Community-Based Tuberculosis Preventive Treatment Among Child and Adolescent Household Contacts in Ethiopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Description of Interventions
2.3. Patient Enrollment in the Study
2.4. Adherence Support and Follow-Up
2.5. Supportive Supervision
2.6. Data Management and Analysis
2.7. Ethical Considerations
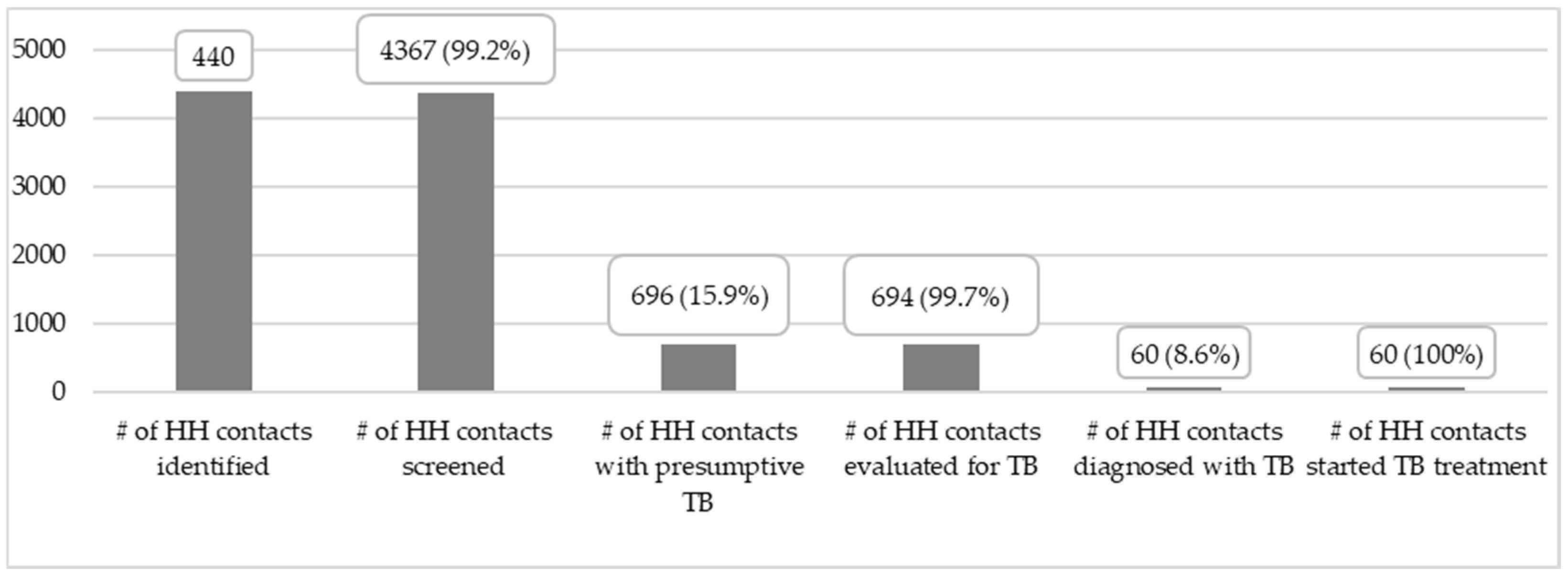
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOTs | Directly observed treatment |
| DR-TB | Drug-resistant TB |
| HEWs | Health extension workers |
| 3HP | Rifapentine and isoniazid |
| PHCUs | Primary health care units |
| SNNPR | Southern Nations, Nationalities, and Peoples’ Region |
| PTB | Pulmonary tuberculosis |
| TB | Tuberculosis |
| TPT | Tuberculosis preventive treatment |
References
- Bagcchi, S. WHO’s global tuberculosis report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Amisi, J.A.; Carter, E.J.; Masini, E.; Szkwarko, D. Closing the loop in child TB contact management: Completion of TB preventive therapy outcomes in western Kenya. BMJ Open 2021, 11, e040993. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.; Dobler, C.; Marais, B.; Denholm, J. Preventive therapy for latent tuberculosis infection—The promise and the challenges. Int. J. Infect. Dis. 2017, 56, 68–76. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment-Drug-Resistant Tuberculosis Treatment, 2022 Update; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Marais, B.J.; Verkuijl, S.; Casenghi, M.; Triasih, R.; Hesseling, A.C.; Mandalakas, A.M.; Marcy, O.; Seddon, J.A.; Graham, S.M.; Amanullah, F. Paediatric tuberculosis—New advances to close persistent gaps. Int. J. Infect. Dis. 2021, 113, S63–S67. [Google Scholar] [CrossRef] [PubMed]
- Moyo, N.; Tay, E.L.; Nolan, A.; Graham, H.R.; Graham, S.M.; Denholm, J.T. TB contact tracing for young children: An Australian cascade of care review. Public Health. Action 2021, 11, 91–96. [Google Scholar] [CrossRef]
- Heuvelings, C.; de Vries, S.; Grobusch, M. Tackling TB in low-incidence countries: Improving diagnosis and management in vulnerable populations. Int. J. Infect. Dis. 2017, 56, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Szkwarko, D.; Hirsch-Moverman, Y.; Du Plessis, L.; Du Preez, K.; Carr, C.; Mandalakas, A.M. Child contact management in high tuberculosis burden countries: A mixed-methods systematic review. PLoS ONE 2017, 12, e0182185. [Google Scholar] [CrossRef]
- Vasiliu, A.; Salazar-Austin, N.; Trajman, A.; Lestari, T.; Mtetwa, G.; Bonnet, M.; Casenghi, M. Child contact case management—A major policy-practice gap in high-burden countries. Pathogens 2021, 11, 1. [Google Scholar] [CrossRef]
- Jerene, D.; Assefa, D.; Tesfaye, K.; Bayu, S.; Seid, S.; Aberra, F.; Bedru, A.; Khan, A.; Creswell, J. Effectiveness of women-led community interventions in improving tuberculosis preventive treatment in children: Results from a comparative, before–after study in Ethiopia. BMJ Open 2022, 12, e062298. [Google Scholar] [CrossRef]
- Sandoval, M.; Mtetwa, G.; Devezin, T.; Vambe, D.; Sibanda, J.; Dube, G.S.; Dlamini-Simelane, T.; Lukhele, B.; Mandalakas, A.M.; Kay, A. Community-based tuberculosis contact management: Caregiver experience and factors promoting adherence to preventive therapy. PLOS Glob. Public Health 2023, 3, e0001920. [Google Scholar] [CrossRef]
- Jo, Y.; Gomes, I.; Flack, J.; Salazar-Austin, N.; Churchyard, G.; Chaisson, R.E.; Dowdy, D.W. Cost-effectiveness of scaling up short course preventive therapy for tuberculosis among children across 12 countries. eClinicalMedicine 2021, 31, 100707. [Google Scholar] [CrossRef] [PubMed]
- Mafirakureva, N.; Mukherjee, S.; Tchounga, B.; Atwine, D.; Youngui, B.T.; Ssekyanzi, B.; Okello, R.; Leonie, S.; Cohn, J.; Casenghi, M.; et al. Household costs incurred under community- and facility-based service-delivery models of tuberculosis preventive therapy for children: A survey in Cameroon and Uganda. J. Glob. Health Rep. 2023, 7, e2023072. [Google Scholar] [CrossRef]
- Federal Democratic Republic of Ethiopia Ministry of Health. Guidelines for Clinical and Programmatic Management of TB, TB/HIV, DR-TB and Leprosy in Ethiopia; Addis Ababa 2021. Available online: http://dataverse.nipn.ephi.gov.et/bitstream/handle/123456789/1662/Guidelines_for_Clinical_and_Programmatic_Management_of_TB_TBHIV.pdf?sequence=1 (accessed on 2 April 2025).
- Kay, A.W.; Sandoval, M.; Mtetwa, G.; Mkhabela, M.; Ndlovu, B.; Devezin, T.; Sikhondze, W.; Vambe, D.; Sibanda, J.; Dube, G.S.; et al. Vikela Ekhaya: A novel, community-based, tuberculosis contact management program in a high burden setting. Clin. Infect. Dis. 2021, 74, 1631–1638. [Google Scholar] [CrossRef]
- Bonnet, M.; Kyakwera, C.; Kyomugasho, N.; Atwine, D.; Mugabe, F.; Nansumba, M.; Ii, Y.B.; Mwanga-Amumpaire, J.; Kiwanuka, J. Prospective cohort study of the feasibility and yield of household child tuberculosis contact screening in Uganda. Int. J. Tuberc. Lung Dis. 2017, 21, 862–868. [Google Scholar] [CrossRef]
- Chabala, C.; Chongwe, G.; Jumbe-Marsden, E.; Somwe, S.W. Missed opportunities for screening child contacts of smear-positive tuberculosis in Zambia, a high-prevalence setting. Int. J. Tuberc. Lung Dis. 2017, 21, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, V.; Koura, K.; Adjobimey, M.; Gnanou, S.; Wandji, A.; Gody, J.C.; Delacourt, C.; Detjen, A.; Graham, S.M.; Mass-erey, E. Child contact investigation and TPT: How to operationalise normative guidance in real life in resource-limited settings and increase uptake of TPT and TPT completion. Int. J. Tuberc. Lung Dis. 2020, 24, 452–460. [Google Scholar] [CrossRef]
- Sulis, G.; Combary, A.; Getahun, H.; Gnanou, S.; Giorgetti, P.F.; Konseimbo, A.; Capone, S.; Hamada, Y.; Baddeley, A.; Matteelli, A. Implementation of tuberculosis prevention for exposed children, Burkina Faso. Bull. World Health Organ. 2018, 96, 386–392. [Google Scholar] [CrossRef]
- Acuña-Villaorduña, C.; Jones-López, E.C.; Marques-Rodrigues, P.; Fregona, G.; Gaeddert, M.; Ribeiro-Rodrigues, R.; Vinhas, S.; Palaci, M.; Salgame, P.; Dietze, R.; et al. Sustained effect of isoniazid preventive therapy among household contacts in Brazil. Int. J. Tuberc. Lung Dis. 2022, 26, 406–411. [Google Scholar] [CrossRef]
- Blok, L.; Sahu, S.; Creswell, J.; Alba, S.; Stevens, R.; Bakker, M.I. Comparative meta-analysis of tuberculosis contact investigation interventions in eleven high burden countries. PLoS ONE 2015, 10, e0119822. [Google Scholar] [CrossRef]
- Otero, L.; Battaglioli, T.; Ríos, J.; De la Torre, Z.; Trocones, N.; Ordoñez, C.; Seas, C.; Van der Stuyft, P. Contact evaluation and isoniazid preventive therapy among close and household contacts of tuberculosis patients in Lima, Peru: An analysis of routine data. Trop. Med. Int. Health 2019, 25, 346–356. [Google Scholar] [CrossRef]
- Datiko, D.G.; Yassin, M.A.; Theobald, S.J.; Cuevas, L.E. A community-based isoniazid preventive therapy for the prevention of childhood tuberculosis in Ethiopia. Int. J. Tuberc. Lung Dis. 2017, 21, 1002–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Egere, U.; Togun, T.; Sillah, A.; Mendy, F.; Otu, J.; Hoelscher, M.; Heinrich, N.; Hill, P.C.; Kampmann, B. Identifying children with tuberculosis among household contacts in The Gambia. Int. J. Tuberc. Lung Dis. 2017, 21, 46–52. [Google Scholar] [CrossRef]
- Hall, C.; Sukijthamapan, P.; dos Santos, R.; Nourse, C.; Murphy, D.; Gibbons, M.; Francis, J.R. Challenges to delivery of isoniazid preventive therapy in a cohort of children exposed to tuberculosis in Timor-Leste. Trop. Med. Int. Health 2015, 20, 730–736. [Google Scholar] [CrossRef]
- Jaganath, D.; Zalwango, S.; Okware, B.; Nsereko, M.; Kisingo, H.; Malone, L.; Lancioni, C.; Okwera, A.; Joloba, M.; Mayanja-Kizza, H.; et al. Contact investigation for active tuberculosis among child contacts in Uganda. Clin. Infect. Dis. 2013, 57, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Tefera, F.; Barnabee, G.; Sharma, A.; Feleke, B.; Atnafu, D.; Haymanot, N.; O’malley, G.; Feleke, G. Evaluation of facility and community-based active household tuberculosis contact investigation in Ethiopia: A cross-sectional study. BMC Health Serv. Res. 2019, 19, 234. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Yirdaw, K.D.; Datiko, D.G.; Cuevas, L.E.; Yassin, M.A. Yield of household contact investigation of patients with pulmonary tuberculosis in southern Ethiopia. BMC Public Health 2020, 20, 737. [Google Scholar] [CrossRef]
- Souza, A.B.; Arriaga, M.B.; Amorim, G.; Araújo-Pereira, M.; Nogueira, B.M.F.; Queiroz, A.T.L.; Figueiredo, M.C.; Rocha, M.S.; Benjamin, A.; Moreira, A.S.R.; et al. Determinants of losses in the latent tuberculosis infection cascade of care in Brazil. BMJ Glob. Health 2021, 6, e005969. [Google Scholar] [CrossRef]
- Wysocki, A.D.; Villa, T.C.S.; Arakawa, T.; Brunello, M.E.F.; Vendramini, S.H.F.; Monroe, A.A.; Kritski, A.L. Latent tuberculosis infection diagnostic and treatment cascade among contacts in primary health care in a city of Sao Paulo state, Brazil: Cross-sectional study. PLoS ONE 2016, 11, e0155348. [Google Scholar] [CrossRef]
- Bonnet, M.; Vasiliu, A.; Tchounga, B.K.; Cuer, B.; Fielding, K.; Ssekyanzi, B.; Youngui, B.T.; Cohn, J.; Dodd, P.J.; Tiendrebeogo, G.; et al. Effectiveness of a community-based approach for the investigation and management of children with household tuberculosis contact in Cameroon and Uganda: A cluster-randomised trial. Lancet Glob. Health 2023, 11, e1911–e1921. [Google Scholar] [CrossRef]
- Nyirenda, M.; Sinfield, R.; Haves, S.; Molyneux, E.M.; Graham, S.M. Poor attendance at a child TB contact clinic in Malawi [Notes from the Field]. Int. J. Tuberc. Lung Dis. 2006, 10, 585–587. [Google Scholar]
- Salazar-Austin, N.; Cohn, S.; Barnes, G.L.; Tladi, M.; Motlhaoleng, K.; Swanepoel, C.; Motala, Z.; Variava, E.; Martinson, N.; Chaisson, R.E. Improving Tuberculosis Preventive Therapy Uptake: A Cluster-randomized Trial of Symptom-based Versus Tuberculin Skin Test–based Screening of Household Tuberculosis Contacts Less Than 5 Years of Age. Clin. Infect. Dis. 2020, 70, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Heuvelings, C.C.; de Vries, S.G.; Greve, P.F.; Visser, B.J.; Bélard, S.; Janssen, S.; Cremers, A.L.; Spijker, R.; Shaw, B.; A Hill, R.; et al. Effectiveness of interventions for diagnosis and treatment of tuberculosis in hard-to-reach populations in countries of low and medium tuberculosis incidence: A systematic review. Lancet Infect. Dis. 2017, 17, e144–e158. [Google Scholar] [CrossRef] [PubMed]


| Key Interventions # | Preintervention (Routine Practice) |
|---|---|
| 1.0 Capacity building | |
|
|
| 2.0 Contact screening and evaluation practice | |
|
|
| 3.0 TPT initiation and follow-up | |
|
|
| 4.0 Program monitoring and support | |
|
|
| Variables | N (%) |
|---|---|
| Age (n = 4403) | |
| <15 years | 1735 (39.4) |
| ≥15 years | 2668 (60.6) |
| Period of enrolment | |
| Intervention | 4403 |
| Preintervention | 412 |
| Cascade of contact screening | |
| Index bacteriologically confirmed PTB cases | 1069 |
| Households visited | 1050 (98.2) |
| Contacts screened for TB (n = 4374) | |
| <15 years | 1723 (39.4) |
| ≥15 years | 2651 (60.6) |
| Contacts with positive TB screen result (n = 696) | |
| <15 years | 81 (11.6) |
| ≥15 years | 615 (88.4) |
| Contacts evaluated for TB (n = 687) | |
| <15 years | 79 (11.4) |
| ≥15 years | 608 (88.6) |
| Contacts diagnosed with active TB (n = 60) | |
| <15 years | 4 (6.7) |
| ≥15 years | 56 (93.3) |
| Contacts put on TB treatment (n = 60) | |
| <15 years | 4 (6.7) |
| ≥15 years | 56 (93.3) |
| Variables | N (%) |
|---|---|
| TPT initiation | |
| Age | |
| <5 years | 328 (20.9) |
| 5–14 years | 1239 (79.1) |
| Total | 1567 (100.0) |
| Period of enrolment | |
| Intervention | 1567 (95.4) |
| Preintervention | 85 (92.9) |
| Type of TPT regimen (n = 1567) | |
| 3RH | 1528 (97.5) |
| 3HP | 39 (2.5) |
| Site of TPT initiation (n = 1567) | |
| Health center | 175 (11.2) |
| Health post | 1392 (88.8) |
| Total | |
| TPT completion | |
| Age (n = 1537) | |
| <5 years | 320 (20.8) |
| 5–14 years | 1217 (79.2) |
| Period of enrolment | |
| Intervention | 1537 (98.1) |
| Preintervention | 79 (95.4) |
| Type of TPT regimen (n = 1537) | |
| 3RH | 1498 (98.0) |
| 3HP | 39 (100.0) |
| Site of adherence support (n = 1537) | |
| Health post | 1383 (99.4) |
| Health center | 154 (88.0) |
| Variables | Yes (n, %) | No (n, %) | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Contacts screened for TB | |||||
| Preintervention | 394 (95.6) | 18 (4.4) | 5.54 | 2.93–10.13 | <0.001 |
| Intervention | 4367 (99.1) | 36 (0.9) | |||
| TB diagnosed among contacts | |||||
| Preintervention | 2 (8.3) | 22 (91.7) | 1.06 | 0.25–9.54 | 0.93 |
| Intervention | 60 (8.8) | 621 (91.2) | |||
| TPT initiation | |||||
| <5 years | 328 (94.0) | 21 (6.0) | 0.71 | 0.41–1.25 | 0.18 |
| 5–14 years | 1239 (95.7) | 54 (4.3) | |||
| Preintervention | 85 (92.9) | 19 (7.1) | 4.67 | 2.54–8.23 | <0.001 |
| Intervention | 1567 (98.1) | 75 (1.9) | |||
| TPT completion | |||||
| <5 years | 320 (97.6) | 8 (2.4) | 0.72 | 0.31–1.90 | 0.44 |
| 5–14 years | 1217 (98.2) | 22 (1.8) | |||
| Intervention | 1537 (98.1) | 30 (1.9) | 3.89 | 1.28–9.86 | 0.002 |
| Preintervention | 79 (95.4) | 6 (4.6) | |||
| Adherence at health post | 1383 (99.4) | 9 (0.6) | 20.95 | 8.97–52.71 | <0.001 |
| Adherence Health Center | 154 (88.0) | 21 (12.0) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abelti, E.; Dememew, Z.; Gebreyohannes, A.; Alemayehu, Y.; Terfassa, T.; Janfa, T.; Jerene, D.; Suarez, P.; Datiko, D. Community-Based Tuberculosis Preventive Treatment Among Child and Adolescent Household Contacts in Ethiopia. Trop. Med. Infect. Dis. 2025, 10, 102. https://doi.org/10.3390/tropicalmed10040102
Abelti E, Dememew Z, Gebreyohannes A, Alemayehu Y, Terfassa T, Janfa T, Jerene D, Suarez P, Datiko D. Community-Based Tuberculosis Preventive Treatment Among Child and Adolescent Household Contacts in Ethiopia. Tropical Medicine and Infectious Disease. 2025; 10(4):102. https://doi.org/10.3390/tropicalmed10040102
Chicago/Turabian StyleAbelti, Eshetu, Zewdu Dememew, Asfawesen Gebreyohannes, Yohannes Alemayehu, Tilay Terfassa, Taye Janfa, Degu Jerene, Pedro Suarez, and Daniel Datiko. 2025. "Community-Based Tuberculosis Preventive Treatment Among Child and Adolescent Household Contacts in Ethiopia" Tropical Medicine and Infectious Disease 10, no. 4: 102. https://doi.org/10.3390/tropicalmed10040102
APA StyleAbelti, E., Dememew, Z., Gebreyohannes, A., Alemayehu, Y., Terfassa, T., Janfa, T., Jerene, D., Suarez, P., & Datiko, D. (2025). Community-Based Tuberculosis Preventive Treatment Among Child and Adolescent Household Contacts in Ethiopia. Tropical Medicine and Infectious Disease, 10(4), 102. https://doi.org/10.3390/tropicalmed10040102





