A Case of Persistent KSHV Viremia in the Context of HIV, SARS-CoV-2, and Other Co-Infections
Abstract
1. Introduction
2. Materials and Methods
3. Results
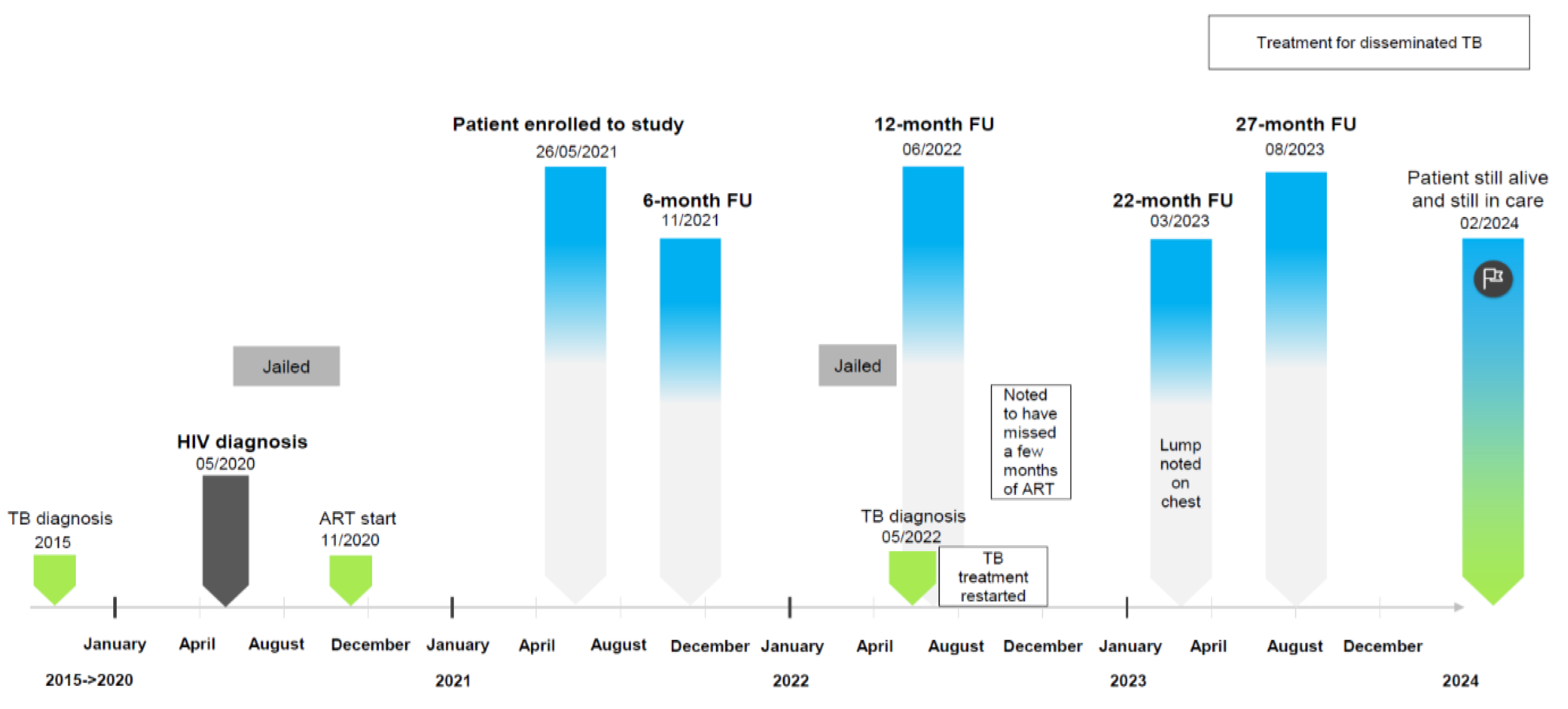
3.1. General Description of the Case in the Context of Routine HIV Clinical Care
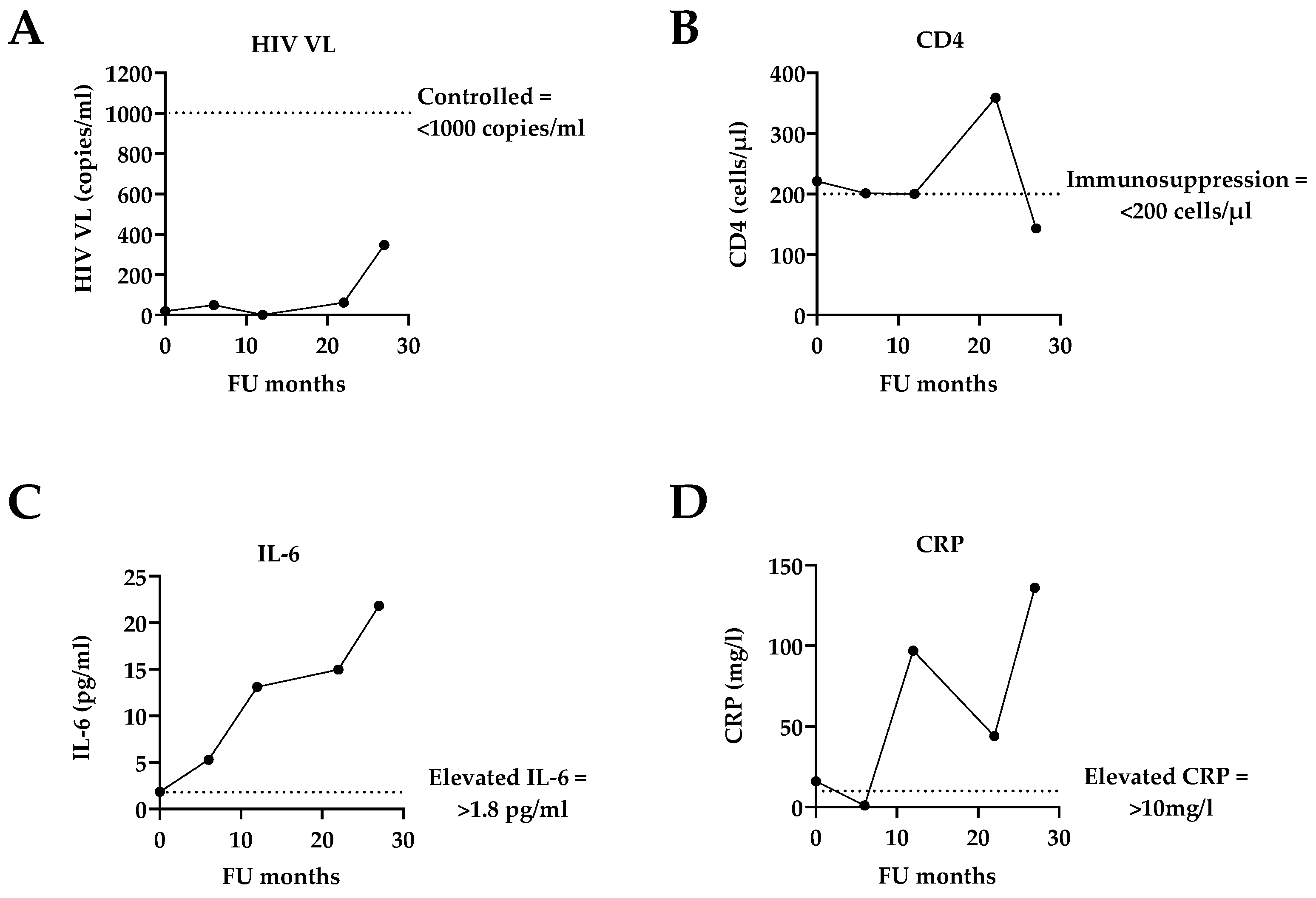
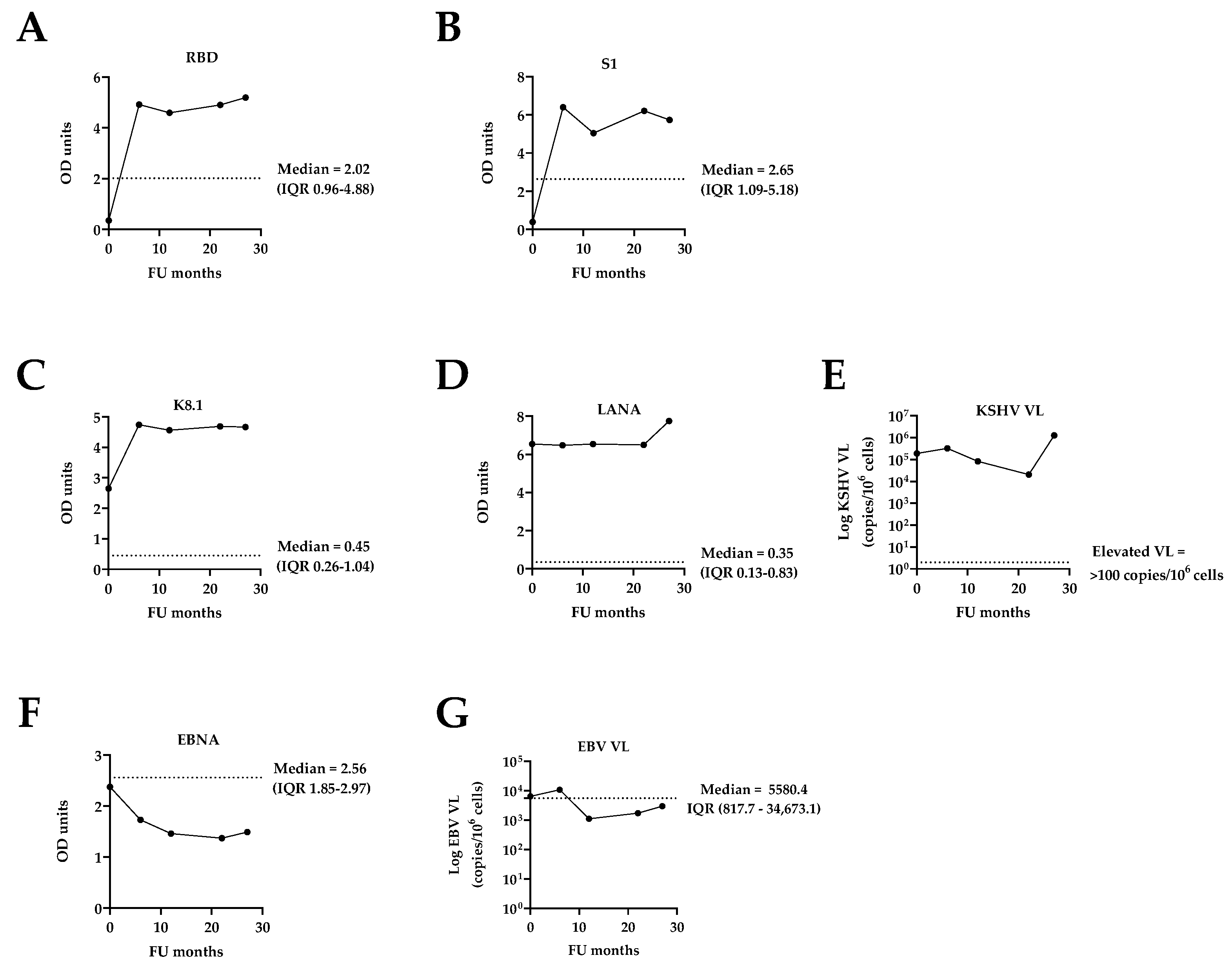
3.2. Assessment of Co-Infections: SARS-CoV-2, KSHV, and EBV
4. Discussion
5. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesarman, E.; Chadburn, A.; Rubinstein, P.G. KSHV/HHV8-mediated hematologic diseases. Blood 2022, 139, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Polizzotto, M.N.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma-associated herpesvirus-associated malignancies: Epidemiology, pathogenesis, and advances in treatment. Semin. Oncol. 2015, 42, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Minhas, V.; Wood, C. Epidemiology and transmission of Kaposi’s sarcoma-associated herpesvirus. Viruses 2014, 6, 4178–4194. [Google Scholar] [CrossRef]
- Chinna, P.; Bratl, K.; Lambarey, H.; Blumenthal, M.J.; Schäfer, G. The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. Int. J. Mol. Sci. 2023, 24, 13066. [Google Scholar] [CrossRef] [PubMed]
- Majaya, E.; Girdler-Brown, B.V.; Muchengeti, M.; Singh, E. The impact of the South African antiretroviral treatment programme on the age-standardised incidence rate of Kaposi sarcoma, 1999-2016: An interrupted time series analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 102, 20–27. [Google Scholar] [CrossRef]
- Motlhale, M.; Sitas, F.; Bradshaw, D.; Chen, W.C.; Singini, M.G.; de Villiers, C.B.; Lewis, C.M.; Muchengeti, M.; Waterboer, T.; Mathew, C.G.; et al. Epidemiology of Kaposi’s sarcoma in sub-Saharan Africa. Cancer Epidemiol. 2022, 78, 102167. [Google Scholar] [CrossRef]
- Powles, T.; Stebbing, J.; Bazeos, A.; Hatzimichael, E.; Mandalia, S.; Nelson, M.; Gazzard, B.; Bower, M. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann. Oncol. 2009, 20, 775–779. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Uldrick, T.S.; Wyvill, K.M.; Aleman, K.; Marshall, V.; Wang, V.; Whitby, D.; Pittaluga, S.; Jaffe, E.S.; Millo, C.; et al. Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 730–738. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Uldrick, T.S.; Hu, D.; Yarchoan, R. Clinical Manifestations of Kaposi Sarcoma Herpesvirus Lytic Activation: Multicentric Castleman Disease (KSHV-MCD) and the KSHV Inflammatory Cytokine Syndrome. Front. Microbiol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Choi, M.H.; Leung, C.S.; To, P.Y.; Lai, S.W.; Chan, M.C.; Chik, T.S.H.; Lau, D.P.L.; Au, E.Y.L.; Yuen, K.Y.; Hung, F.N.I.; et al. Kaposi Sarcoma Herpesvirus (KSHV) inflammatory cytokine syndrome (KICS): A case study. Res. Sq. 2025. preprint. [Google Scholar] [CrossRef]
- Lambarey, H.; Blumenthal, M.J.; Chetram, A.; Joyimbana, W.; Jennings, L.; Orrell, C.; Schäfer, G. Reactivation of Kaposi’s sarcoma-associated herpesvirus (KSHV) by SARS-CoV-2 in non-hospitalised HIV-infected patients. Ebiomedicine 2024, 100, 104986. [Google Scholar] [CrossRef] [PubMed]
- Labo, N.; Marshall, V.; Miley, W.; Davis, E.; McCann, B.; Stolka, K.B.; Ndom, P.; Hemingway-Foday, J.J.; Abassora, M.; Newton, R.; et al. Mutual detection of Kaposi’s sarcoma-associated herpesvirus and Epstein-Barr virus in blood and saliva of Cameroonians with and without Kaposi’s sarcoma. Int. J. Cancer 2019, 145, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- South African National Department of Health. 2023 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy and Breastfeeding, Adolescents, Children, Infants and Neonates; Version 3; Republic of South Africa National Department of Health: Pretoria, South Africa, 2023.
- Donzel, M.; Bonjour, M.; Combes, J.; Broussais, F.; Sesques, P.; Traverse-Glehen, A. Lymphomas associated with Epstein-Barr virus infection in 2020: Results from a large, unselected case series in France. eClinicalMedicine 2022, 54, 101674. [Google Scholar] [CrossRef] [PubMed]
- Diakite, M.; Shaw-Saliba, K.; Lau, C.-Y. Malignancy and viral infections in Sub-Saharan Africa: A review. Front. Virol. 2023, 3, 1103737. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein–Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef]
- Ichikawa, A.; Arakawa, F.; Kiyasu, J.; Sato, K.; Miyoshi, H.; Niino, D.; Kimura, Y.; Takeuchi, M.; Yoshida, M.; Ishibashi, Y. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: Histology, Epstein–Barr virus, and clonality are important predictors of disease progression and regression. Eur. J. Haematol. 2013, 91, 20–28. [Google Scholar] [CrossRef]
- Sausen, D.G.; Bhutta, M.S.; Gallo, E.S.; Dahari, H.; Borenstein, R. Stress-Induced Epstein-Barr Virus Reactivation. Biomolecules 2021, 11, 1380. [Google Scholar] [CrossRef]
- Vetsika, E.-K.; Callan, M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev. Mol. Med. 2004, 6, 1–16. [Google Scholar] [CrossRef]
- Blumenthal, M.J.; Lambarey, H.; Chetram, A.; Riou, C.; Wilkinson, R.J.; Schäfer, G. Kaposi’s Sarcoma-Associated Herpesvirus, but Not Epstein-Barr Virus, Co-infection Associates with Coronavirus Disease 2019 Severity and Outcome in South African Patients. Front. Microbiol. 2021, 12, 795555. [Google Scholar] [CrossRef]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.E.; Burrel, S.; Mokrani, D.; Pineton de Chambrun, M.; Luyt, D.; Chommeloux, J.; Guiraud, V.; Bréchot, N.; Schmidt, M.; Hekimian, G.; et al. Herpesviridae lung reactivation and infection in patients with severe COVID-19 or influenza virus pneumonia: A comparative study. Ann. Intensive Care 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Naendrup, J.-H.; Garcia Borrega, J.; Eichenauer, D.A.; Shimabukuro-Vornhagen, A.; Kochanek, M.; Böll, B. Reactivation of EBV and CMV in Severe COVID-19—Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically ill Patients. J. Intensive Care Med. 2021, 37, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, S.; Cassaniti, I.; Novazzi, F.; Fiorina, L.; Piralla, A.; Comolli, G.; Bruno, R.; Maserati, R.; Gulminetti, R.; Novati, S.; et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 104, 315–319. [Google Scholar] [CrossRef]
- Saade, A.; Moratelli, G.; Azoulay, E.; Darmon, M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect. Dis. Now. 2021, 51, 676–679. [Google Scholar] [CrossRef]
- Simonnet, A.; Engelmann, I.; Moreau, A.S.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now. 2021, 51, 296–299. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, S.; Dong, H.; Lv, H.; Teng, X.; Zhang, J.; Wang, T.; Zhang, X.; Qin, Y.; Chai, Y.; et al. Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation. BMC Infect. Dis. 2021, 21, 955. [Google Scholar] [CrossRef]
- Kumwenda, T.; Hodson, D.Z.; Rambiki, K.; Rambiki, E.; Fedoriw, Y.; Tymchuk, C.; Wallrauch, C.; Heller, T.; Painschab, M.S. Diagnosis and Management of Kaposi Sarcoma-Associated Herpesvirus Inflammatory Cytokine Syndrome in Resource-Constrained Settings: A Case Report and an Adapted Case Definition. Trop. Med. Infect. Dis. 2024, 9, 307. [Google Scholar] [CrossRef]
- Blumenthal, M.J.; Schutz, C.; Barr, D.; Locketz, M.; Marshall, V.; Whitby, D.; Katz, A.A.; Uldrick, T.; Meintjes, G.; Schäfer, G. The Contribution of Kaposi’s Sarcoma-Associated Herpesvirus to Mortality in Hospitalized Human Immunodeficiency Virus-Infected Patients Being Investigated for Tuberculosis in South Africa. J. Infect. Dis. 2019, 220, 841–851. [Google Scholar] [CrossRef]
- Tibenderana, R.M.; Blumenthal, M.J.; Bukajumbe, E.; Schäfer, G.; Mohamed, Z. Clinical Significance of Elevated KSHV Viral Load in HIV-Related Kaposi’s Sarcoma Patients in South Africa. Viruses 2024, 16, 189. [Google Scholar] [CrossRef]
| FU Month | 0 | 6 | 12 | 22 | 27 | |
|---|---|---|---|---|---|---|
| Symptoms at presentation (self-reported) | None | Respiratory symptoms, coughing, weight loss, night sweats, arthralgia, myalgia | Fatigue, oedema, cachexia, respiratory symptoms, coughing, gastrointestinal disturbance, altered mental state, weight loss, night sweats, neuropathy, radiographic abnormalities, arthralgia, myalgia | Oedema, cachexia, gastrointestinal disturbance, weight loss, night sweats, arthralgia, myalgia, neuropathy with pain (post TB treatment) | Respiratory symptoms, coughing, night sweats, arthralgia, myalgia | |
| Weight (kg) | No information | 61.6 | 43 | 54 | 45 | |
| WHO stage | 2 | 2 | 3 | 4 | 4 | |
| ART regimen | TDF/FTC/EFV | TDF/FTC/EFV | TDF/3TC/DTG | ABC/3TC/DTG | ABC/3TC/DTG | |
| TB diagnosis | No | No | Yes | Yes | Yes | |
| COVID-19 vaccination (self-reported) | No | No | No | No | No | |
| Laboratory blood analysis (chemical pathology and haematology): | ||||||
| Sodium (mmol/L) | 135 | 136 | 140 | 138 | 135 | |
| Creatinine (µmol/L) | 74 | 76 | 115 | 175 | 150 | |
| Platelet count (×109/L) | 269 | 184 | 275 | 245 | 162 | |
| Absolute CD4 (cells/µL) | 221 | 201 | 200 | 359 | 143 | |
| Virology (serology and VL): | ||||||
| HIV VL (copies/mL) | <20 | <50 | 1 * | 61 | 347 | |
| SARS-CoV-2 serology (OD) | RBD | 0.35 | 4.92 | 4.59 | 4.90 | 5.19 |
| S1 | 0.39 | 6.40 | 5.05 | 6.21 | 5.74 | |
| KSHV serology (OD) | K8.1 | 2.65 | 4.74 | 4.56 | 4.69 | 4.66 |
| LANA | 6.54 | 6.48 | 6.54 | 6.50 | 7.75 | |
| EBV serology–EBNA (OD units) | 2.38 | 1.73 | 1.46 | 1.37 | 1.49 | |
| KSHV VL (copies/106 cells) | 189,946.3 | 325,019.4 | 83,107.2 | 20,434.6 | 1,278,350.5 | |
| EBV VL (copies/106 cells) | 6450.0 | 10,800.0 | 1120.0 | 1730.0 | 3000.0 | |
| Inflammatory markers: | ||||||
| IL-6 (pg/mL) | 1.96 | 5.28 | 13.11 | 14.99 | 21.83 | |
| CRP (mg/L) | 16 | 1 | 97 | 44 | 136 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambarey, H.; Blumenthal, M.J.; Chinna, P.; Naude, V.N.; Jennings, L.; Orrell, C.; Schäfer, G. A Case of Persistent KSHV Viremia in the Context of HIV, SARS-CoV-2, and Other Co-Infections. Trop. Med. Infect. Dis. 2025, 10, 53. https://doi.org/10.3390/tropicalmed10020053
Lambarey H, Blumenthal MJ, Chinna P, Naude VN, Jennings L, Orrell C, Schäfer G. A Case of Persistent KSHV Viremia in the Context of HIV, SARS-CoV-2, and Other Co-Infections. Tropical Medicine and Infectious Disease. 2025; 10(2):53. https://doi.org/10.3390/tropicalmed10020053
Chicago/Turabian StyleLambarey, Humaira, Melissa J. Blumenthal, Prishanta Chinna, Vincent N. Naude, Lauren Jennings, Catherine Orrell, and Georgia Schäfer. 2025. "A Case of Persistent KSHV Viremia in the Context of HIV, SARS-CoV-2, and Other Co-Infections" Tropical Medicine and Infectious Disease 10, no. 2: 53. https://doi.org/10.3390/tropicalmed10020053
APA StyleLambarey, H., Blumenthal, M. J., Chinna, P., Naude, V. N., Jennings, L., Orrell, C., & Schäfer, G. (2025). A Case of Persistent KSHV Viremia in the Context of HIV, SARS-CoV-2, and Other Co-Infections. Tropical Medicine and Infectious Disease, 10(2), 53. https://doi.org/10.3390/tropicalmed10020053








