitology, lost? Parasitol. Res. 2009, 105, 1759–1762. [Google Scholar] [CrossRef]
- International Organization for Migration (IOM). DTM Italy—Flow Monitoring Surveys with Migrants Arriving to Italy; IOM: Rome, Italy, 2024; Available online: https://dtm.iom.int/sites/g/files/tmzbdl1461/files/reports/DTM_Report_Flow%20Monitoring%20Surveys%20with%20migrants%20arriving%20in%20Italy_2023_1.pdf (accessed on 11 December 2024).
- Decreto del Presidente del Consiglio dei Ministri 12 Gennaio 2017. Definizione e Aggiornamento dei Livelli Essenziali di Assistenza 2017. Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=58669 (accessed on 11 December 2024).
- Decreto del Presidente del Consiglio dei Ministri 2 Novembre 2015. Disposizioni Relative ai Requisiti di Qualita’ e Sicurezza del Sangue e Degli Emocomponenti. Available online: https://www.gazzettaufficiale.it/eli/id/2015/12/28/15A09709/sg (accessed on 11 December 2024).
- Pati, I.; Cruciani, M.; Masiello, F.; Barone, F.; Silvioli, G.; La Raja, M.; Pupella, S.; De Angelis, V. Chagas Disease and Transfusion Risk in Italy: The Results of a National Survey. Pathogens 2022, 1, 1229. [Google Scholar] [CrossRef]
- Calleri, G.; Angheben, A.; Albonico, M. Neglected tropical diseases in Europe: Rare diseases and orphan drugs? Infection 2019, 47, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Comelli, A.; Angheben, A.; Albonico, M.; Calleri, G.; Zammarchi, L.; Napoli, C.; Marrone, R. Schistosomiasis in migrants: Bridging the gap in Italy’s treatment guidelines and access. J. Travel. Med. 2024, 32, taae107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).