Epidemiology of HIV in Remote Equatorial Regions of Cameroon: High Prevalence in Older Adults and Regional Disparities
Abstract
1. Introduction
2. Materials and Methods
2.1. Villages and Study Population
2.2. HIV Testing
2.3. Statistical Analyses
2.4. Ethics Considerations
3. Results
3.1. Description of Serosurveys
3.2. Characteristics of Study Participants
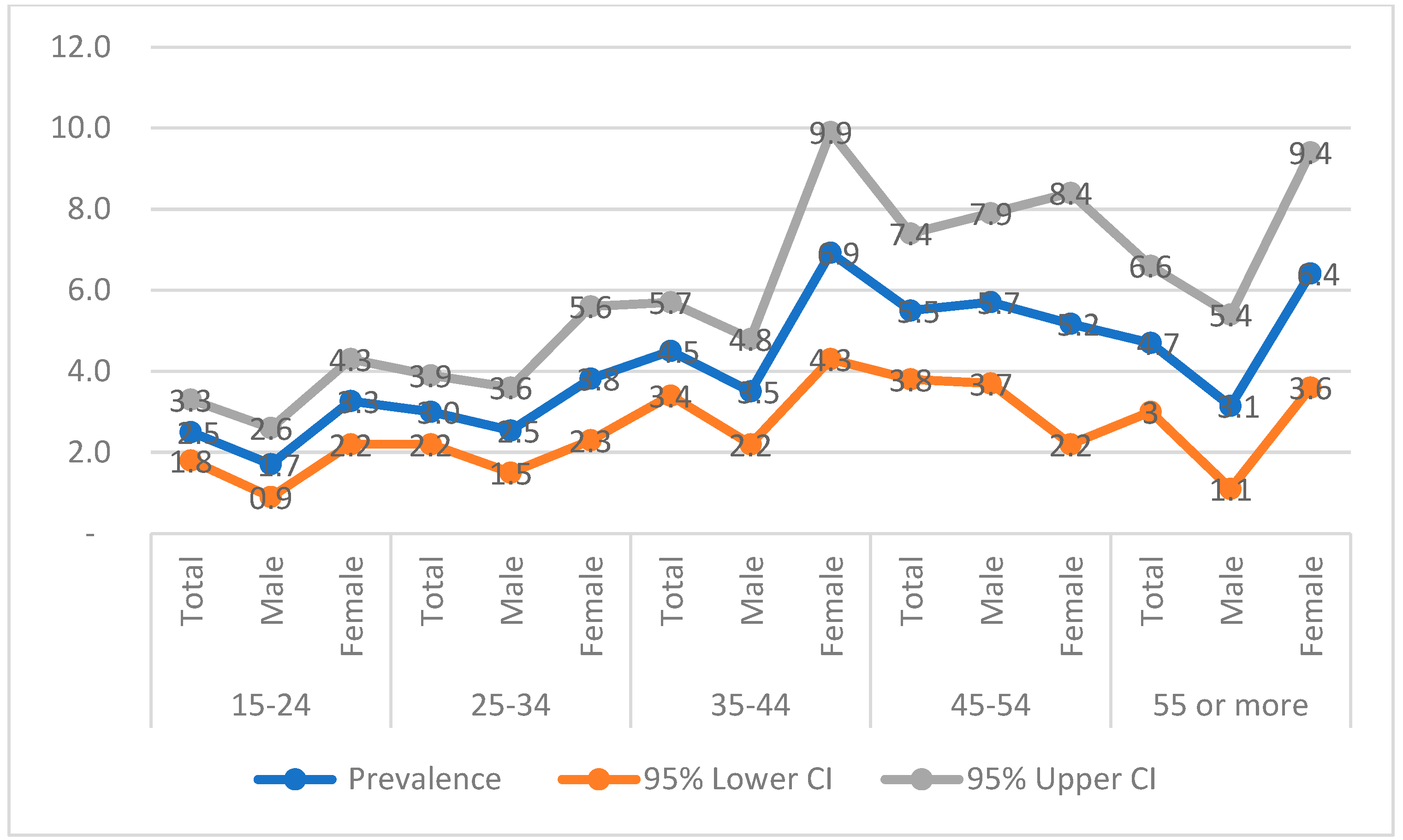
3.3. HIV Prevalence in Remote Communities of Cameroon
3.4. Bivariate Analyses
3.5. Multivariate Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joint United Nations Programme on HIV/AIDS. AIDS, Crisis and the Power to Transform: UNAIDS Global AIDS Update 2025; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2025. [Google Scholar]
- Global Statistics. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 22 July 2025).
- Rapport Mondial D’Avancement sur la Lutte Contre le Sida 2020; World Health Organization: Geneva, Switzerland, 2020.
- UNAIDS. 2004: Report on the Global AIDS Epidemic: 4th Global Report; Joint United Nations Programme on HIV, AIDS, Ed.; UNAIDS: Geneva, Switzerland, 2004; ISBN 978-92-9173-355-2. [Google Scholar]
- Zekeng, L.; Yanga, D.; Trebucq, A.; Sokal, D.; Salla, R.; Kaptue, L. HIV Prevalence in Patients with Sexually Transmitted Diseases in Yaounde, (Cameroon) in 1989 and 1990: Necessity of an STD Control Programme. Sex. Transm. Infect. 1992, 68, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Kuaban, C.; Bercion, R. HIV seroprevalence in adults with pulmonary tuberculosis in Yaounde, Cameroon. Med. Trop. 1996, 56, 357–360. [Google Scholar]
- Buvé, A.; Caraël, M.; Hayes, R.J.; Auvert, B.; Ferry, B.; Robinson, N.J.; Anagonou, S.; Kanhonou, L.; Laourou, M.; Abega, S.; et al. Multicentre Study on Factors Determining Differences in Rate of Spread of HIV in Sub-Saharan Africa: Methods and Prevalence of HIV Infection. AIDS 2001, 15 (Suppl. S4), S5–S14. [Google Scholar] [CrossRef] [PubMed]
- Nyambi, P.; Zekeng, L.; Kenfack, H.; Tongo, M.; Nanfack, A.; Nkombe, I.; Ndonko, F.; Shang, J.; Burda, S.; Mbah, H.; et al. HIV Infection in Rural Villages of Cameroon. J. Acquir. Immune Defic. Syndr. 2002, 31, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.F.; Van Heuverswyn, F.; Li, Y.; Bailes, E.; Takehisa, J.; Santiago, M.L.; Bibollet-Ruche, F.; Chen, Y.; Wain, L.V.; Liegeois, F.; et al. Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1. Science 2006, 313, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Van Heuverswyn, F.; Li, Y.; Neel, C.; Bailes, E.; Keele, B.F.; Liu, W.; Loul, S.; Butel, C.; Liegeois, F.; Bienvenue, Y.; et al. Human Immunodeficiency Viruses: SIV Infection in Wild Gorillas. Nature 2006, 444, 164. [Google Scholar] [CrossRef] [PubMed]
- D’arc, M.; Ayouba, A.; Esteban, A.; Learn, G.H.; Boué, V.; Liegeois, F.; Etienne, L.; Tagg, N.; Leendertz, F.H.; Boesch, C.; et al. Origin of the HIV-1 Group O Epidemic in Western Lowland Gorillas. Proc. Natl. Acad. Sci. USA 2015, 112, E1343–E1352. [Google Scholar] [CrossRef] [PubMed]
- Tongo, M.; Martin, D.P.; Dorfman, J.R. Elucidation of Early Evolution of HIV-1 Group M in the Congo Basin Using Computational Methods. Genes 2021, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé Publique. Directives Nationales sur la Prise en Charge du VIH; Ministère de la Santé Publique: Yaoundé, Cameroun, 2021. [Google Scholar]
- Comité National de Lutte contre le Sida. Plan Stratégique National de Lutte Contre le VIH/Sida et les IST 2021–2023 du Cameroun; Ministère de la Santé Publique/Comité National de Lutte contre le Sida: Yaoundé, Cameroun, 2020; Available online: http://www.cnls.cm/uploads/docs/2024-11-01-psn-2021-2023-vesion-francaise.pdf (accessed on 22 July 2025).
- Enquête Démographique et de Santé du Cameroun 2018; Institut National de la Statistique (INS): Yaoundé, Cameroun; ICF: Rockville, MD, USA, 2020; Available online: https://dhsprogram.com/pubs/pdf/FR360/FR360.pdf (accessed on 22 July 2025).
- Enquête Démographique et de Santé et à Indicateurs Multiples du Cameroun 2011; Institut National de la Statistique (INS): Yaoundé, Cameroun; ICF International: Calverton, MD, USA, 2012; Available online: https://dhsprogram.com/pubs/pdf/fr260/fr260.pdf (accessed on 22 July 2025).
- Bekolo, C.E.; Kouanfack, C.; Ateudjieu, J.; Bechem, E.T.; Ndeso, S.A.; Tendengfor, N.; Nsagha, D.S.; Choukem, S.P. The Declining Trend in HIV Prevalence from Population-Based Surveys in Cameroon between 2004 and 2018: Myth or Reality in the Universal Test and Treat Era? BMC Public Health 2023, 23, 479. [Google Scholar] [CrossRef] [PubMed]
- Sandie, A.B.; Tchatchueng Mbougua, J.B.; Nlend, A.E.N.; Thiam, S.; Nono, B.F.; Fall, N.A.; Senghor, D.B.; Sylla, E.H.M.; Faye, C.M. Hot-Spots of HIV Infection in Cameroon: A Spatial Analysis Based on Demographic and Health Surveys Data. BMC Infect. Dis. 2022, 22, 334. [Google Scholar] [CrossRef] [PubMed]
- Edoul, G.; Chia, J.E.; Vidal, N.; Guichet, E.; Montavon, C.; Delaporte, E.; Mpoudi Ngole, E.; Ayouba, A.; Peeters, M. High HIV Burden and Recent Transmission Chains in Rural Forest Areas in Southern Cameroon, Where Ancestors of HIV-1 Have Been Identified in Ape Populations. Infect. Genet. Evol. 2020, 84, 104358. [Google Scholar] [CrossRef]
- Consolidated Guidelines on HIV Testing Services, 2019; World Health Organization: Geneva, Switzerland, 2020.
- Statistical Software for Data Science|Stata. Available online: https://www.stata.com/ (accessed on 25 August 2025).
- Barros, A.J.; Hirakata, V.N. Alternatives for Logistic Regression in Cross-Sectional Studies: An Empirical Comparison of Models That Directly Estimate the Prevalence Ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Pinilla, M.; Villalba-Niño, S.; Olaya-Galán, N.N. Negative Log-Binomial Model with Optimal Robust Variance to Estimate the Prevalence Ratio, in Cross-Sectional Population Studies. BMC Med. Res. Methodol. 2023, 23, 219. [Google Scholar] [CrossRef] [PubMed]
- Godwe, C.; Goni, O.H.; San, J.E.; Sonela, N.; Tchakoute, M.; Nanfack, A.; Koro, F.K.; Butel, C.; Vidal, N.; Duerr, R.; et al. Phylogenetic Evidence of Extensive Spatial Mixing of Diverse HIV-1 Group M Lineages within Cameroon but Not between Its Neighbours. Virus Evol. 2024, 10, veae070. [Google Scholar] [CrossRef] [PubMed]
- Évaluation de l’impact du VIH sur la Population au Cameroun (CAMPHIA) 2017–2018: Rapport Final; Ministère de la santé (MINSANTE), Division de la Recherche Opérationnelle en Santé (DROS): Yaoundé, Cameroon, 2020. Available online: https://stacks.cdc.gov/view/cdc/120051 (accessed on 22 July 2025).
- Ngoume, Y.F.; Teagho, U.C.; Eselacha, B.; Goni, O.H.; Kenfack, D.-D.; Tchakoute, M.; Nguefack-Tsague, G.; Tongo, M. Differences in HIV Infection Trends in Two Regions of Cameroon with a Longstanding HIV Epidemic: Insights from 2012 and 2022. Front. Public Health 2025, 13, 1517213. [Google Scholar] [CrossRef] [PubMed]
- Comité National de Lutte Contre le Sida. Epidémiologie de L’Infection à VIH au Cameroun: Premier Semestre 2018; Comité National de Lutte Contre le Sida: Yaoundé, Cameroon, 2018. [Google Scholar]
- Mosoko, J.J.; Macauley, I.B.; Zoungkanyi, A.-C.B.; Bella, A.; Koulla-Shiro, S. Human Immunodeficiency Virus Infection and Associated Factors among Specific Population Subgroups in Cameroon. AIDS Behav. 2009, 13, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Sample Size and Its Importance in Research. Indian J. Psychol. Med. 2020, 42, 102–103. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Negative n (%) | Positive n (%) | Total | PR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age (Years, p = 0.017, Quasi Likelihood under Independence Model Criterion (QIC) = 1265.76) | |||||
| 15–19 | 1022(98.2) | 19(1.8) | 1041 | 1 | |
| 20–24 | 703(96.4) | 26(3.6) | 729 | 1.95(1.09–3.50) | 0.025 |
| 25–29 | 676(96.7) | 23(3.3) | 699 | 1.80(1.00–3.28) | 0.05 |
| 30–34 | 719(97.3) | 20(2.7) | 739 | 1.48(0.80–2.76) | 0.21 |
| 35–39 | 661(95.4) | 32(4.6) | 693 | 2.53(1.45–4.43) | 0.001 |
| 40–44 | 477(95.6) | 22(4.4) | 499 | 2.42(1.32–4.42) | 0.004 |
| 45–49 | 309(94.5) | 18(5.5) | 327 | 3.02(1.60–5.68) | <0.001 |
| 50–54 | 274(94.5) | 16(5.5) | 290 | 3.02(1.58–5.80) | <0.001 |
| 55–59 | 164(94.8) | 9(5.2) | 173 | 2.85(1.31–6.20) | 0.008 |
| 60–64 | 129(96.3) | 5(3.7) | 134 | 2.04(0.78–5.38) | 0.15 |
| 65 or more | 240(95.2) | 12(4.8) | 252 | 2.61(1.28–5.30) | 0.008 |
| Age (Years, p = 0.025, Quasi Likelihood under Independence Model Criterion (QIC) = 1204.63) | |||||
| 15–49 | 4567(96.6) | 160(3.4) | 4727 | 1 | |
| 50 or more | 807(95.1) | 42(4.9) | 849 | 1.46(1.05–2.04) | 0.025 |
| Sex (p = 0.005, Quasi Likelihood under Independence Model Criterion (QIC) = 1191.79) | |||||
| Male | 3261(97.0) | 102(3.0) | 3363 | 1 | |
| Female | 2085(95.5) | 98(4.5) | 2183 | 1.48(1.13–1.94) | 0.005 |
| Region (p < 0.001, Quasi Likelihood under Independence Model Criterion (QIC) = 1207.65) | |||||
| Centre | 810(97.7) | 19(2.3) | 829 | 1 | |
| East | 1927(97.2) | 56(2.8) | 1983 | 1.23(0.74–2.06) | 0.43 |
| Littoral | 831(96.7) | 28(3.3) | 859 | 1.42(0.80–2.53) | 0.23 |
| South | 1806(94.8) | 99(5.2) | 1905 | 2.27(1.40–3.68) | <0.001 |
| Marital Status (p < 0.001, Quasi Likelihood under Independence Model Criterion (QIC) = 1003.47) | |||||
| Single | 1829(96.8) | 60(3.2) | 1889 | 1 | |
| Married | 1423(96.9) | 46(3.1) | 1469 | 0.99(0.68–1.44) | 0.94 |
| Divorced/Separated /Widow(er) | 115(89.8) | 13(10.2) | 128 | 3.20(1.80–5.67) | <0.001 |
| Living together | 1278(96.7) | 43(3.3) | 1321 | 1.02(0.70–1.51) | 0.90 |
| Education (p = 0.5, Quasi Likelihood under Independence Model Criterion (QIC) = 843.54) | |||||
| No education | 187(97.9) | 4(2.1) | 191 | 1 | |
| Primary | 1326(96.4) | 49(3.6) | 1375 | 1.86(0.68–5.10) | 0.23 |
| Secondary or higher | 2497(96.9) | 80(3.1) | 2577 | 1.62(0.60–4.38) | 0.34 |
| Characteristics | aPR (95% CI) | aP-Value |
|---|---|---|
| Test of model main effects: Age (p = 0.17, Wald chi-square = 14.04) | ||
| 15–19 | 1 | |
| 20–24 | 1.61(0.85–3.05) | 0.14 |
| 25–29 | 1.64(0.84–3.23) | 0.15 |
| 30–34 | 1.42(0.69–2.93) | 0.34 |
| 35–39 | 2.15(1.10–4.20) | 0.02 |
| 40–44 | 2.04(0.97–4.31) | 0.06 |
| 45–49 | 3.11(1.46–6.64) | 0.003 |
| 50–54 | 2.76(1.24–6.15) | 0.013 |
| 55–59 | 2.98(1.23–7.20) | 0.015 |
| 60–64 | 2.29(0.82–6.40) | 0.11 |
| 65 or more | 1.82(0.69–4.80) | 0.22 |
| Test of model main effects: Sex (p = 0.007, Wald chi-square = 7.24) | ||
| Male | 1 | |
| Female | 1.53(1.12–2.08) | 0.007 |
| Test of model main effects: Region (p = 0.167, Wald chi-square = 5.07) | ||
| Centre | 1 | |
| East | 1.38(0.79–2.41) | 0.25 |
| Littoral | 1.51(0.81–2.81) | 0.196 |
| South | 1.82(1.04–3.18) | 0.035 |
| Test of model main effects: Marital status (p = 0.019, Wald chi-square = 10.00) | ||
| Single | 1 | |
| Married | 0.65(0.41–1.02) | 0.059 |
| Divorced/Separated/Widow(er) | 1.70(0.80–3.58) | 0.165 |
| Living together | 0.82(0.53–1.26) | 0.367 |
| Goodness of fit: Quasi Likelihood under Independence Model Criterion (QIC) = 1109.62 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tongo, M.; Ngoume, Y.F.; Tanko, R.F.; Teagho, U.C.; Eselacha, B.; Goni, O.H.; Kenfack, D.-D.; Tchakoute, M.; Nguefack-Tsague, G. Epidemiology of HIV in Remote Equatorial Regions of Cameroon: High Prevalence in Older Adults and Regional Disparities. Trop. Med. Infect. Dis. 2025, 10, 334. https://doi.org/10.3390/tropicalmed10120334
Tongo M, Ngoume YF, Tanko RF, Teagho UC, Eselacha B, Goni OH, Kenfack D-D, Tchakoute M, Nguefack-Tsague G. Epidemiology of HIV in Remote Equatorial Regions of Cameroon: High Prevalence in Older Adults and Regional Disparities. Tropical Medicine and Infectious Disease. 2025; 10(12):334. https://doi.org/10.3390/tropicalmed10120334
Chicago/Turabian StyleTongo, Marcel, Yannick F. Ngoume, Ramla F. Tanko, Urmes C. Teagho, Brice Eselacha, Oumarou H. Goni, Dell-Dylan Kenfack, Mérimé Tchakoute, and Georges Nguefack-Tsague. 2025. "Epidemiology of HIV in Remote Equatorial Regions of Cameroon: High Prevalence in Older Adults and Regional Disparities" Tropical Medicine and Infectious Disease 10, no. 12: 334. https://doi.org/10.3390/tropicalmed10120334
APA StyleTongo, M., Ngoume, Y. F., Tanko, R. F., Teagho, U. C., Eselacha, B., Goni, O. H., Kenfack, D.-D., Tchakoute, M., & Nguefack-Tsague, G. (2025). Epidemiology of HIV in Remote Equatorial Regions of Cameroon: High Prevalence in Older Adults and Regional Disparities. Tropical Medicine and Infectious Disease, 10(12), 334. https://doi.org/10.3390/tropicalmed10120334






