A Brief History of the Use of Insecticides in Brazil to Control Vector-Borne Diseases, and Implications for Insecticide Resistance
Abstract
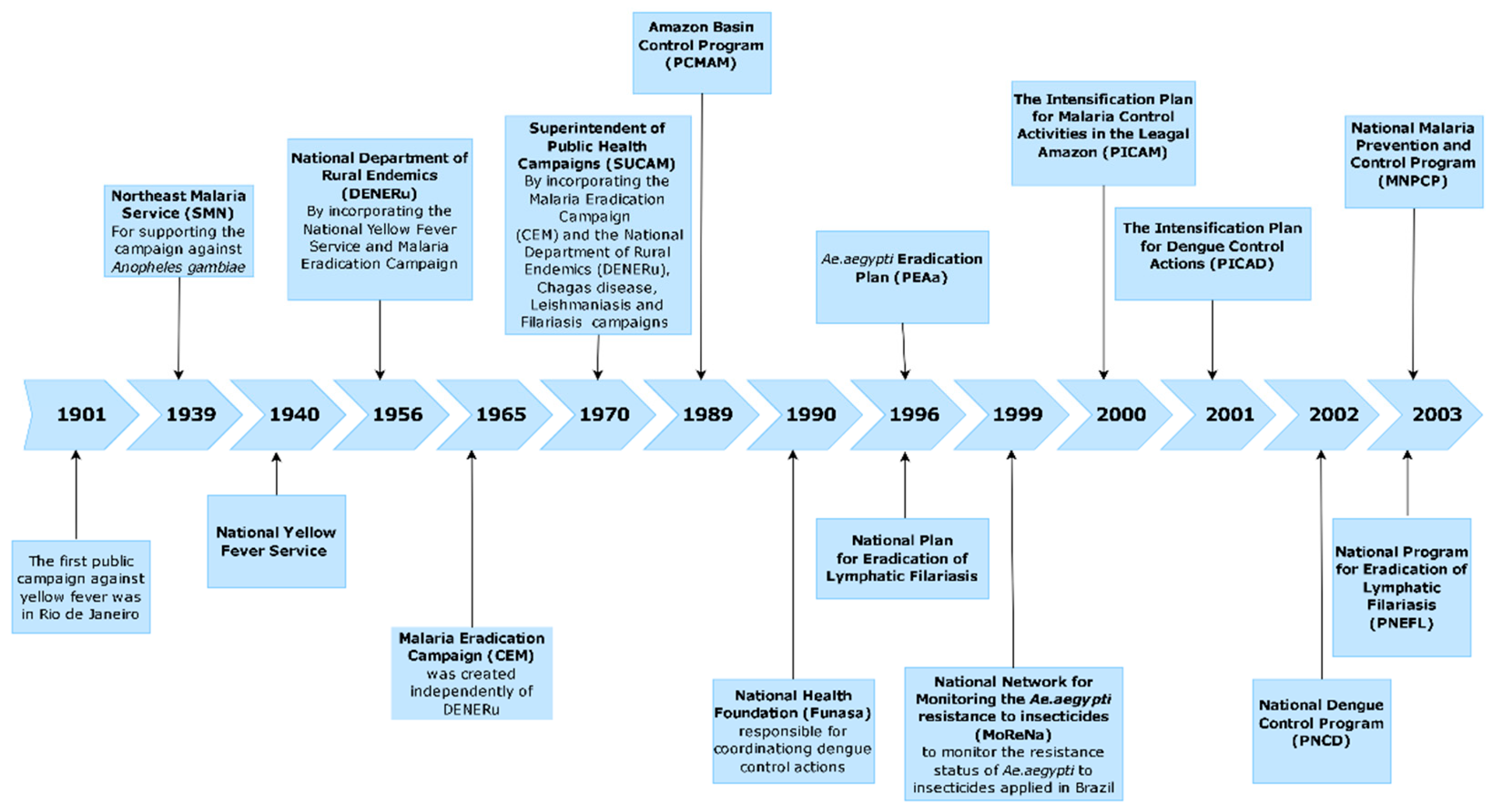
1. Introduction
2. Materials and Methods
3. Results
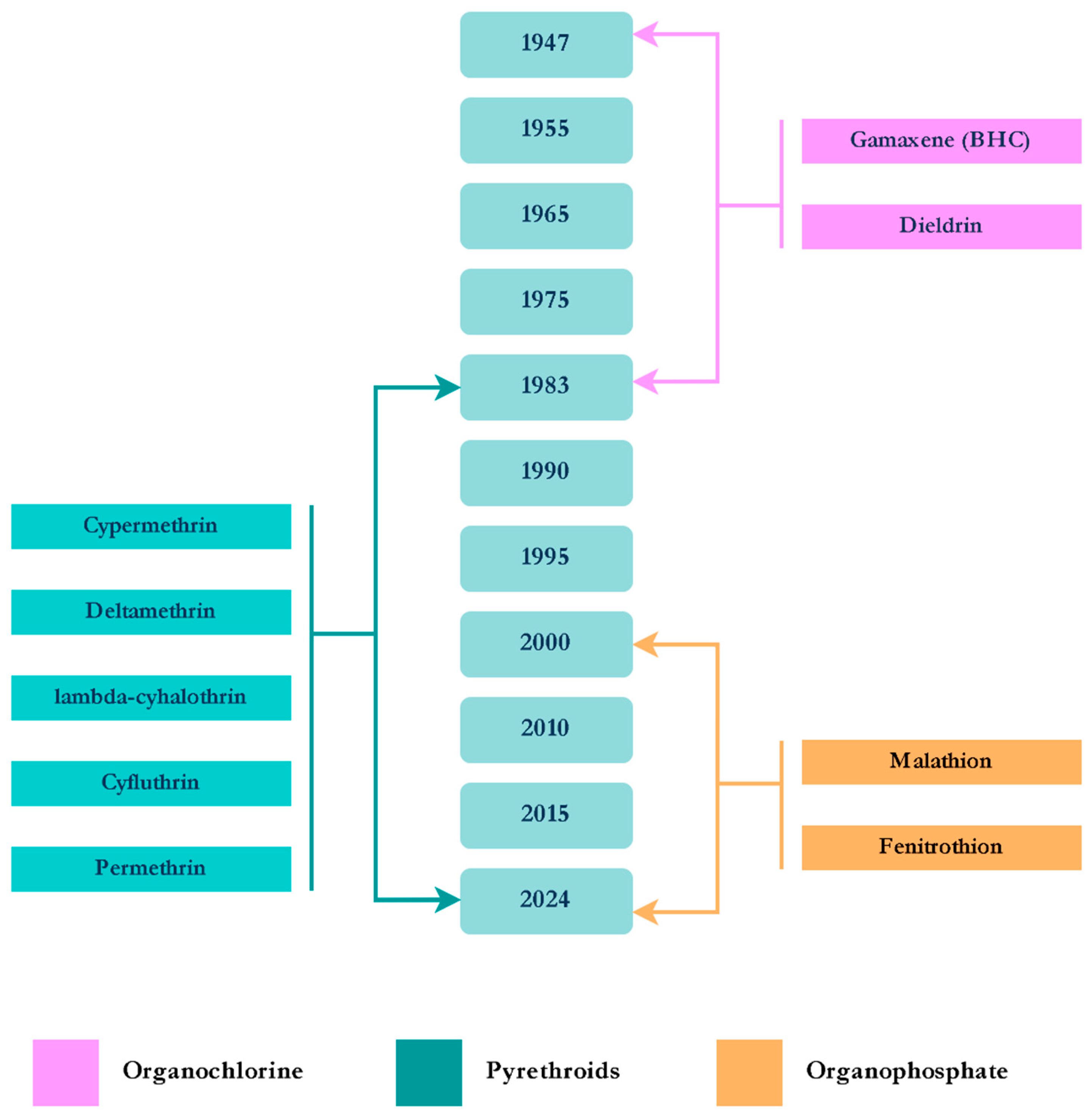
3.1. Aedes aegypti Linnaeus, 1762
3.2. Other Vector Species of Medical Importance
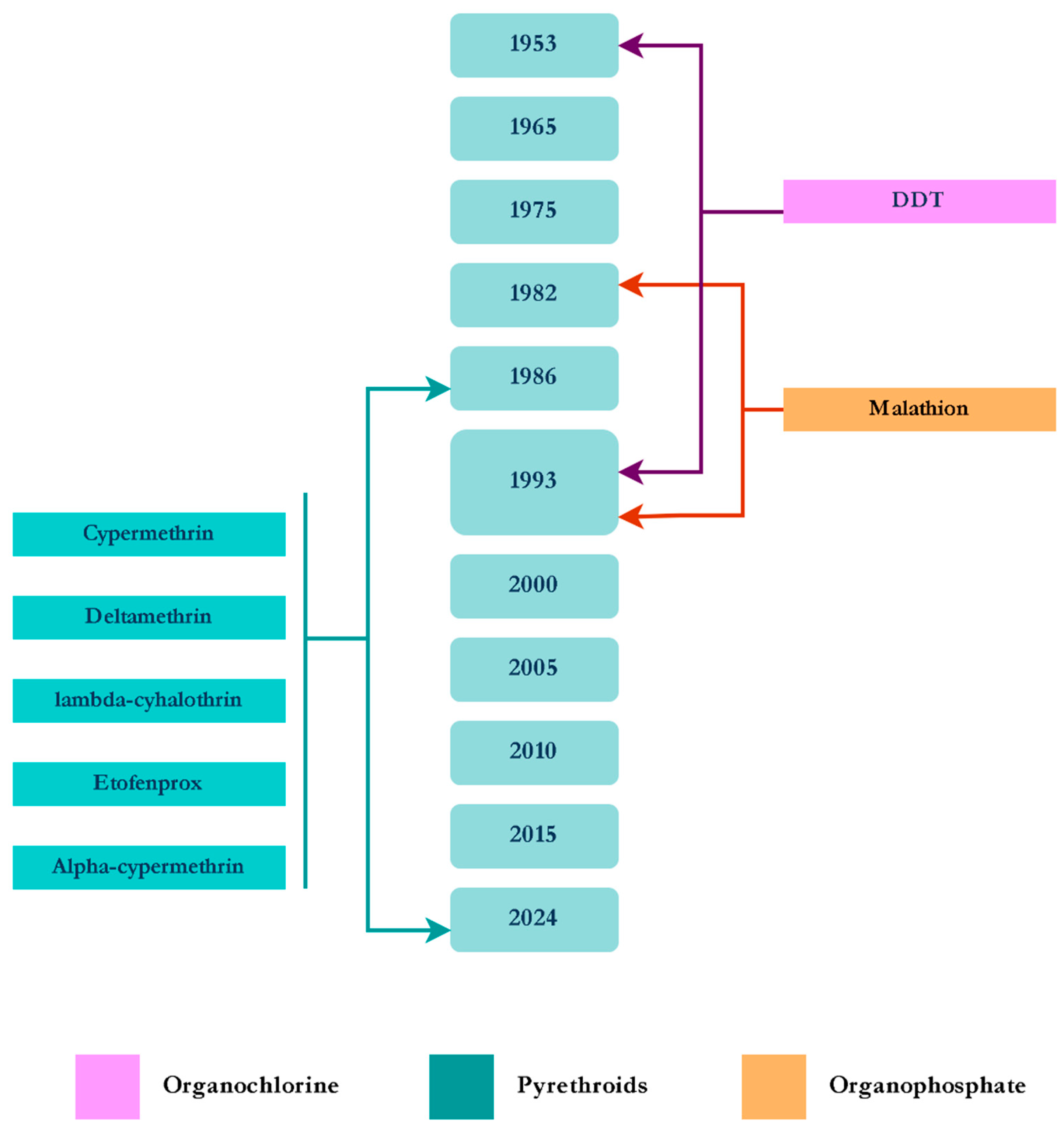
3.2.1. Anopheles spp.
3.2.2. Culex quinquefasciatus Say, 1823
3.2.3. Triatomines
3.2.4. Phlebotomines
4. Discussion and Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHC | Benzene Hexachloride |
| BTi | Bacillus thuringiensis israelensis |
| CCl4 | Carbon Tetrachloride |
| CL | Cutaneous Leishmaniasis |
| CSI | Chitin Synthesis Inhibitor |
| DDT | Dichloro-Diphenyl-Trichloroethane |
| DEC | Diethylcarbamazine |
| DENERu | Departamento Nacional de Endemias Rurais (Brazilian National Department for Rural Endemics) |
| IGR | Insect Growth Regulator |
| ITN | Insecticide-Treated Net |
| IVM | Ivermectin |
| JHA | Juvenile Hormone Analogue |
| LF | Lymphatic Filariasis |
| Lsp | Lysinibacillus sphaericus |
| MoReNAa | Monitoramento da Resistência de Aedes aegypti a Inseticidas (Brazilian insecticide resistance monitoring network) |
| MoH | Ministry of Health |
| OP | Organophosphate |
| ORS | Outdoor Residual Spraying |
| PAHO | Pan American Health Organization |
| PNCD | Programa Nacional de Controle da Dengue (Brazilian National Dengue Control Program) |
| PY | Pyrethroids |
| ULV | Ultra-Low Volume |
| VBD | Vector-Borne Disease |
| VL | Visceral Leishmaniasis |
| WHO | World Health Organization |
| YF | Yellow Fever |
References
- Golding, N.; Wilson, A.L.; Moyes, C.L.; Cano, J.; Pigott, D.M.; Velayudhan, R.; Brooker, S.J.; Smith, D.L.; Hay, S.I.; Lindsay, S.W. Integrating Vector Control across Diseases. BMC Med. 2015, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Files, M.A.; Hansen, C.A.; Herrera, V.C.; Schindewolf, C.; Barrett, A.D.T.; Beasley, D.W.C.; Bourne, N.; Milligan, G.N. Baseline Mapping of Oropouche Virology, Epidemiology, Therapeutics, and Vaccine Research and Development. NPJ Vaccines 2022, 7, 38. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). A Global Strategy to Eliminate Yellow Fever Epidemics (EYE) 2017–2026; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization (WHO). Japanese encephalitis vaccines. Wkly. Epidemiol. Rec. 2006, 81, 331–340. [Google Scholar]
- Torres-Flores, J.M.; Reyes-Sandoval, A.; Salazar, M.I. Dengue Vaccines: An Update. BioDrugs 2022, 36, 325–336. [Google Scholar] [CrossRef]
- Ly, H. Ixchiq (VLA1553): The First FDA-Approved Vaccine to Prevent Disease Caused by Chikungunya Virus Infection. Virulence 2024, 15, 2301573. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Koren, M.A.; Lin, L.; Eckels, K.H.; De La Barrera, R.; Dussupt, V.; Donofrio, G.; Sondergaard, E.L.; Mills, K.T.; Robb, M.L.; Lee, C.; et al. Safety and Immunogenicity of a Purified Inactivated Zika Virus Vaccine Candidate in Adults Primed with a Japanese Encephalitis Virus or Yellow Fever Virus Vaccine in the USA: A Phase 1, Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Lancet Infect. Dis. 2023, 23, 1175–1185. [Google Scholar] [CrossRef]
- Kallás, E.G.; Cintra, M.A.T.; Moreira, J.A.; Patiño, E.G.; Braga, P.E.; Tenório, J.C.V.; Infante, V.; Palacios, R.; de Lacerda, M.V.G.; Batista Pereira, D.; et al. Live, Attenuated, Tetravalent Butantan-Dengue Vaccine in Children and Adults. N. Engl. J. Med. 2024, 390, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.Y.; Yang, S.; Lu, H.Z.; Wang, L.M.; Li, N.; Zhang, H.T.; Xing, S.Y.; Du, Y.N.; Deng, S.Q. A review on Zika vaccine development. Pathog. Dis. 2024, 82, ftad036. [Google Scholar] [CrossRef]
- Murray, A.; Ignaszak, A. Mapping Climate Change-Driven Epidemics. Front. Epidemiol. 2025, 5, 1605058. [Google Scholar] [CrossRef]
- Montenegro, D.; Cortés-Cortés, G.; Balbuena-Alonso, M.G.; Warner, C.; Camps, M. Wolbachia-Based Emerging Strategies for Control of Vector-Transmitted Disease. Acta Trop. 2024, 260, 107410. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Vector-Borne Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 18 September 2025).
- Lima-Camara, T.N. Dengue Is a Product of the Environment: An Approach to the Impacts of the Environment on the Aedes aegypti Mosquito and Disease Cases. Rev. Bras. Epidemiol. 2024, 27, e240048. [Google Scholar] [CrossRef]
- Kerr, J.A. História da Febre-Amarela no Brasil. Am. J. Trop. Med. Hyg. 1970, 19, 891–894. [Google Scholar] [CrossRef]
- Fraga, C. Sobre o Surto Epidêmico de Febre Amarela no Rio de. Janeiro. Boletín De La Oficina Sanit. Panam. 1928, 7. [Google Scholar]
- World Health Organization (WHO). DDT and Its Use in Malaria Control; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Pan American Health Organization (PAHO). Topic 21: Status of Aedes aegypti Eradication in the Americas. In Proceedings of the 15th Pan American Sanitary Conference, San Juan, Puerto Rico, 21 September–3 October 1958; Available online: https://iris.paho.org/handle/10665.2/29106 (accessed on 18 September 2025).
- Brasil Ministério da Saúde. Endemias Rurais: Métodos de Trabalho Adotados pelo DNERu; Departamento Nacional de Endemias Rurais: Brasília, Brazil, 1968. [Google Scholar]
- Benchimol, J.L.; Gualandi, F.C.; Barreto, D.C.S.; Pinheiro, L.A. Leishmanioses: Sua configuração histórica no Brasil, com ênfase na doença visceral nos anos 1930 a 1960. Bol. Mus. Para. Emílio Goeldi Ciênc. Hum. 2019, 14, 611–626. [Google Scholar] [CrossRef]
- Chediak, M.; Pimenta Jr, F.G.; Coelho, G.E.; Braga, I.A.; Lima, J.B.P.; Cavalcante, K.R.L.; de Sousa, L.C.; de Melo-Santos, M.A.V.; Macoris, M.L.G.; de Araújo, A.P.; et al. Spatial and temporal country-wide survey of temephos resistance in Brazilian populations of Aedes aegypti. Mem. Inst. Oswaldo Cruz 2016, 111, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.B.; Martins, A.J.; Rodovalho, C.d.M.; Bellinato, D.F.; Dias, L.D.S.; Macoris, M.d.L.d.G.; Andrighetti, M.T.M.; Lima, J.B.P.; Obara, M.T. Assessment of the Susceptibility Status of Aedes aegypti (Diptera: Culicidae) Populations to Pyriproxyfen and Malathion in a Nation-Wide Monitoring of Insecticide Resistance Performed in Brazil from 2017 to 2018. Parasites Vectors 2020, 13, 531. [Google Scholar] [CrossRef]
- Schatzmayr, H.G.; Nogueira, R.M.; Travassos da Rosa, A.P. An Outbreak of Dengue Virus at Rio de Janeiro—1986. Mem. Inst. Oswaldo Cruz 1986, 81, 245–246. [Google Scholar] [CrossRef]
- Macoris, M.; Andrighetti, M.T.; Takaku, L.; Glasser, C.M.; Garbeloto, V.C.; Cirino, V.C. Changes in susceptibility of Aedes aegypti to organophosphates in municipalities in the state of São Paulo, Brazil. Rev. Saude Publica 1999, 33, 521–522. [Google Scholar] [CrossRef]
- da-Cunha, M.P.; Lima, J.B.P.; Brogdon, W.G.; Moya, G.E.; Valle, D. Monitoring of Resistance to the Pyrethroid Cypermethrin in Brazilian Aedes aegypti (Diptera: Culicidae) Populations Collected between 2001 and 2003. Mem. Inst. Oswaldo Cruz 2005, 100, 441–444. [Google Scholar] [CrossRef]
- Montella, I.R.; Martins, A.J.; Viana-Medeiros, P.F.; Lima, J.B.; Braga, I.A.; Valle, D. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am. J. Trop. Med. Hyg. 2007, 77, 467–477. [Google Scholar] [CrossRef]
- Lima, J.B.P. Aedes Aegypti e Anopheles Neotropicais, Vetores de Importāncia Médica no Brasil: Aspectos Básicos de Biologia e Controle. Ph.D. Thesis, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, Brazil, 2003. [Google Scholar]
- Braga, I.A.; Valle, D. Aedes aegypti: Vigilância, Monitoramento da Resistência e Alternativas de Controle no Brasil. Epidemiol. Serv. Saude 2007, 16, 295–302. [Google Scholar] [CrossRef]
- Mazzari, M.B.; Georghiou, G.P. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J. Am. Mosq. Control Assoc. 1995, 11, 315–322. [Google Scholar]
- Melo-Santos, M.A.V.; Varjal-Melo, J.J.M.; Araújo, A.P.; Gomes, T.C.; Paiva, M.H.; Regis, L.N.; Furtado, A.F.; Magalhaes, T.; Macoris, M.L.; Andrighetti, M.T.; et al. Resistance to the organophosphate temephos: Mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop. 2010, 113, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Menezes, H.S.G.; Oliveira, L.H.G.; Araújo, A.P.; Carvalho, K.S.; Silva-Filha, M.H.N.L. Aedes aegypti strain selected with Bacillus thuringiensis var. israelensis larvicide for 50 generations remains susceptible and exhibited increased fitness. Parasites Vectors 2025, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Rique, H.L.; Menezes, H.S.G.; Melo-Santos, M.A.V.; Silva-Filha, M.H.N.L. Evaluation of a long-lasting microbial larvicide against Culex quinquefasciatus and Aedes aegypti under laboratory and semi-field trial conditions. Parasites Vectors 2024, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Bellinato, D.F.; Viana-Medeiros, P.F.; Araújo, S.C.; Martins, A.J.; Lima, J.B.P.; Valle, D. Resistance Status to the Insecticides Temephos, Deltamethrin, and Diflubenzuron in Brazilian Aedes aegypti Populations. Biomed Res. Int. 2016, 2016, 8603263. [Google Scholar] [CrossRef]
- Amorim, L.B.; Helvecio, E.; de Oliveira, C.M.F.; Ayres, C.F.J. Susceptibility Status of Culex Quinquefasciatus (Diptera: Culicidae) Populations to the Chemical Insecticide Temephos in Pernambuco, Brazil. Pest Manag. Sci. 2013, 69, 1307–1314. [Google Scholar] [CrossRef]
- Araújo, A.P.; Araujo Diniz, D.F.; Helvecio, E.; de Barros, R.A.; de Oliveira, C.M.F.; Ayres, C.F.J.; de Melo-Santos, M.A.V.; Regis, L.N.; Silva-Filha, M.H.N.L. The Susceptibility of Aedes aegypti Populations Displaying Temephos Resistance to Bacillus thuringiensis israelensis: A Basis for Management. Parasit. Vectors 2013, 6, 297. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Imunização e Doenças Transmissíveis, Coordenação-Geral de Vigilância de Arboviroses. Nota Informativa Nº 103/2019-CGARB/DEIDT/SVS/MS: Recomendações Para Manejo da Resistência de Aedes aegypti a Inseticidas; Brasil Ministério da Saúde: Brasília, Brazil, 2019. [Google Scholar]
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Martins Junior, A. de J. Resistance to Temephos and Deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem. Inst. Oswaldo Cruz 2019, 114, e180544. [Google Scholar] [CrossRef]
- Dias, L.D.S.; Martins, A.J.; Rodovalho, C.d.M.; Bellinato, D.F.; de Ázara, T.M.F.; do Nascimento, A.M.R.; Corbel, V.; Macoris, M.d.L.d.G.; Andrighetti, M.T.M.; Lima, J.B.P. Susceptibility of Aedes aegypti to Spinosad Larvicide and Space Spray Adulticides in Brazil. Mem. Inst. Oswaldo Cruz 2025, 120, e240270. [Google Scholar] [CrossRef]
- Garcia, G.A.; Hoffmann, A.A.; Maciel-de-Freitas, R.; Villela, D.A.M. Aedes aegypti Insecticide Resistance Underlies the Success (and Failure) of Wolbachia Population Replacement. Sci. Rep. 2020, 10, 63. [Google Scholar] [CrossRef]
- Carlos, B.C.; Rona, L.D.P.; Christophides, G.K.; Souza-Neto, J.A. A Comprehensive Analysis of Malaria Transmission in Brazil. Pathog. Glob. Health 2019, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Deane, L.M. Malaria Studies and Control in Brazil. Am. J. Trop. Med. Hyg. 1988, 38, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, J.L.; Sá, M.R. (Eds.) Adolpho Lutz e a Entomologia Médica no Brasil = Adolpho Lutz Medical Entomology in Brazil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 2006; Volume 2, Book 3; 508p, ISBN 85-7541-043-1. [Google Scholar]
- Chagas, C. Luta Contra a Malária: Conferencia Proferida no Núcleo Colonial São Bento. April 1933. Available online: https://arca.fiocruz.br/items/9fc7967f-2232-447e-b283-93f86c877984/full (accessed on 6 October 2025).
- Soper, F.L. Paris Green in the Eradication of Anopheles Gambiae: Brazil; Biodiversity Heritage Library: Alexandria, Egypt, 1940. [Google Scholar]
- Baia-da-Silva, D.C.; Brito-Sousa, J.D.; Rodovalho, S.R.; Peterka, C.; Moresco, G.; Lapouble, O.M.M.; Melo, G.C.d.; Sampaio, V.d.S.; Alecrim, M.d.G.C.; Pimenta, P.; et al. Current Vector Control Challenges in the Fight against Malaria in Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180542. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.A.; Lima, J. O Uso de Cortinas Impregnadas com Deltametrina no Controle da Malária em Garimpos no Território Federal do Amapá: Nota Prévia. Rev. Bras. Malariol. Doencas. Trop. 1986, 38, 137–139. [Google Scholar]
- Salgado-Cavalcante, E.T.; Tadei, W.P.; Pinto, C.T.; Xavier, P.A.; Lima, I.E.N.S. Efeitos da Ação Residual da Deltametrina, em Cortinas de Ráfia e Sarrapilha no Controle da Malária, em áreas de Garimpo, no Estado do Amapá. Rev. Da Soc. Bras. De Med. Trop. 1992, 25, 6–7. [Google Scholar]
- Santos, J.B.; Santos, F.d.; Macêdo, V. Variação da Densidade Anofélica com o Uso de Mosquiteiros Impregnados com Deltametrina em uma área Endêmica de Malária na Amazônia Brasileira. Cad. Saude Publica 1999, 15, 281–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, R.L.C.; Fayal, A.S.; Aguiar, A.E.F.; Vieira, D.B.R.; Póvoa, M.M. Avaliação do efeito residual de piretróides sobre anofelinos da Amazônia brasileira. Rev. Saúde Pública 2007, 41, 276–283. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Guia para Gestão Local do Controle da Malária; Brasil Ministério da Saúde: Brasília, Brazil, 2009; p. 60. [Google Scholar]
- Galardo, A.K.R.; Zimmerman, R.; Galardo, C.D. Larval Control of Anopheles (Nyssorhinchus) darlingi using Granular Formulation of Bacillus sphaericus in Abandoned Gold-Miners Excavation Pools in the Brazilian Amazon Rainforest. Rev. Soc. Bras. Med. Trop. 2013, 46, 172–177. [Google Scholar] [CrossRef][Green Version]
- Vieira, G.D.D.; Basano, S.D.A.; Katsuragawa, T.H.; Camargo, L.M.A. Insecticide-Treated Bed Nets in Rondônia, Brazil: Evaluation of Their Impact on Malaria Control. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 493–497. [Google Scholar] [CrossRef]
- da Silva Ferreira Lima, A.C.; Galardo, A.K.R.; Müller, J.N.; de Andrade Corrêa, A.P.S.; Ribeiro, K.A.N.; Silveira, G.A.; Hijjar, A.V.; Soares da Roch Bauzer, L.G.; Lima, J.B.P. Evaluation of Long-Lasting Insecticidal Nets (LLINs) for Malaria Control in an Endemic Area in Brazil. Parasites Vectors 2023, 16, 162. [Google Scholar] [CrossRef]
- Brasil Conselho Nacional de Secretários de Saúde. Nota Técnica nº 46/2011: Projeto de Expansão do Acesso às Medidas de Prevenção e Controle da Malária; Brasil Conselho Nacional de Secretários de Saúde: Brasília, Brazil, 2011. [Google Scholar]
- Galardo, A.K.R.; Póvoa, M.M.; Sucupira, I.M.C.; Galardo, C.D.; Santos, R.L.C.D. Anopheles darlingi and Anopheles marajoara (Diptera: Culicidae) Susceptibility to Pyrethroids in an Endemic Area of the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2015, 48, 765–769. [Google Scholar] [CrossRef]
- Corrêa, A.P.S.A. Avaliação Residual de Inseticidas Para o Controle da Malária em Diferentes Superfícies, e do Status de Suscetibilidade. Ph.D. Thesis, Instituto Oswaldo Cruz/Fundação Oswaldo Cruz, Rio de Janeiro, Brazil, 2019; 184p. [Google Scholar]
- Sucupira, I.M.C.; Santos, M.M.M.d.; Póvoa, M.M. Mosquitos anofelinos envolvidos na transmissão da malária humana no município de Cruzeiro do Sul, estado do Acre, Amazônia brasileira. Rev. Panamazonica Saude 2022, 13, e202201224. [Google Scholar] [CrossRef]
- Amorim, Q.S.; Rodovalho, C.M.; Loureiro, A.C.; Serravale, P.; Bellinato, D.F.; Guimarães, P.; Corbel, V.; Martins, A.J.; Lima, J.B.P. First Large-Scale Assessment of Pyrethroid Resistance in Anopheles darlingi (Diptera: Culicidae) in Brazil (2021–2024): A Crucial Step in Informing Decision-Making in Malaria Control. Malar. J. 2025, 24, 155. [Google Scholar] [CrossRef] [PubMed]
- Brasil Ministério da Saúde. Guia de Vigilância Epidemiológica, Caderno 6: Aids, Hepatites Virais, Sífilis Congênita, Sífilis em Gestantes; Brasil. Ministério da Saúde: Brasília, Brazil, 2009. [Google Scholar]
- Hochman, G. From Autonomy to Partial Alignment: National Malaria Programs in the Time of Global Eradication, Brazil, 1941–1961. Can. Bull. Med. Hist. 2008, 25, 161–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Regis, L.; Silva-Filha, M.H.N.L.; Oliveira, C.M.F.d.; Rios, E.M.; Silva, S.B.d.; Furtado, A.F. Integrated Control Measures against Culex quinquefasciatus, the Vector of Filariasis in Recife. Mem. Inst. Oswaldo Cruz 1995, 90, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Regis, L.; Oliveira, C.M.F.; Silva-Filha, M.H.; Silva, S.B.; Maciel, A.; Furtado, A.F. Efficacy of Bacillus sphaericus in Control of the Filariasis Vector Culex quinquefasciatus in an Urban Area of Olinda, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 488–492. [Google Scholar] [CrossRef]
- Santos, E.M.d.M.; Regis, L.N.; Silva-Filha, M.H.N.L.; Barbosa, R.M.R.; Melo-Santos, M.A.V.d.; Gomes, T.C.S.; Oliveira, C.M.F.d. The Effectiveness of a Combined Bacterial Larvicide for Mosquito Control in an Endemic Urban Area in Brazil. Biol. Control 2018, 121, 190–198. [Google Scholar] [CrossRef]
- Bracco, J.E.; Barata, J.M.; Marinotti, O. Evaluation of Insecticide Resistance and Biochemical Mechanisms in a Population of Culex quinquefasciatus (Diptera: Culicidae) from São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 1999, 94, 115–120. [Google Scholar] [CrossRef]
- Andrade, C.F.S.; Campos, J.C.; Cabrini, I.; Marques Filho, C.A.M.; Hibi, S. Suscetibilidade de populações de Culex quinquefasciatus Say (Diptera: Culicidae) sujeitas ao controle com Bacillus sphaericus Neide no rio Pinheiros, São Paulo. BioAssay 2007, 2, 4. [Google Scholar]
- Lopes, R.P.; Lima, J.B.P.; Martins, A.J. Insecticide Resistance in Culex quinquefasciatus Say, 1823 in Brazil: A Review. Parasites Vectors 2019, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filha, M.H.N.L.; de Melo Chalegre, K.D.; Anastacio, D.B.; de Oliveira, C.M.F.; da Silva, S.B.; Acioli, R.V.; Hibi, S.; de Oliveira, D.C.; Parodi, E.S.M.; Filho, C.A.M.M.; et al. Culex quinquefasciatus Field Populations Subjected to Treatment with Bacillus sphaericus Did Not Display High Resistance Levels. Biol. Control 2008, 44, 227–234. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Lymphatic Filariasis Elimination in the Americas. In Proceedings of the Regional LF Elimination Program Managers’ Meeting, Santo Domingo, Dominican Republic, 9–11 August 2000; Pan American Health Organization (PAHO): Washington, DC, USA, 2000. [Google Scholar]
- Rocha, A.; Dos Santos, E.M.; Oliveira, P.; Brandão, E. Histórico das ações de controle da filariose linfática em Olinda, Pernambuco, Brasil. Rev. Patol. Trop. 2016, 45, 339. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Brasil Elimina a Filariose Linfática Como Problema de Saúde Pública. 2024. Available online: https://www.paho.org/pt/noticias/30-9-2024-brasil-elimina-filariose-linfatica-como-problema-saude-publica (accessed on 18 September 2025).
- Brandão, E.; Oliveira, P.; da Silva, M.A.L.; Rocha, A. Brazil Was Certified by the World Health Organization for Having Eliminated Lymphatic filariasis: What Now? Parasites Vectors 2025, 18, 123. [Google Scholar] [CrossRef]
- Coura, J.R.; Dias, J.C.P. Epidemiology, Control and Surveillance of Chagas Disease: 100 Years after Its Discovery. Mem. Inst. Oswaldo Cruz 2009, 104, 31–40. [Google Scholar] [CrossRef]
- Dias, E. Um Ensaio de Profilaxia da Moléstia de Chagas; Impr. Nacional: Rio de Janeiro, Brazil, 1945. [Google Scholar]
- Aragão, M.B.; Souza, S.A. Triatoma infestans Colonizando em Domicílios da Baixada Fluminense, Estado do Rio de Janeiro, Brasil. Rev. Soc. Bras. Med. Trop. 1971, 5, 115–121. [Google Scholar] [CrossRef]
- Dias, J.C.P. Os primórdios do controle da doença de Chagas (em homenagem a Emmanuel Dias, pioneiro do controle, no centenário de seu nascimento). Rev. Soc. Bras. Med. Trop. 2011, 44 (Suppl. S2), 12–18. [Google Scholar] [CrossRef]
- Dias, J.C.P.; Schofield, C.J. The Evolution of Chagas Disease Control after 90 Years since Carlos Chagas’ Discovery. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. S1), 103–121. [Google Scholar] [CrossRef]
- Dias, J.C. Control of Chagas Disease in Brazil. Parasitol. Today 1987, 3, 336–341. [Google Scholar] [CrossRef]
- Dobbin, J.E., Jr.; Cruz, A.E. Some data on the triatominae of Pernambuco. Rev. Bras. Malariol. Doencas Trop. 1966, 18, 261–267. [Google Scholar]
- Garcia-Zapata, M.T.; Marsden, P.D.; Virgens, D.d.; Penna, R.; Soares, V.; Brasil, I.A.d.; Castro, C.N.d.; Prata, A.; Macêdo, V. O Controle Da Transmissão Da Doença de Chagas Em Mambaí—Goiás, Brasil (1982–1984). Rev. Soc. Bras. Med. Trop. 1986, 19, 219–225. [Google Scholar] [CrossRef]
- Schiavi, A.; Lima, A.; Ramos, A.S. A Desinsetização Da área Central Do Estado de São Paulo Visando Vetores Da Moléstia de Chagas. Arq. Hig 1952, 17, 117–121. [Google Scholar]
- Sherlock, I.A.; Muniz, T.M.; Guitton, N. A Ação Do Malathion Sobre Os Ovos de Triatomíneos Vetores de Doença de Chagas. Rev. Soc. Bras. Med. Trop. 1976, 10, 77–84. [Google Scholar] [CrossRef][Green Version]
- Oliveira Filho, A.M. New Alternatives for Chagas’ Disease Control. Mem. Inst. Oswaldo Cruz 1984, 79, 117–123. [Google Scholar] [CrossRef]
- Oliveira Filho, A.M. Development of Insecticide Formulations and Determination of Dosages and Application Schedules to Fit Specific Situations. Rev. Argent. Microbiol. 1988, 20, 39–48. [Google Scholar]
- Oliveira Filho, A.M.; Melo, M.T.V.; Santos, C.E.; Faria Filho, O.F.; Carneiro, F.C.F.; Oliveira-Lima, J.W.; Vieira, J.B.F.; Gadelha, F.V.; Ishihata, J. Tratamentos Focais e Totais com Inseticidas de Ação Residual para o Controle de Triatoma brasiliensis e Triatoma pseudomaculata no Nordeste Brasileiro. Cad. Saude Publica 2000, 16, S105–S111. [Google Scholar] [CrossRef]
- Dias, J.C.P. Evolution of Chagas Disease Screening Programs and Control Programs: Historical Perspective. Glob. Heart 2015, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Diotaiuti, L.; Faria Filho, O.F.; Carneiro, F.C.F.; Dias, J.C.P.; Pires, H.H.R.; Schofield, C.J. Aspectos Operacionais Do Controle Do Triatoma brasiliensis. Cad. Saude Publica 2000, 16, S61–S67. [Google Scholar] [CrossRef]
- Pessoa, G.C.D.; Vinãs, P.A.; Rosa, A.C.L.; Diotaiuti, L. History of Insecticide Resistance of Triatominae Vectors. Rev. Soc. Bras. Med. Trop. 2015, 48, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Filho, J.D.; Reis, A.S.; Monteiro, C.C.; Shimabukuro, P.H.F. Online catalogue of the Coleção de Flebotomíneos (FIOCRUZ/COLFLEB), a biological collection of American sand flies (Diptera: Psychodidae, Phlebotominae) held at Fiocruz Minas, Brazil. GigaByte 2022, 2022, gigabyte52. [Google Scholar] [CrossRef]
- Feliciangeli, M.D. Natural Breeding Places of Phlebotomine Sandflies. Med. Vet. Entomol. 2004, 18, 71–80. [Google Scholar] [CrossRef]
- Teodoro, U.; Silveira, T.G.V.; dos Santos, D.R.; dos Santos, E.S.; dos Santos, A.R.; de Oliveira, O.; Kühl, J.B.; Alberton, D. Influence of rearrangement and cleaning of the peridomiciliary area and building disinsectization on sandfly population density in the municipality of Doutor Camargo, Paraná State, Brazil. Cad. Saude Publica 2003, 19, 1801–1813. [Google Scholar] [CrossRef]
- Nascimento, M.d.D.S.B.; Costa, J.M.L.; Fiori, B.I.P.; Viana, G.M.C.; Filho, M.S.G.; Alvim, A.d.C.; Bastos, O.C.; Nakatani, M.; Reed, S.; Badaró, R.; et al. Aspectos Epidemiológicos Determinantes Na Manutenção Da Leishmaniose Visceral No Estado Do Maranhão—Brasil. Rev. Soc. Bras. Med. Trop. 1996, 29, 233–240. [Google Scholar] [CrossRef]
- Assis, T.M.d.; Azeredo-da-Silva, A.L.F.d.; Cota, G.; Rocha, M.F.; Werneck, G.L. Cost-Effectiveness of a Canine Visceral Leishmaniasis Control Program in Brazil Based on Insecticide-Impregnated Collars. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200680. [Google Scholar] [CrossRef]
- Falcão, A.L.; Falcão, A.R.; Pinto, C.T.; Gontijo, C.M.; Falqueto, A. Effect of Deltamethrin Spraying on the Sandfly Populations in a Focus of American Cutaneous Leishmaniasis. Mem. Inst. Oswaldo Cruz 1991, 86, 399–404. [Google Scholar] [CrossRef]
- Passerat de Silans, L.N.M.; Dedet, J.-P.; Arias, J.R. Field Monitoring of Cypermethrin Residual Effect on the Mortality Rates of the Phlebotomine Sand Fly Lutzomyia longipalpis in the State of Paraíba, Brazil. Mem. Inst. Oswaldo Cruz 1998, 93, 339–344. [Google Scholar] [CrossRef]
- Alves, E.B.; Figueiredo, F.B.; Rocha, M.F.; Castro, M.C.; Werneck, G.L. Effectiveness of Insecticide-Impregnated Collars for the Control of Canine Visceral Leishmaniasis. Prev. Vet. Med. 2020, 182, 105104. [Google Scholar] [CrossRef] [PubMed]
- Brazuna, J.C.M. Estudos Sobre Leishmaniose Visceral Humana E Canina No Município de Campo Grande, MS, Brasil. Ph.D. Thesis, Campo Grande, Mato Grosso do Sul, Brasil, 2012. [Google Scholar]
- Silva, R.A.; Andrade, A.J.d.; Quint, B.B.; Raffoul, G.E.S.; Werneck, G.L.; Rangel, E.F.; Romero, G.A.S. Effectiveness of Dog Collars Impregnated with 4% Deltamethrin in Controlling Visceral Leishmaniasis in Lutzomyia longipalpis (Diptera: Psychodidade: Phlebotominae) Populations. Mem. Inst. Oswaldo Cruz 2018, 113, e170377. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; de Souza, C.F.; Rontani, R.B.; Pereira, A.; Farnes, K.B.; Gorsich, E.E.; Silva, R.A.; Brazil, R.P.; Hamilton, J.G.; Courtenay, O. Community Deployment of a Synthetic Pheromone of the Sand Fly Lutzomyia longipalpis Co-Located with Insecticide Reduces Vector Abundance: Implications for Control of Leishmania Infantum. PLoS Negl. Trop. Dis. 2021, 15, e0009080. [Google Scholar] [CrossRef]
- Alexander, B.; Barros, V.C.; de Souza, S.F.; Barros, S.S.; Teodoro, L.P.; Soares, Z.R.; Gontijo, N.F.; Reithinger, R. Susceptibility to Chemical Insecticides of Two Brazilian Populations of the Visceral Leishmaniasis Vector Lutzomyia longipalpis (Diptera: Psychodidae). Trop. Med. Int. Health 2009, 14, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Félix de Lima, M.; Albuquerque e Silva, R.; de Almeida Rocha, D.; de Oliveira Mosqueira, G.; Gurgel-Gonçalves, R.; Takashi Obara, M. Insecticide-Impregnated Dog Collars for the Control of Visceral Leishmaniasis: Evaluation of the Susceptibility of Field Lutzomyia longipalpis Populations to Deltamethrin. Parasites Vectors 2024, 17, 468. [Google Scholar] [CrossRef] [PubMed]
- Falcao, A.R. DDT and dieldrin susceptibility of a natural population of Phlebotomus longipalpis in Minas Gerais, Brazil. Rev. Bras. Malariol. Doencas Trop. 1963, 15, 411–415. [Google Scholar]
- Falcão, A.R.; Pinto, C.T.; Gontijo, C.M. Susceptibility of Lutzomyia longipalpis to Deltamethrin. Mem. Inst. Oswaldo Cruz 1988, 83, 395–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González, M.A.; Bell, M.J.; Bernhardt, S.A.; Brazil, R.P.; Dilger, E.; Courtenay, O.; Hamilton, J.G.C. Susceptibility of Wild-Caught Lutzomyia longipalpis (Diptera: Psychodidae) Sand Flies to Insecticide after an Extended Period of Exposure in Western São Paulo, Brazil. Parasites Vectors 2019, 12, 110. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Boletim Epidemiológico: Monitoramento das arboviroses e balanço de encerramento do COE Dengue e outras Arboviroses 2024; Brasil Ministério da Saúde: Brasília, Brazil, 2024; Volume 55, p. 11. [Google Scholar]
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Diretrizes Nacionais para Prevenção e Controle das Arboviroses Urbanas: Vigilância Entomológica e Controle Vetorial. Brasília: Ministério da Saúde. 2025; 190p. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_nacionais_arboviroses_urbanas.pdf (accessed on 31 October 2025).
- Almeida, C.E.; Faucher, L.; Lavina, M.; Costa, J.; Harry, M. Molecular Individual-Based Approach on Triatoma brasiliensis: Inferences on Triatomine Foci, Trypanosoma cruzi Natural Infection Prevalence, Parasite Diversity and Feeding Sources. PLoS Negl. Trop. Dis. 2016, 10, e0004447. [Google Scholar] [CrossRef]
- Barbosa, L.M.C.; Scarpassa, V.M. Bionomics and Population Dynamics of Anopheline Larvae from an Area Dominated by Fish Farming Tanks in Northern Brazilian Amazon. PLoS ONE 2023, 18, e0288983. [Google Scholar] [CrossRef]
- Ribeiro, G., Jr.; dos Santos, C.G.S.; Lanza, F.; Reis, J.; Vaccarezza, F.; Diniz, C.; Miranda, D.L.P.; de Araújo, R.F.; Cunha, G.M.; de Carvalho, C.M.M.; et al. Wide Distribution of Trypanosoma cruzi-Infected Triatomines in the State of Bahia, Brazil. Parasites Vectors 2019, 12, 604. [Google Scholar] [CrossRef]
- Rocha, E.M.; Katak, R.d.M.; Campos de Oliveira, J.; Araujo, M.d.S.; Carlos, B.C.; Galizi, R.; Tripet, F.; Marinotti, O.; Souza-Neto, J.A. Vector-Focused Approaches to Curb Malaria Transmission in the Brazilian Amazon: An Overview of Current and Future Challenges and Strategies. Trop. Med. Infect. Dis. 2020, 5, 161. [Google Scholar] [CrossRef]
- Sánchez-Ribas, J.; Oliveira-Ferreira, J.; Gimnig, J.E.; Pereira-Ribeiro, C.; Santos-Neves, M.S.A.; Silva-do-Nascimento, T.F. Environmental Variables Associated with Anopheline Larvae Distribution and Abundance in Yanomami Villages within Unaltered Areas of the Brazilian Amazon. Parasites Vectors 2017, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.Q.d.; Afonso, M.M.d.S.; Freire, L.J.M.; Santana, A.L.F.d.; Pereira-Colavite, A.; Rangel, E.F. Ecological Aspects of the Phlebotominae Fauna (Diptera: Psychodidae) among Forest Fragments and Built Areas in an Endemic Area of American Visceral Leishmaniasis in João Pessoa, Paraíba, Brazil. Insects 2022, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Tadei, W.P.; Dutary Thatcher, B. Malaria Vectors in the Brazilian Amazon: Anopheles of the Subgenus Nyssorhynchus. Rev. Inst. Med. Trop. Sao Paulo 2000, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Valença-Barbosa, C.; Andrade, I.M.d.; de Simas, F.D.T.; Neto, O.C.C.; Silva, N.A.d.; Costa, C.F.; Moreira, B.O.B.; Finamore-Araujo, P.; Alvarez, M.V.N.; Borges-Veloso, A.; et al. New Approaches to the Ecology of Triatoma sordida in Peridomestic Environments of an Endemic Area of Minas Gerais, Brazil. Pathogens 2025, 14, 178. [Google Scholar] [CrossRef]
- Pontes, S.R.L.; Anastácio, L.F. Incidence of Pesticide Poisoning in Brazil between 2014 and 2024/Incidência de Intoxicações por Agrotóxicos no Brasil entre 2014 e 2024. Agrotecnologia 2025, 14, 50–61. [Google Scholar]
- Perobelli, J.E. Pesticides and Public Health: Discussing Risks in Brazilian Agro-Industrial Growth. Front. Toxicol. 2025, 7, 1442801. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharif, B.; Melo-Santos, M.A.V.; Barbosa, R.M.R.; Junqueira Ayres, C.F. A Brief History of the Use of Insecticides in Brazil to Control Vector-Borne Diseases, and Implications for Insecticide Resistance. Trop. Med. Infect. Dis. 2025, 10, 336. https://doi.org/10.3390/tropicalmed10120336
Alsharif B, Melo-Santos MAV, Barbosa RMR, Junqueira Ayres CF. A Brief History of the Use of Insecticides in Brazil to Control Vector-Borne Diseases, and Implications for Insecticide Resistance. Tropical Medicine and Infectious Disease. 2025; 10(12):336. https://doi.org/10.3390/tropicalmed10120336
Chicago/Turabian StyleAlsharif, Bashir, Maria Alice Varjal Melo-Santos, Rosângela Maria Rodrigues Barbosa, and Constância Flávia Junqueira Ayres. 2025. "A Brief History of the Use of Insecticides in Brazil to Control Vector-Borne Diseases, and Implications for Insecticide Resistance" Tropical Medicine and Infectious Disease 10, no. 12: 336. https://doi.org/10.3390/tropicalmed10120336
APA StyleAlsharif, B., Melo-Santos, M. A. V., Barbosa, R. M. R., & Junqueira Ayres, C. F. (2025). A Brief History of the Use of Insecticides in Brazil to Control Vector-Borne Diseases, and Implications for Insecticide Resistance. Tropical Medicine and Infectious Disease, 10(12), 336. https://doi.org/10.3390/tropicalmed10120336





