Climate, Interventions, and Malaria Outcomes in a Warming World: Towards Climate-Smart Malaria Control in Kenya
Abstract
1. Introduction
2. Main Text
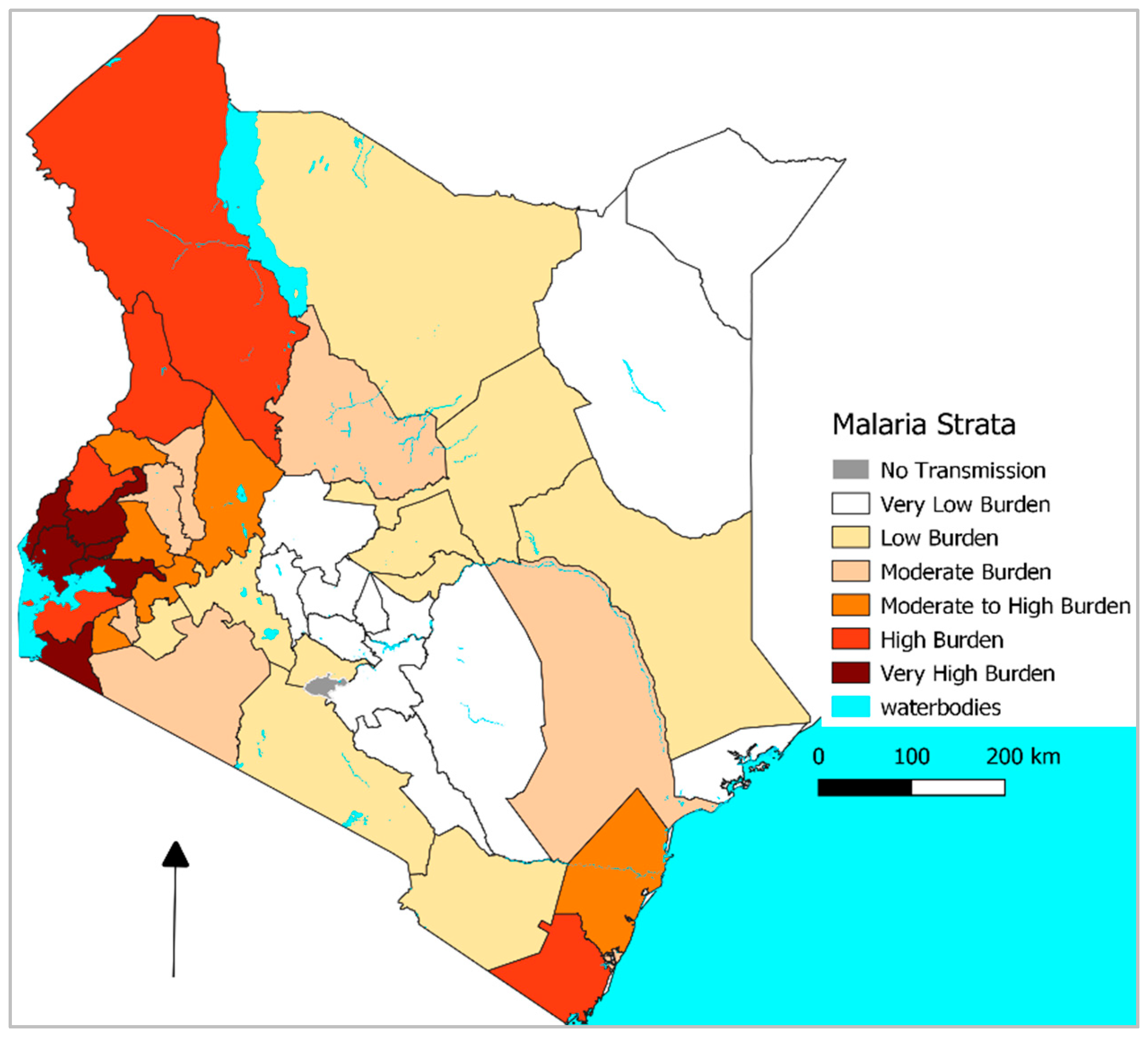
What the Recent Evidence Shows
3. Mechanisms and Interactions
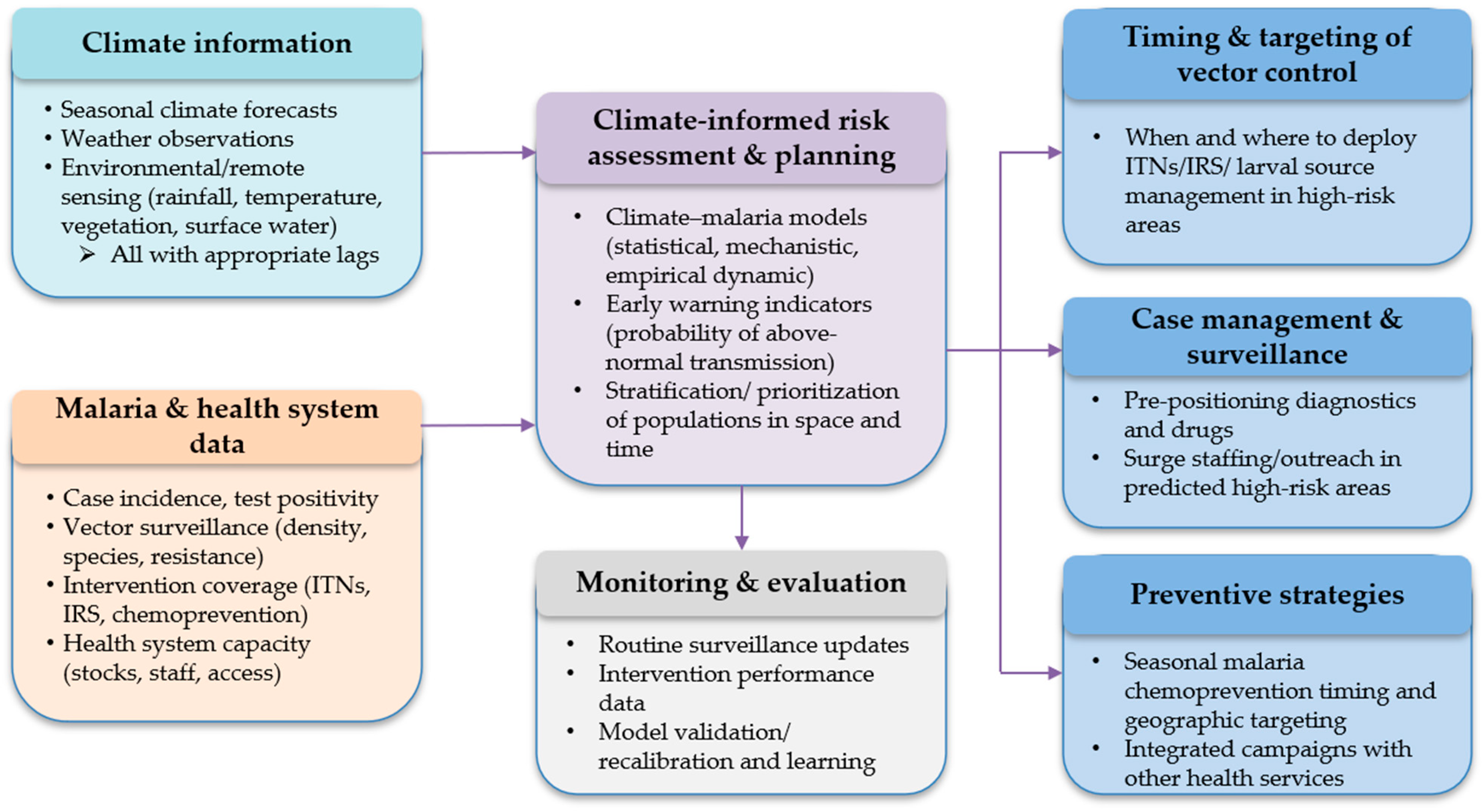
4. Policy and Practice: A Climate-Smart Agenda for Kenya
5. Limitations of the Current Evidence
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 21 September 2025).
- Ministry of Health Kenya. Kenya Malaria Strategy 2019–2023; Ministry of Health Kenya: Nairobi, Kenya, 2019.
- Nyawanda, B.O.; Khagayi, S.; Ochomo, E.; Bigogo, G.; Kariuki, S.; Munga, S.; Vounatsou, P. The Influence of Malaria Control Interventions and Climate Variability on Changes in the Geographical Distribution of Parasite Prevalence in Kenya between 2015 and 2020. Int. J. Health Geogr. 2024, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Alegana, V.A.; Macharia, P.M.; Muchiri, S.; Mumo, E.; Oyugi, E.; Kamau, A.; Chacky, F.; Thawer, S.; Molteni, F.; Rutazanna, D.; et al. Plasmodium falciparum Parasite Prevalence in East Africa: Updating Data for Malaria Stratification. PLoS Glob. Public Health 2021, 1, e0000014. [Google Scholar] [CrossRef] [PubMed]
- Macharia, P.M.; Giorgi, E.; Noor, A.M.; Waqo, E.; Kiptui, R.; Okiro, E.A.; Snow, R.W. Spatio-Temporal Analysis of Plasmodium falciparum Prevalence to Understand the Past and Chart the Future of Malaria Control in Kenya. Malar. J. 2018, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Kenya. Kenya Malaria Strategy 2023–2027; Ministry of Health Kenya: Nairobi, Kenya, 2024.
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal Biology of Mosquito-Borne Disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef]
- Matsushita, N.; Kim, Y.; Ng, C.F.S.; Moriyama, M.; Igarashi, T.; Yamamoto, K.; Otieno, W.; Minakawa, N.; Hashizume, M. Differences of Rainfall-Malaria Associations in Lowland and Highland in Western Kenya. Int. J. Environ. Res. Public Health 2019, 16, 3693. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Wainwright, C.M.; Marsham, J.H.; Keane, R.J.; Rowell, D.P.; Finney, D.L.; Black, E.; Allan, R.P. ‘Eastern African Paradox’ Rainfall Decline Due to Shorter Not Less Intense Long Rains. npj Clim. Atmos. Sci. 2019, 2, 34. [Google Scholar] [CrossRef]
- Ongoma, V.; Chen, H.; Gao, C. Projected Changes in Mean Rainfall and Temperature over East Africa Based on CMIP5 Models. Int. J. Climatol. 2018, 38, 1375–1392. [Google Scholar] [CrossRef]
- Nyawanda, B.O.; Kariuki, S.; Khagayi, S.; Bigogo, G.; Danquah, I.; Munga, S.; Vounatsou, P. Forecasting Malaria Dynamics Based on Causal Relations between Control Interventions, Climatic Factors, and Disease Incidence in Western Kenya. J. Glob. Health 2024, 14, 04208. [Google Scholar] [CrossRef]
- Nissan, H.; Ukawuba, I.; Thomson, M. Climate-Proofing a Malaria Eradication Strategy. Malar. J. 2021, 20, 190. [Google Scholar] [CrossRef]
- Blanford, J.I.; Kioko, C. A Multidimensional Space-Time Geospatial Analysis for Examining the Spatial Trends of Vector-Borne Diseases: 20 Years of Malaria in Kenya. Acta Trop. 2025, 271, 107839. [Google Scholar] [CrossRef]
- Samake, J.N.; Athinya, D.K.; Milanoi, S.; Ramaita, E.; Muchoki, M.; Omondi, S.; Abong’o, B.; Matoke-Muhia, D.; Mbogo, C.; Keitany, K.; et al. Spatial Distribution and Population Structure of the Invasive Anopheles stephensi in Kenya from 2022 to 2024. Sci. Rep. 2025, 15, 19878. [Google Scholar] [CrossRef]
- Kabaria, C.W.; Gilbert, M.; Noor, A.M.; Snow, R.W.; Linard, C. The Impact of Urbanization and Population Density on Childhood Plasmodium falciparum Parasite Prevalence Rates in Africa. Malar. J. 2017, 16, 49. [Google Scholar] [CrossRef]
- Nyawanda, B.O.; Beloconi, A.; Khagayi, S.; Bigogo, G.; Obor, D.; Otieno, N.A.; Lange, S.; Franke, J.; Sauerborn, R.; Utzinger, J.; et al. The Relative Effect of Climate Variability on Malaria Incidence after Scale-up of Interventions in Western Kenya: A Time-Series Analysis of Monthly Incidence Data from 2008 to 2019. Parasite Epidemiol. Control 2023, 21, e00297. [Google Scholar] [CrossRef]
- Tariq, A.; Bisanzio, D.; Mutuku, F.; Ndenga, B.; Jembe, Z.; Maina, P.; Chebii, P.; Ronga, C.; Okuta, V.; LaBeaud, A.D. Modelling the Effects of Precipitation and Temperature on Malaria Incidence in Coastal and Western Kenya. Malar. J. 2025, 24, 208. [Google Scholar] [CrossRef] [PubMed]
- Sewe, M.; Rocklöv, J.; Williamson, J.; Hamel, M.; Nyaguara, A.; Odhiambo, F.; Laserson, K. The Association of Weather Variability and Under Five Malaria Mortality in KEMRI/CDC HDSS in Western Kenya 2003 to 2008: A Time Series Analysis. Int. J. Environ. Res. Public Health 2015, 12, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Nyawanda, B.O.; Khagayi, S.; Obor, D.; Odhiambo, S.B.; Beloconi, A.; Otieno, N.A.; Bigogo, G.; Kariuki, S.; Munga, S.; Vounatsou, P. The Effects of Climatic and Non-Climatic Factors on Malaria Mortality at Different Spatial Scales in Western Kenya, 2008–2019. BMJ Glob. Health 2024, 9, e014614. [Google Scholar] [CrossRef] [PubMed]
- Beloconi, A.; Nyawanda, B.O.; Bigogo, G.; Khagayi, S.; Obor, D.; Danquah, I.; Kariuki, S.; Munga, S.; Vounatsou, P. Malaria, Climate Variability, and Interventions: Modelling Transmission Dynamics. Sci. Rep. 2023, 13, 7367. [Google Scholar] [CrossRef]
- Njotto, L.L.; Senyoni, W.; Cronie, O.; Stensgaard, A.-S. Bayesian Spatio-Temporal Modeling and Prediction of Malaria Cases in Tanzania Mainland (2016–2023): Unveiling Associations with Climate and Intervention Factors. Int. J. Health Geogr. 2025, 24, 20. [Google Scholar] [CrossRef]
- Gbaguidi, G.J.; Topanou, N.; Filho, W.L.; Begedou, K.; Ketoh, G.K. Environmental and Socio-Economic Determinants of Malaria Transmission in West Africa: A Systematic Review. One Health Outlook 2025, 7, 47. [Google Scholar] [CrossRef]
- Ye, H.; Beamish, R.J.; Glaser, S.M.; Grant, S.C.H.; Hsieh, C.; Richards, L.J.; Schnute, J.T.; Sugihara, G. Equation-Free Mechanistic Ecosystem Forecasting Using Empirical Dynamic Modeling. Proc. Natl. Acad. Sci. USA 2015, 112, E1569–E1576. [Google Scholar] [CrossRef]
- Sewe, M.O.; Tozan, Y.; Ahlm, C.; Rocklöv, J. Using Remote Sensing Environmental Data to Forecast Malaria Incidence at a Rural District Hospital in Western Kenya. Sci. Rep. 2017, 7, 2589. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.L.M.; Whitehead, S.A.; Thomas, M.B. Quantifying the Effects of Temperature on Mosquito and Parasite Traits That Determine the Transmission Potential of Human Malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef] [PubMed]
- Division of National Malaria Programme. Kenya Malaria Indicator Survey 2020; Division of National Malaria Programme: Nairobi, Kenya, 2021.
- Alegana, V.A.; Maina, J.; Ouma, P.O.; Macharia, P.M.; Wright, J.; Atkinson, P.M.; Okiro, E.A.; Snow, R.W.; Tatem, A.J. National and Sub-National Variation in Patterns of Febrile Case Management in Sub-Saharan Africa. Nat. Commun. 2018, 9, 4994. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyawanda, B.O.; Ochomo, E.; Otieno, J.D.; Keitany, K.; Machini, B.K.; Vounatsou, P. Climate, Interventions, and Malaria Outcomes in a Warming World: Towards Climate-Smart Malaria Control in Kenya. Trop. Med. Infect. Dis. 2025, 10, 335. https://doi.org/10.3390/tropicalmed10120335
Nyawanda BO, Ochomo E, Otieno JD, Keitany K, Machini BK, Vounatsou P. Climate, Interventions, and Malaria Outcomes in a Warming World: Towards Climate-Smart Malaria Control in Kenya. Tropical Medicine and Infectious Disease. 2025; 10(12):335. https://doi.org/10.3390/tropicalmed10120335
Chicago/Turabian StyleNyawanda, Bryan O., Eric Ochomo, James D. Otieno, Kibor Keitany, Beatrice K. Machini, and Penelope Vounatsou. 2025. "Climate, Interventions, and Malaria Outcomes in a Warming World: Towards Climate-Smart Malaria Control in Kenya" Tropical Medicine and Infectious Disease 10, no. 12: 335. https://doi.org/10.3390/tropicalmed10120335
APA StyleNyawanda, B. O., Ochomo, E., Otieno, J. D., Keitany, K., Machini, B. K., & Vounatsou, P. (2025). Climate, Interventions, and Malaria Outcomes in a Warming World: Towards Climate-Smart Malaria Control in Kenya. Tropical Medicine and Infectious Disease, 10(12), 335. https://doi.org/10.3390/tropicalmed10120335





