Canals, Contaminants, and Connections: Exploring the Urban Exposome in a Tropical River System
Abstract
1. Introduction
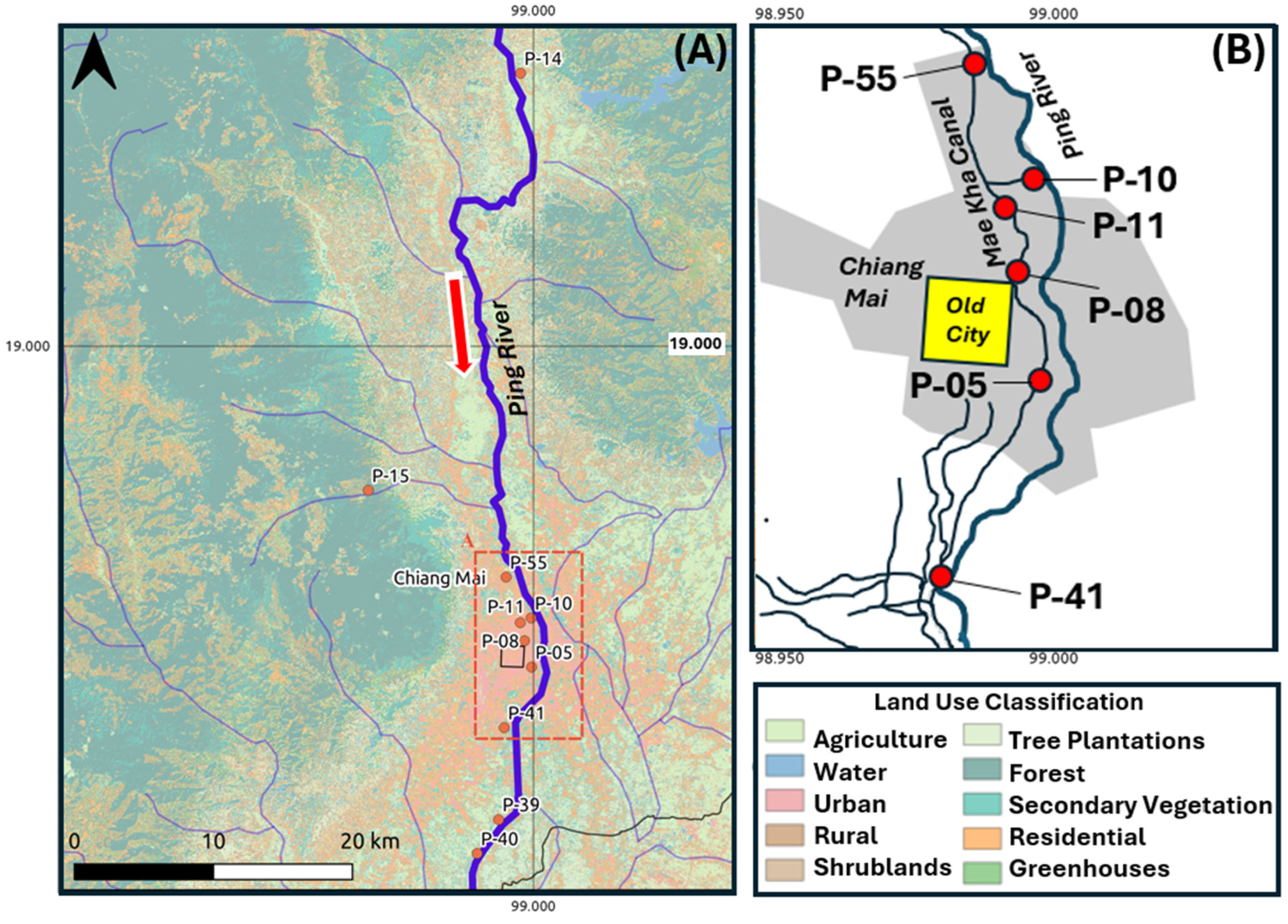
2. Study Area

3. Methods
3.1. Overview
3.2. Analytical Analysis
4. Results
4.1. EPC Concentrations and Detection Frequencies
4.2. Patterns Within the Urban Canal Network
4.3. Potential Enrichment
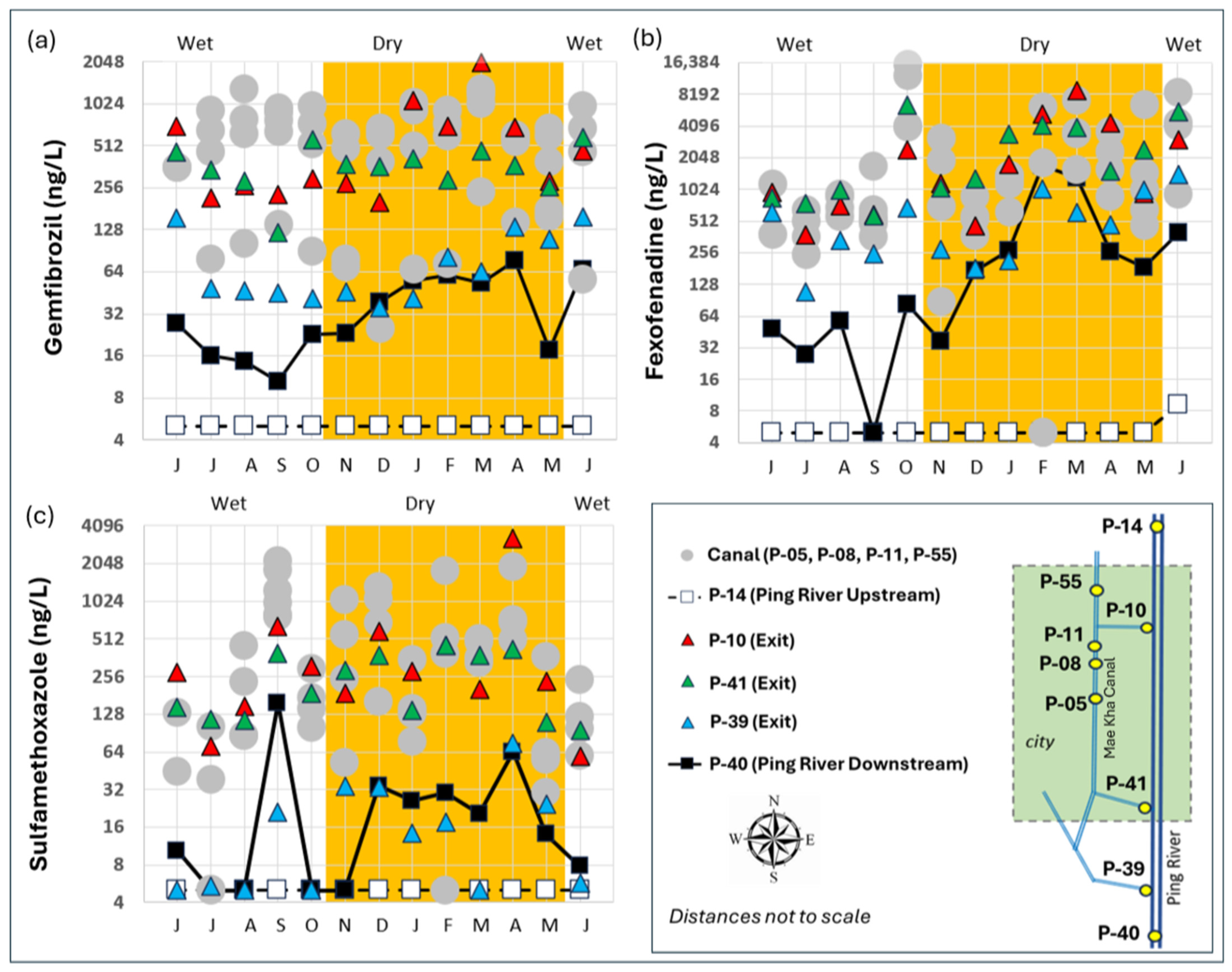
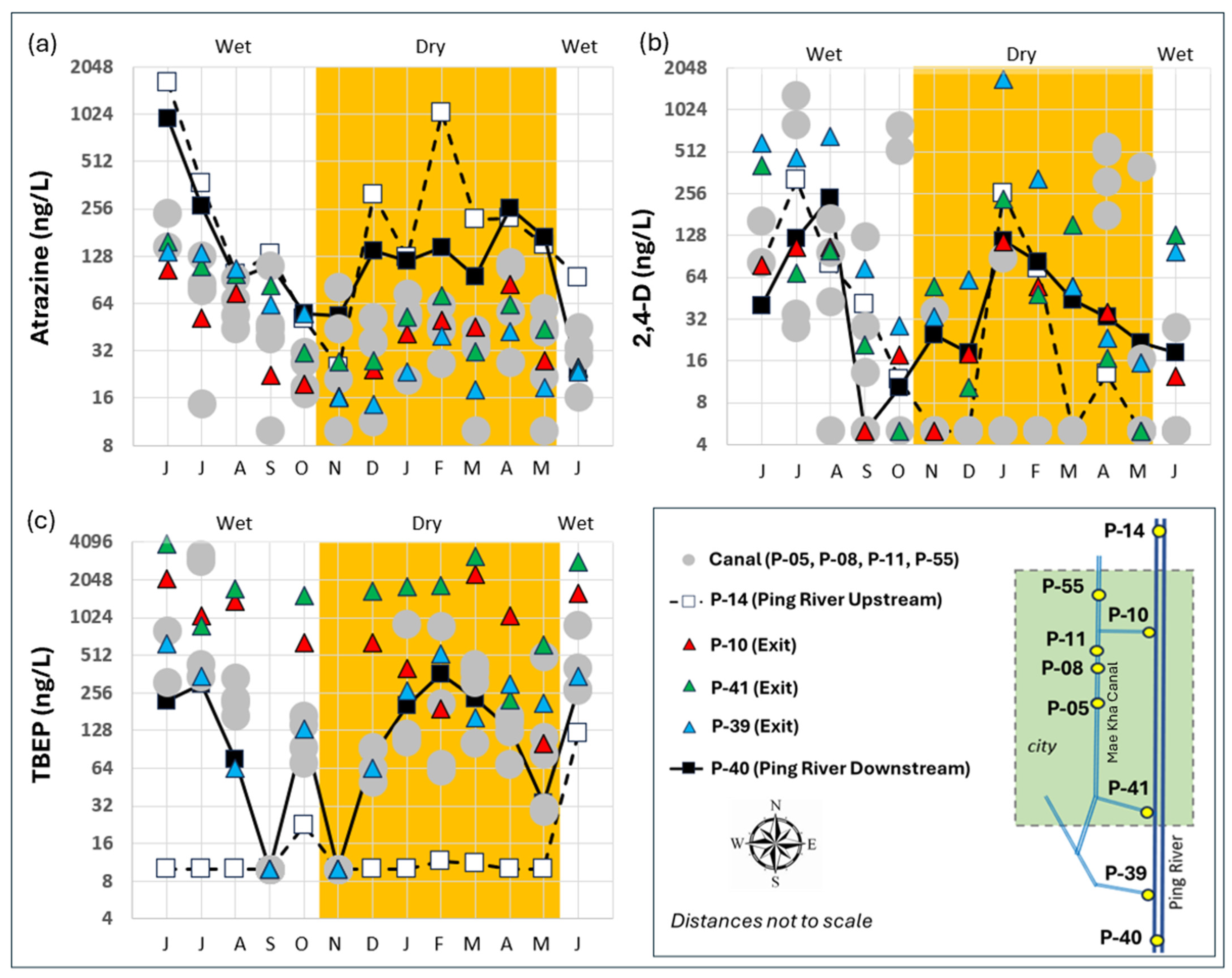
4.4. EPC Time Series
4.5. Ecological Risk
4.6. Non-Targeted Analysis
5. Discussion
5.1. Urban–River Linkages
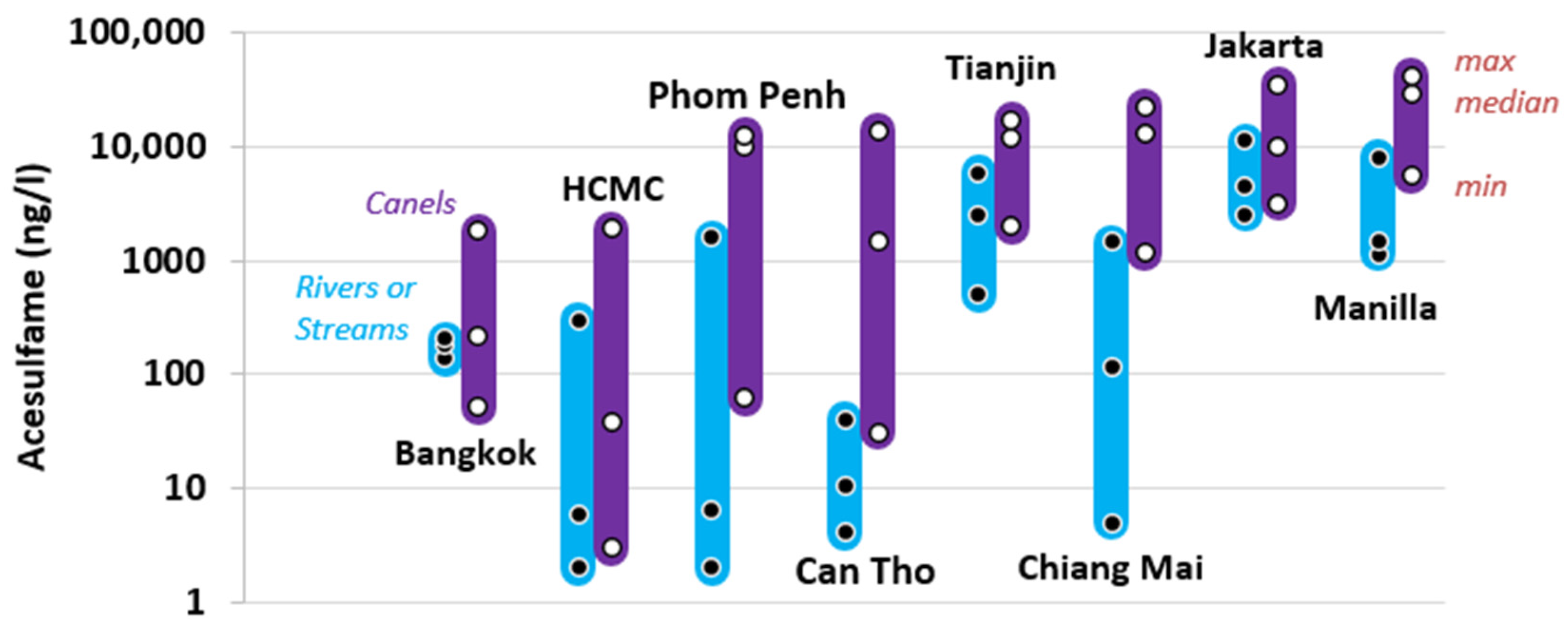
5.2. Urban Canals as Contamination Connectors—An Asian Perspective
5.3. Mae Kha Canal in Focus
5.4. Potential Risk
5.4.1. Single-Compound Concerns
5.4.2. Cumulative and Mixture Risks
- Implement long-term, high-frequency monitoring to capture both chronic background concentrations and short-term contamination pulses.
- Develop region- and species-specific chronic toxicity benchmarks that reflect local ecological sensitivities and community composition.
- Integrate mixture and multistressor effects into risk assessment frameworks using component-based approaches, whole-mixture testing, and next-generation tools such as omics [105].
5.5. Priority Compounds
5.6. Caveats and Limitations
6. Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez, A.; Engman, A.; Rosas, K.G.; Perez-Reyes, O.; Martinó-Cardona, D.M. Urban impacts on tropical island streams: Some key aspects influencing ecosystem response. Urban Ecosyst. 2012, 15, 315–325. [Google Scholar] [CrossRef]
- Capps, K.A.; Bentsen, C.N.; Ramírez, A. Poverty, urbanization, and environmental degradation: Urban streams in the developing world. Freshw. Sci. 2016, 35, 429–435. [Google Scholar] [CrossRef]
- Rico, A.; de Oliveira, R.; Nunes, G.S.d.S.; Rizzi, C.; Villa, S.; López-Heras, I.; Vighi, M.; Waichman, A.V. Pharmaceuticals and other urban contaminants threaten Amazonian freshwater ecosystems. Environ. Int. 2021, 155, 106702. [Google Scholar] [CrossRef]
- Lee, T.H.Y.; Ziegler, A.D.; Marques dos Santos, M.; Srinuansom, K.; Tan, S.Y.; Snyder, S.A. Spatial and temporal patterns of emerging and persistent contaminants in a mixed-use catchment: A case study of the upper Ping in Northern Thailand. ACS EST Water 2024, 4, 1531–1545. [Google Scholar] [CrossRef]
- Lee, T.H.Y.; Chuah, J.; Snyder, S.A. Occurrence of emerging contaminants in Southeast Asian environments: Present status, challenges, and future prospects. ACS EST Water 2022, 2, 907–931. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Pérez-Pereira, A.; Carrola, J.S.; Tiritan, M.E.; Ribeiro, C. Enantioselectivity in ecotoxicity of pharmaceuticals, illicit drugs, and industrial persistent pollutants in aquatic and terrestrial environments: A review. Sci. Total Environ. 2024, 912, 169573. [Google Scholar] [CrossRef]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, personal care products and endocrine disrupting agents in the environment–a review. Clean Soil Air Water 2009, 37, 277–303. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: A review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Santos, D.; Flores, E.M.M.; Ince, N.H. Pharmaceuticals and personal care products (PPCPs): Environmental and public health risks. Environ. Prog. Sustain. Energy 2022, 41, e13821. [Google Scholar] [CrossRef]
- Ranjan, N.; Singh, P.K.; Maurya, N.S. Pharmaceuticals in water as emerging pollutants for river health: A critical review under Indian conditions. Ecotoxicol. Environ. Saf. 2022, 247, 114220. [Google Scholar] [CrossRef]
- Anh, N.T.; Nhan, N.T.; Schmalz, B.; Le Luu, T. Influences of key factors on river water quality in urban and rural areas: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Kumar, M.; Ngasepam, J.; Dhangar, K.; Mahlknecht, J.; Manna, S. Critical review on negative emerging contaminant removal efficiency of wastewater treatment systems: Concept, consistency and consequences. Bioresour. Technol. 2022, 352, 127054. [Google Scholar] [CrossRef] [PubMed]
- González, S.; López-Roldán, R.; Cortina, J.L. Presence and biological effects of emerging contaminants in Llobregat River basin: A review. Environ. Pollut. 2012, 161, 83–92. [Google Scholar] [CrossRef]
- Mandaric, L.; Celic, M.; Marcé, R.; Petrovic, M. Introduction on emerging contaminants in rivers and their environmental risk. In Emerging Contaminants in River Ecosystems: Occurrence and Effects Under Multiple Stress Conditions; Springer: Cham, Switzerland, 2016; pp. 3–25. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef]
- Maculewicz, J.; Kowalska, D.; Świacka, K.; Toński, M.; Stepnowski, P.; Białk-Bielińska, A.; Dołżonek, J. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential. Sci. Total Environ. 2022, 802, 149916. [Google Scholar] [CrossRef]
- Ritter, K.; Solomon, K.; Sibley, P.; Hall, K.; Keen, P.; Mattu, G.; Linton, B. Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the Walkerton inquiry. J. Toxicol. Environ. Health A 2002, 65, 1–142. [Google Scholar] [CrossRef]
- Tong, X.; Mohapatra, S.; Zhang, J.; Tran, N.H.; You, L.; He, Y.; Gin, K.Y.H. Source, fate, transport and modelling of selected emerging contaminants in the aquatic environment: Current status and future perspectives. Water Res. 2022, 217, 118418. [Google Scholar] [CrossRef]
- Bugnot, A.B.; Hose, G.C.; Walsh, C.J.; Floerl, O.; French, K.; Dafforn, K.A.; Hanford, J.; Lowe, E.C.; Hahs, A.K. Urban impacts across realms: Making the case for inter-realm monitoring and management. Sci. Total Environ. 2019, 648, 711–719. [Google Scholar] [CrossRef]
- Kessler, J.; Dawley, D.; Crow, D.; Garmany, R.; Georgel, P.T. Potential health risks linked to emerging contaminants in major rivers and treated waters. Water 2019, 11, 2615. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- Pamuru, S.T.; Forgione, E.; Croft, K.; Kjellerup, B.V.; Davis, A.P. Chemical characterization of urban stormwater: Traditional and emerging contaminants. Sci. Total Environ. 2022, 813, 151887. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.Y.; Li, C.; dos Santos, M.M.; Tan, S.Y.; Sureshkumar, M.; Srinuansom, K.; Ziegler, A.D.; Snyder, S.A. Assessment of emerging and persistent contaminants in an anthropogenic-impacted watershed: Application using targeted, non-targeted, and in vitro bioassay techniques. Chemosphere 2024, 364, 143067. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Srinuansom, K.; Snyder, S.A.; Ziegler, A.D. Atmosphere-transported emerging and persistent contaminants (EPCs) in rainfall and throughfall: Insights from a rural site in Northern Thailand. Atmosphere 2023, 14, 1603. [Google Scholar] [CrossRef]
- Lee, T.H.Y.; Ziegler, A.D.; Li, C.; Srinuansom, K.; Snyder, S.A. Riverine pesticides in an agricultural catchment in Northern Thailand: With focus on atrazine and metabolites. ACS EST Water 2024, 4, 3758–3772. [Google Scholar] [CrossRef]
- Firoz, A.S.M. Water Quality Assessment of Huay Kaew Stream Mae Kha Canal and Mae Ping River. Ph.D. Thesis, Graduate School, Chiang Mai University, Chiang Mai, Thailand, 1996. Available online: http://aunilo.uum.edu.my/Find/Record/th-cmuir.6653943832-32859 (accessed on 29 June 2025).
- Yang, Z. Trace metals in the Mae Kha Canal and the Mae Ping River in Chiang Mai, Northern Thailand. J. Sci. Soc. Thail. 1997, 23, 123–134. [Google Scholar] [CrossRef]
- Ribeiro, G.; Srisuwan, A. Urban development discourses, environmental management and public participation: The case of the Mae Kha Canal in Chiang Mai, Thailand. Environ. Urban. 2005, 17, 171–182. [Google Scholar] [CrossRef]
- Gugino, H.; Irvine, K.; Ngern-Klun, R.; Sukontason, K.; Sukontason, K.; Prangkio, C.; Thunyapar, P. Impacts of the urban environment on area water source: The Klong Mae Kha-Chiang Mai, Thailand. In Proceedings of the 4th International Symposium on Southeast Asian Water Environment, Asian Institute of Technology, Bangkok, Thailand, 6–8 December 2006; pp. 85–88. [Google Scholar]
- Deelder, M. Down the Drain: A Study on the Political Ecology of Wastewater Governance Affecting Slum Settlements in Rapidly Urbanizing Chiang Mai. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2013. Available online: https://studenttheses.uu.nl/handle/20.500.12932/15384 (accessed on 29 June 2025).
- Mettes, C. From the Muddy Banks of the Mae Kha. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2014. Available online: https://studenttheses.uu.nl/handle/20.500.12932/16785 (accessed on 29 June 2025).
- Nuanla-Or, S. Creating Sustainable Future of a Degraded Urban Canal: Mae Kha, in Chiang Mai, Thailand. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2016. Available online: https://digitalcommons.lsu.edu/gradschool_theses/784 (accessed on 29 June 2025).
- Ichikawa, T.; Ongsavangchai, N. Analyzing the space composition and community system of the sustainable village along the Mae Kha Canal. Res. Rep. Fac. Eng. Kindai Univ. 2017, 51, 33–40. Available online: https://cn.bing.com/search?q=Analyzing+the+space+composition+and+community+system+of+the+sustainable+village+along+the+Mae+Kha+Canal&cvid=0d3a9739340e41139047c9553197c241&gs_lcrp=EgRlZGdlKgYIABBFGDkyBggAEEUYOTIHCAEQ6wcYQNIBCDEwOTBqMGo0qAIIsAIB&FORM=ANAB01&adppc=EDGEESS&PC=EDGEESS (accessed on 29 June 2025).
- Manene, O.; Deadman, N.; Techakijvej, C.; Kullasoot, S.; Sapewisut, P.; Sareein, N.; Phalaraksh, C. Ecological health assessment of Mae Kha Canal, Chiang Mai Province, Thailand in 2023. J. Ecol. Environ. 2024, 48, 11. [Google Scholar] [CrossRef]
- Kunacheva, C.; Boontanon, S.K.; Fujii, S.; Tanaka, S.; Musirat, C.; Artsalee, C.; Wongwattana, T. Contamination of perfluorinated compounds (PFCs) in Chao Phraya River and Bangpakong River, Thailand. Water Sci. Technol. 2009, 60, 975–982. [Google Scholar] [CrossRef]
- Brigden, K.; Labunska, I.; Santillo, D. Investigation of Hazardous Chemical Discharges from Two Textile Manufacturing Facilities, and Chemical Contamination of Nearby Canals Connecting to the Lower Chao Phraya River, Thailand, 2010. Greenpeace Research Laboratories Technical Note 06/2010. Available online: https://greenpeace.to/publications/ChaoPhraya_canals_textilefacilities.pdf (accessed on 29 June 2025).
- Li, Y.; Jindal, R.; Choi, K.; Kho, Y.L.; de Bullen, P.G. Pharmaceutical residues in wastewater treatment plants and surface waters in Bangkok. J. Hazard. Toxic Radioact. Waste 2012, 16, 88–91. [Google Scholar] [CrossRef]
- Tewari, S.; Jindal, R.; Kho, Y.L.; Eo, S.; Choi, K. Major pharmaceutical residues in wastewater treatment plants and receiving waters in Bangkok, Thailand, and associated ecological risks. Chemosphere 2013, 91, 697–704. [Google Scholar] [CrossRef]
- Deemoon, S.; Sarin, C.; Juksu, K.; Kritsunankul, C.; Ying, G.-G.; Sriprang, S. Occurrence and estrogenic risks of endocrine disrupting chemicals in wet and dry seasons of the Nan River, Phitsanulok, Thailand. Songklanakarin J. Sci. Technol. 2018, 40, 5. [Google Scholar]
- Deemoon, S.; Sarin, C.; Juksu, K.; GuoYing, G.; Kritsunankul, C.; Sriprang, S. Endocrine disrupting chemicals and estrogenic activity in surface water, sediment, and fish from the Nan River, Phitsanulok, Thailand. J. Health Sci. Thail. 2019, 28, 68–80. [Google Scholar]
- Juksu, K.; Zhao, J.-L.; Liu, Y.-S.; Yao, L.; Sarin, C.; Sreesai, S.; Klomjek, P.; Jiang, Y.-X.; Ying, G.-G. Occurrence, fate and risk assessment of biocides in wastewater treatment plants and aquatic environments in Thailand. Sci. Total Environ. 2019, 690, 1110–1119. [Google Scholar] [CrossRef]
- Juksu, K.; Liu, Y.-S.; Zhao, J.-L.; Yao, L.; Sarin, C.; Sreesai, S.; Klomjek, P.; Traitangwong, A.; Ying, G.-G. Emerging contaminants in aquatic environments and coastal waters affected by urban wastewater discharge in Thailand: An ecological risk perspective. Ecotoxicol. Environ. Saf. 2020, 204, 110952. [Google Scholar] [CrossRef]
- Chan, R.; Wandee, S.; Wang, M.; Chiemchaisri, W.; Chiemchaisri, C.; Yoshimura, C. Fate, transport and ecological risk of antibiotics from pig farms along the Bang Pakong River, Thailand. Agric. Ecosyst. Environ. 2020, 304, 107123. [Google Scholar] [CrossRef]
- Lee, T.H.; Duangnamon, D.; Boontha, T.; Webster, R.D.; Ziegler, A.D. Emerging and persistent contaminants in a remote coastal stream system: Five priority compounds in Southeast Asia. Sustainability 2025, 17, 581. [Google Scholar] [CrossRef]
- Itayama, T.; Hawkins, P.R.; Leelahakriengkrai, P.; Kullasoot, S.; Whangchai, N.; Chitmanat, C.; Peerapornpisal, Y.; Kawabata, Z.I. Bioassessment of dry season water quality in the Ping River around Chiang Mai city, Thailand. Chiang Mai J. Sci. 2015, 42, 349–366. Available online: https://epg.science.cmu.ac.th/ejournal/journal-detail.php?id=5757 (accessed on 29 June 2025).
- Tsuzuki, Y.; Koottatep, T.; Jiawkok, S.; Saengpeng, S. Municipal wastewater characteristics in Thailand and effects of soft intervention measures in households on pollutant discharge reduction. Water Sci. Technol. 2010, 62, 231–244. [Google Scholar] [CrossRef]
- Meier, P. Semi- and Decentralized Solutions for the Treatment of Domestic Wastewater and Wastewater of a Marketplace in Chiang Mai (Thailand). UNESCAP. 2016. Available online: https://unescap.org/sites/default/files/Report_TH_ChiangMai_MarketplaceWastewaterSolutions_Meier_2017_0.pdf (accessed on 29 June 2025).
- Taweesan, A.; Koottatep, T.; Dongo, K. Factors influencing the performance of faecal sludge management services: Case study in Thailand municipalities. Environ. Dev. Sustain. 2017, 19, 125–140. [Google Scholar] [CrossRef]
- Kuraji, K.; Punyatrong, K.; Sirisiyard, I. Six years intensive rainfall observation in Mae Chaem Watershed, Northern Thailand. In Proceedings of the 6th International Study Conference on GEWEX in Asia and GAME, Kyoto, Japan, 3–5 December 2004; Volume 5. Available online: https://hyarc.nagoya-u.ac.jp/game/6thconf/html/abs_html/pdfs/T3KK09Aug04160237.pdf (accessed on 29 June 2025).
- Ziegler, A.D.; Benner, S.G.; Tantasirin, C.; Wood, S.H.; Sutherland, R.A.; Sidle, R.C.; Jachowski, N.; Nullet, M.A.; Xi, L.X.; Snidvongs, A.; et al. Turbidity-based sediment monitoring in northern Thailand: Hysteresis, variability, and uncertainty. J. Hydrol. 2014, 519, 2020–2039. [Google Scholar] [CrossRef]
- Guo, J.; Liu, S.; Zhou, L.; Cheng, B.; Li, Q. Prioritizing pharmaceuticals based on environmental risks in the aquatic environment in China. J. Environ. Manag. 2021, 278, 111479. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dos Santos, M.M.; Tanabe, P.; Harraka, G.T.; Magnuson, J.T.; McGruer, V.; Schlenk, D. Bioassay Guided Analysis Coupled with Non-Target Chemical Screening in Polyethylene Plastic Shopping Bag Fragments after Exposure to Simulated Gastric Juice of Fish. J. Hazard. Mater. 2021, 401, 123421. [Google Scholar] [CrossRef] [PubMed]
- Moschet, C.; Lew, B.M.; Hasenbein, S.; Anumol, T.; Young, T.M. LC- and GC-QTOF-MS as Complementary Tools for a Comprehensive Micropollutant Analysis in Aquatic Systems. Environ. Sci. Technol. 2017, 51, 1553–1561. [Google Scholar] [CrossRef]
- Norman Toxicology Database. Available online: https://www.norman-network.com/nds/ecotox/ (accessed on 25 June 2025).
- Skeffington, R.A.; Halliday, S.J.; Wade, A.J.; Bowes, M.J.; Loewenthal, M. Using high-frequency water quality data to assess sampling strategies for the EU Water Framework Directive. Hydrol. Earth Syst. Sci. 2015, 19, 2491–2504. [Google Scholar] [CrossRef]
- Torres, C.; Gitau, M.W.; Paredes-Cuervo, D.; Engel, B. Evaluation of sampling frequency impact on the accuracy of water quality status as determined considering different water quality monitoring objectives. Environ. Monit. Assess. 2022, 194, 489. [Google Scholar] [CrossRef]
- Cormier, G.; Barbeau, B.; Arp, H.P.H.; Sauvé, S. The degradation behaviour of nine diverse contaminants in urban surface water and wastewater prior to water treatment. Environ. Sci. Process. Impacts 2015, 17, 2051–2065. [Google Scholar] [CrossRef]
- Soller, J.; Stephenson, J.; Olivieri, K.; Downing, J.; Olivieri, A.W. Evaluation of seasonal scale first flush pollutant loading and implications for urban runoff management. J. Environ. Manag. 2005, 76, 309–318. [Google Scholar] [CrossRef]
- Schiff, K.C.; Tiefenthaler, L.L. Seasonal flushing of pollutant concentrations and loads in urban stormwater. JAWRA J. Am. Water Resour. Assoc. 2011, 47, 136–142. [Google Scholar] [CrossRef]
- Chuah, C.J.; Ziegler, A.D. Temporal variability of faecal contamination from on-site sanitation systems in the groundwater of Northern Thailand. Environ. Manag. 2018, 61, 939–953. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). ECOTOX Knowledgebase [Data Set]. Available online: https://cfpub.epa.gov/ecotox/ (accessed on 2 July 2025).
- Judson, R.; Richard, A.; Dix, D.J.; Houck, K.; Martin, M.; Kavlock, R.; Dellarco, V.; Henry, T.; Holderman, T.; Sayre, P.; et al. The toxicity data landscape for environmental chemicals. Environ. Health Perspect. 2009, 117, 685–695. [Google Scholar] [CrossRef]
- Swedish Chemicals Agency (KEMI). PRIO—A Tool for Identifying Hazardous Substances. Available online: https://www.kemi.se/en/prio-start (accessed on 2 July 2025).
- Biswas, P.; Vellanki, B.P. Occurrence of emerging contaminants in highly anthropogenically influenced river Yamuna in India. Sci. Total Environ. 2021, 782, 146741. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubair, M.; Mustafa, G.; Nazar, M.F.; Sun, Q. Occurrence, Spatial Variation and Risk Assessment of Pharmaceuticals and Personal Care Products in Urban Wastewater, Canal Surface Water, and Their Sediments: A Case Study of Lahore, Pakistan. Sci. Total Environ. 2019, 688, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and Risk Assessment of Multiple Classes of Antibiotics in Urban Canals and Lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.I.A.; Fernando, B.A.V.W.; Dehini, G.K. Assessment of Pollution Sources, Fate of Pollutants, and Potential Instream Interventions to Mitigate Pollution of Earthen Canals of Urban to Rural-Urban Fringe. Water Air Soil Pollut. 2019, 230, 262. [Google Scholar] [CrossRef]
- Caracciolo, R. Spatial and Temporal Dynamics of Multi-Class Emerging Contaminants in the Saigon-Dongnai River and Estuarine System, Vietnam. Ph.D. Thesis, Université Grenoble Alpes, Auvergne-Rhône-Alpes, France, 2023. Available online: https://theses.hal.science/tel-04861161/ (accessed on 22 June 2025).
- Sumida, T.; Takada, H.; Takei, A.; Yoshimatsu, K.; Imai, S.; Koike, T.; Banno, M.; Fujisawa, M.; Isogai, S.; Alidoust, M.; et al. Artificial sweeteners in surface waters from Asian, African and Middle Eastern countries: Utility as molecular markers and water pollution status in 2010–2019. Environ. Monit. Contam. Res. 2024, 4, 1–18. [Google Scholar] [CrossRef]
- Gan, Z.; Sun, H.; Feng, B.; Wang, R.; Zhang, Y. Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Res. 2013, 47, 4928–4937. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Q.; Yang, X. Occurrence and removal of acesulfame and sucralose in the drinking water treatment plants along the Yangtze River. Water Supply 2019, 19, 1305–1312. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Zhao, J.-L.; Liu, Y.-S.; Liu, W.-R.; Zhang, Q.-Q.; Yao, L.; Hu, L.-X.; Zhang, J.-N.; Jiang, Y.-X.; Ying, G.-G. Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci. Total Environ. 2018, 616, 816–823. [Google Scholar] [CrossRef]
- Buerge, I.J.; Buser, H.R.; Kahle, M.; Muller, M.D.; Poiger, T. Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: An ideal chemical marker of domestic wastewater in groundwater. Environ. Sci. Technol. 2009, 43, 4381–4385. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Q.; Thomas, K.V.; Dang, A.K.; Binh, V.N.; Anh, N.T.K.; Thai, P.K. Use of artificial sweeteners and caffeine in a population of Hanoi: An assessment by wastewater-based epidemiology. Sci. Total Environ. 2023, 868, 161515. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, F.; Pal, A.; Gin, K.Y.H.; Reinhard, M. Occurrence of emerging organic contaminants in a tropical urban catchment in Singapore. Chemosphere 2011, 83, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Hu, J.; Li, J.; Ong, S.L. Suitability of artificial sweeteners as indicators of raw wastewater contamination in surface water and groundwater. Water Res. 2014, 48, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Buser, H.R.; Poiger, T.; Müller, M.D. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals in the Thames catchment. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef]
- Carlsson, C.; Johansson, A.K.; Alvan, G.; Bergman, K.; Kühler, T. Are pharmaceuticals potent environmental pollutants? Part I: Environmental risk assessments of selected active pharmaceutical ingredients. Sci. Total Environ. 2006, 364, 67–87. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-steroidal anti-inflammatory drugs in the environment: Where were we and how far we have come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef]
- Rodrigues, F.; Durães, L.; Simões, N.E.; Pereira, A.M.; Silva, L.J.; Feio, M.J. Pharmaceuticals in urban streams: A review of their detection and effects in the ecosystem. Water Res. 2024, 268, 122657. [Google Scholar] [CrossRef]
- Batucan, N.S.P.; Tremblay, L.A.; Northcott, G.L.; Matthaei, C.D. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environ. Adv. 2022, 7, 100164. [Google Scholar] [CrossRef]
- Kookana, R.S.; Williams, M.; Boxall, A.B.A.; Larsson, D.G.J.; Gaw, S.; Choi, K.; Yamamoto, H.; Thatikonda, S.; Zhu, Y.-G.; Carriquiriborde, P. Potential Ecological Footprints of Active Pharmaceutical Ingredients: An Examination of Risk Factors in Low-, Middle- and High-Income Countries. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130586. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Yuzir, A.; Al-Qaim, F.F.; Yahaya, N.K.E. Occurrence and distribution of 17 targeted human pharmaceuticals in various aquatic environmental matrices in Southeast Asia with particular reference to Malaysia: A comprehensive review. J. Mex. Chem. Soc. 2021, 65, 434–456. [Google Scholar] [CrossRef]
- Hanafiah, Z.M.; Mohtar, W.H.M.W.; Manan, T.S.A.; Bachi, N.A.; Abu Tahrim, N.; Hamid, H.H.A.; Ghanim, A.; Ahmad, A.; Rasdi, N.W.; Aziz, H.A. Determination and risk assessment of pharmaceutical residues in the urban water cycle in Selangor Darul Ehsan, Malaysia. PeerJ 2023, 11, e14719. [Google Scholar] [CrossRef]
- Villanueva, A.C.; Garcia, M.V.; Resurreccion, A.C. Fate and transport of ibuprofen in a tropical river system. Philipp. J. Sci. 2017, 146, 457–465. Available online: https://iaras.org/iaras/filedownloads/ijes/2017/008-0037(2017).pdf (accessed on 29 June 2025).
- Astuti, N.F.Y.; Sundana, P.T.; Nisaa, A.F.; Mardyanto, M.A. The presence of pharmaceutical compounds in Surabaya rivers: Potential sources and correlation with other water quality parameters. IOP Conf. Ser. Earth Environ. Sci. 2024, 1307, 012017. [Google Scholar] [CrossRef]
- Waleng, N.J.; Nomngongo, P.N. Occurrence of pharmaceuticals in the environmental waters: African and Asian perspectives. Environ. Chem. Ecotoxicol. 2022, 4, 50–66. [Google Scholar] [CrossRef]
- Trianda, Y.; Adityosulindro, S.; Moersidik, S.S. Ibuprofen as an emerging contaminant of concern: Occurrence in Southeast Asia water environment. E3S Web Conf. 2024, 530, 02007. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, X.; Wang, Z.; Xie, D.; Li, Z. Water quality criteria derivation and ecological risk assessment for ibuprofen, a common antifever drug in China. Mar. Dev. 2023, 1, 5. [Google Scholar] [CrossRef]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef]
- Shanmugam, G.; Sampath, S.; Selvaraj, K.K.; Larsson, D.J.; Ramaswamy, B.R. Non-steroidal anti-inflammatory drugs in Indian rivers. Environ. Sci. Pollut. Res. 2014, 21, 921–931. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Panchangam, S.C.; Chen, H.Y. Implications of human pharmaceutical occurrence in the Sindian River of Taiwan: A strategic study of risk assessment. J. Environ. Monit. 2010, 12, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Blasco, J.; Trombini, C. Ibuprofen and diclofenac in the marine environment—A critical review of their occurrence and potential risk for invertebrate species. Water Emerg. Contam. Nanoplast. 2023, 2, 14. [Google Scholar] [CrossRef]
- Huang, Q.; Bu, Q.; Zhong, W.; Shi, K.; Cao, Z.; Yu, G. Derivation of aquatic predicted no-effect concentration (PNEC) for ibuprofen and sulfamethoxazole based on various toxicity endpoints and the associated risks. Chemosphere 2018, 193, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-González, A.B. Ibuprofen as an emerging pollutant on non-target aquatic invertebrates: Effects on Chironomus riparius. Environ. Toxicol. Pharmacol. 2021, 81, 103537. [Google Scholar] [CrossRef]
- Gallego-Ríos, S.E.; Peñuela, G.A. Evaluation of ibuprofen and diclofenac in the main rivers of Colombia and striped catfish Pseudoplatystoma magdaleniatum. Environ. Monit. Assess. 2021, 193, 210. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Pan, L.; Zhou, X.; Zhao, L.; Mou, X.; Zhou, H.; Liu, J.; Wang, X. Evaluation of ibuprofen contamination in local urban rivers and its effects on immune parameters of juvenile grass carp. Fish Physiol. Biochem. 2021, 47, 1405–1413. [Google Scholar] [CrossRef]
- Mastrángelo, M.M.; Valdés, M.E.; Eissa, B.; Ossana, N.A.; Barceló, D.; Sabater, S.; Rodríguez-Mozaz, S.; Giorgi, A.D.N. Occurrence and accumulation of pharmaceutical products in water and biota of urban lowland rivers. Sci. Total Environ. 2022, 828, 154303. [Google Scholar] [CrossRef]
- Godoi, F.G.; Dias, M.A.; Montagner, C.C.; Lo Nostro, F.L.; Moreira, R.G. Review of the nonsteroidal anti-inflammatory drug consumption, occurrence, potential impacts on environmental health, and insights into regulatory decision-making Brazilian aquatic ecosystems. ACS Omega 2025, 10, 26250–26265. [Google Scholar] [CrossRef]
- Canle, M.; Antão-Geraldes, A.M. A snapshot on the occurrence and risk assessment of organic pollutants in an urban river. Appl. Sci. 2022, 13, 146. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Barinova, S.; Lozano, V.L.; Afanasyev, S.; Leite, T.; Branco, P.; Gomez Isaza, D.F.; Geist, J.; Tegos, A.; et al. Multi-Interacting Natural and Anthropogenic Stressors on Freshwater Ecosystems: Their Current Status and Future Prospects for 21st Century. Water 2024, 16, 1483. [Google Scholar] [CrossRef]
- Kidd, K.A.; Backhaus, T.; Brodin, T.; Inostroza, P.A.; McCallum, E.S. Environmental Risks of Pharmaceutical Mixtures in Aquatic Ecosystems: Reflections on a Decade of Research. Environ. Toxicol. Chem. 2024, 43, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and trophic transfer of pharmaceuticals in food webs from a large freshwater lake. Environ. Pollut. 2017, 225, 230–238. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F. What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1453–1496. [Google Scholar] [CrossRef]
- Rico, A.; Oliveira, R.; McDonough, S.; Matser, A.; Khatikarn, J.; Satapornvanit, K.; Nogueira, A.J.; Soares, A.M.; Domingues, I.; Van den Brink, P.J. Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ. Pollut. 2014, 191, 8–16. [Google Scholar] [CrossRef]
- Suzuki, S.; Ogo, M.; Takada, H.; Seki, K.; Mizukawa, K.; Kadoya, A.; Yokokawa, T.; Sugimoto, Y.; Sato-Takabe, Y.; Boonla, C.; et al. Contamination of antibiotics and sul and tet (M) genes in veterinary wastewater, river, and coastal sea in Thailand. Sci. Total Environ. 2021, 791, 148423. [Google Scholar] [CrossRef]
- Chan, R.; Chiemchaisri, C.; Chiemchaisri, W.; Boonsoongnern, A.; Tulayakul, P. Occurrence of antibiotics in typical pig farming and its wastewater treatment in Thailand. Emerg. Contam. 2022, 8, 21–29. [Google Scholar] [CrossRef]
- Kruawal, K.; Sacher, F.; Werner, A.; Müller, J.; Knepper, T.P. Chemical Water Quality in Thailand and Its Impacts on the Drinking Water Production in Thailand. Sci. Total Environ. 2005, 340, 57–70. [Google Scholar] [CrossRef]
- Boontanon, S.K.; Kunacheva, C.; Boontanon, N.; Musirat, N.; Fujii, S.; Tanaka, S. Occurrence of perfluorooctane sulfonate in the water environment of Bangkok, Thailand. J. Environ. Eng. 2013, 139, 588–593. [Google Scholar] [CrossRef]
- Ng, K.; Alygizakis, N.; Nika, M.-C.; Galani, A.; Oswald, P.; Oswaldova, M.; Čirka, Ľ.; Kunkel, U.; Macherius, A.; Sengl, M.; et al. Wide-scope target screening characterization of legacy and emerging contaminants in the Danube River Basin by liquid and gas chromatography coupled with high-resolution mass spectrometry. Water Res. 2023, 230, 119539. [Google Scholar] [CrossRef]
- Nika, M.; Ntaiou, K.; Elytis, K.; Thomaidi, V.; Gatidou, G.; Kalantzi, O.; Thomaidis, N.; Stasinakis, A. Wide-scope target analysis of emerging contaminants in landfill leachates and risk assessment using Risk Quotient methodology. J. Hazard. Mater. 2020, 394, 122493. [Google Scholar] [CrossRef]
- Perazzolo, C.; Morasch, B.; Kohn, T.; Magnet, A.; Thonney, D.; Chèvre, N. Occurrence and fate of micropollutants in the Vidy Bay of Lake Geneva, Switzerland. Part I: Priority list for environmental risk assessment of pharmaceuticals. Environ. Toxicol. Chem. 2010, 29, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, M.; Godlewska, K.; Lis, H.; Caban, M.; Białk-Bielińska, A.; Stepnowski, P. Advances in suspect screening and non-target analysis of polar emerging contaminants in the environmental monitoring. TrAC Trends Anal. Chem. 2022, 154, 116671. [Google Scholar] [CrossRef]
- Shafi, M.; Jan, R.; Gani, K.M. Selection of priority emerging contaminants in surface waters of India, Pakistan, Bangladesh, and Sri Lanka. Chemosphere 2023, 341, 139976. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.P.; Groff, L.C., II; Sobus, J.R. Quantitative non-targeted analysis: Bridging the gap between contaminant discovery and risk characterization. Environ. Int. 2022, 158, 107011. [Google Scholar] [CrossRef]
- Hoang, A.T.; Do, M.C.; Kim, K.W. Environmental risk assessment of selected pharmaceuticals in hospital wastewater in northern Vietnam. Chemosphere 2024, 356, 141973. [Google Scholar] [CrossRef]
- Niu, J.; Lu, Y.; Wang, H.; Qiao, X.; Wang, H.; Ma, C.; Liu, Y. Occurrence, removal and environmental risk assessment of pharmaceutical active compounds (PhACs) and metabolites in hospital wastewater. J. Hazard. Mater. 2024, 480, 136348. [Google Scholar] [CrossRef]
- Anceno, A.J.; Ozaki, M.; Dang, Y.N.D.; Chuluun, B.; Shipin, O.V. Canal networks as extended waste stabilization ponds: Fate of pathogens in constructed waterways in Pathumthani Province, Thailand. Water Sci. Technol. 2007, 55, 143–156. [Google Scholar] [CrossRef]
- Honda, R.; Hara, Y.; Sekiyama, M.; Hiramatsu, A. Impacts of housing development on nutrients flow along canals in a peri-urban area of Bangkok, Thailand. Water Sci. Technol. 2010, 61, 1073–1080. [Google Scholar] [CrossRef]
- Wongaree, M. Water Quality Assessment by Using of Water Quality Index for Mak Khaeng Canal, Udon Thani Province, Thailand. EnvironmentAsia 2019, 12, 93–106. [Google Scholar] [CrossRef]
- Eamrat, R.; Taweesan, A.; Pussayanavin, T. Assessment of Microplastics Distribution and Related Water Quality in an Urban Canal, Thailand. Pollution 2022, 8, 1172–1184. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, R.C.; Katz, A.J. Why is a small sample size not enough? Oncologist 2024, 29, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Di Cecco, G.J.; Gouhier, T.C. Increased spatial and temporal autocorrelation of temperature under climate change. Sci. Rep. 2018, 8, 14850. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, K.; Miller, C.; Scott, E.M.; Willows, R.; Pope, L.; Douglass, J. Flow-directed PCA for monitoring networks. Environmetrics 2017, 28, e2434. [Google Scholar] [CrossRef] [PubMed]
- Looi, L.J.; Aris, A.Z.; Yusoff, F.M.; Isa, N.M.; Haris, H. Application of enrichment factor, geoaccumulation index, and ecological risk index in assessing the elemental pollution status of surface sediments. Environ. Geochem. Health 2019, 41, 27–42. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Rieckermann, J.; Joss, A. Sampling for Pharmaceuticals and Personal Care Products (PPCPs) and Illicit Drugs in Wastewater Systems: Are Your Conclusions Valid? A Critical Review. Environ. Sci. Technol. 2010, 44, 6024–6035. [Google Scholar] [CrossRef]
- Gerrity, D.; Trenholm, R.A.; Snyder, S.A. Temporal variability of pharmaceuticals and illicit drugs in wastewater and the effects of a major sporting event. Water Res. 2011, 45, 5399–5411. [Google Scholar] [CrossRef]
- Rozemeijer, J.; Jordan, P.; Hooijboer, A.; Kronvang, B.; Glendell, M.; Hensley, R.; Rinke, K.; Stutter, M.; Bieroza, M.; Turner, R.; et al. Best practice in high-frequency water quality monitoring for improved management and assessment; a novel decision workflow. Environ. Monit. Assess. 2025, 197, 353. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]


| P-14 | Canals | Exits to River | P-40 | PNEC | |
|---|---|---|---|---|---|
| Sample Number | 13 | 53 | 39 | 13 | |
| Atenolol | bdl | 122 [bdl–231] 0.85 | 46 [bdl–416] 0.64 | bdl [bdl–10] 0.08 | 150,000 |
| Carbamazepine | bdl | 19 [bdl–79] 0.91 | 16 [bdl–59] 0.90 | bdl [bdl–10] 0.23 | 2000 |
| Estrone | bdl | bdl [bdl–22] 0.30 | bdl [bdl–15] 0.46 | bdl | <1 |
| Metoprolol | bdl | 151 [bdl–663] 0.92 | 92 [bdl–592] 0.87 | 8 [bdl–17] 0.69 | 8600 |
| Diclofenac | bdl | 59 [bdl–251] 0.74 | 33 [bdl–274] 0.72 | bdl [bdl–7] 0.08 | 40 |
| Ibuprofen | bdl | 634 [bdl–1558] 0.98 | 203 [bdl–1727] 0.77 | bdl [bdl–32] 0.31 | 11 |
| Naproxen | bdl | bdl [bdl–294] 0.38 | 42 [bdl–192] 0.67 | bdl [bdl–22] 0.23 | 1700 |
| Diphenhydramine | bdl | 71 [bdl–778] 0.53 | 26 [bdl–525] 0.51 | bdl [bdl–30] 0.08 | 990 |
| Acetaminophen | bdl | 3526 [bdl–9908] 0.89 | 259 [bdl–3595] 0.64 | bdl [bdl–30] 0.08 | 46,000 |
| Fexofenadine | bdl [bdl–9] 0.08 | 1019 [bdl–15,932] 0.96 | 1015 [108–8883] 1.0 | 173 [bdl–1730] 0.92 | 200,000 |
| Gabapentin | bdl | 3727 [bdl–11,771] 0.94 | 2595 [bdl–12,287] 0.97 | bdl [bdl–1337] 0.38 | 1,000,000 |
| Gemfibrozil | bdl | 648 [25–1318] 1.0 | 274 [35–2008] 1.0 | 28 [10–77] 1.0 | 500 |
| Metformin | bdl [bdl–259] 0.08 | 915 [bdl–4145] 0.62 | 410 [bdl–19,977] 0.62 | bdl [bdl–538] 0.38 | 160,000 |
| Sulfamethoxazole | bdl | 294 [bdl–1922] 0.96 | 137 [bdl–3223] 0.90 | 14 [bdl–157] 0.69 | 600 |
| Valsartan | bdl | 212 [81–1506] 1.0 | 190 [bdl–1086] 0.85 | bdl [bdl–68] 0.38 | 560,000 |
| Acesulfame | 32 [bdl–72] 0.62 | 13,221 [1170–22,707] 1.0 | 4128 [403–21,469] 1.0 | 516 [165–1474] 1.0 | 72,400 |
| Caffeine | bdl [bdl–1171] 0.31 | 4619 [102–11,556] 1.0 | 1588 [202–5750] 1.0 | 268 [121–1118] 1.0 | 1200 |
| Sucralose | bdl [bdl–46] 0.08 | 4083 [bdl–38,335] 0.94 | 1395 [bdl–10,695] 0.87 | 375 [bdl–2048] 0.62 | 29,700 |
| 4-Nitrophenol | bdl [bdl–75] 0.31 | 441 [bdl–8727] 0.89 | 21 [bdl–1681] 0.74 | bdl [bdl–61] 0.31 | 5000 |
| TBEP | bdl [bdl–121] 0.31 | 112 [bdl–3223] 0.81 | 522 [bdl–3950] 0.85 | 131 [bdl–363] 0.85 | 44,800 |
| Tolyltriazole | bdl | 91 [bdl–2191] 0.81 | 41 [bdl–681] 0.87 | 8 [bdl–58] 0.54 | 8000 (m) |
| 2,4-D | 13 [bdl–322] 0.62 | bdl [bdl–1303] 0.45 | 55 [bdl–1712] 0.85 | 33 [bdl–238] 0.92 | 600 |
| Atrazine | 150 [25–1612] 1.0 | 45 [bdl–240] 0.92 | 42 [15–157] 1.0 | 119 [23–963] 1.0 | 600 |
| Fenobucarb | bdl [bdl–183] 0.31 | bdl [bdl–1159] 0.42 | bdl [bdl–493] 0.36 | 8 [bdl–997] 0.54 | 2130 |
| P-55 | P-11 | P-08 | P-05 | PNEC | |
|---|---|---|---|---|---|
| Sample Number | 12 | 13 | 14 | 14 | |
| Atenolol | 8 [bdl–209] 0.5 | 117 [bdl–231] 0.92 | 134 [24–198] 1.0 | 102 [bdl–143] 0.93 | 150,000 |
| Carbamazepine | 23 [bdl–40] 0.92 | 16 [bdl–33] 0.92 | 24 [bdl–79] 0.86 | 20 [bdl–42] 0.93 | 2000 |
| Estrone | bdl [bdl–12] 0.33 | bdl [bdl–22] 0.46 | bdl [bdl–16] 0.36 | bdl [bdl–6] 0.07 | <1 |
| Metoprolol | 201 [55–609] 1.0 | 109 [bdl–467] 0.92 | 215 [bdl–546] 0.86 | 155 [bdl–663] 0.93 | 8600 |
| Diclofenac | 29 [bdl–87] 0.75 | 81 [bdl–251] 0.69 | 79 [bdl–114] 0.71 | 40 [bdl–88] 0.79 | 40 |
| Ibuprofen | 85 [bdl–617] 0.92 | 712 [379–1558] 1.0 | 755 [101–1380] 1.0 | 467 [95–1263] 1.0 | 11 |
| Naproxen | 38 [bdl–168] 0.92 | bdl [bdl–132] 0.23 | bdl [bdl–294] 0.14 | bdl [bdl–95] 0.29 | 1700 |
| Diphenhydramine | bdl [bdl–72] 0.08 | 147 [bdl–500] 0.69 | bdl [bdl–778] 0.43 | 73 [bdl–325] 0.86 | 990 |
| Acetaminophen | 284 [bdl–2857] 0.75 | 3330 [bdl–9908] 0.85 | 5423 [781–7663] 1.0 | 2695 [4884] 0.93 | 46,000 |
| Fexofenadine | 911 [361–4007] 1.0 | 1930 [519–15,932] 1.0 | 612 [bdl–12,460] 0.93 | 661 [bdl–4383] 0.93 | 200,000 |
| Gabapentin | 2900 [548–9202] 1.0 | 3552 [bdl–11,771] 0.92 | 4648 [bdl–6438] 0.93 | 2844 [bdl–4872] 0.93 | 1,000,000 |
| Gemfibrozil | 85 [25–701] 1.0 | 648 [362–1191] 1.0 | 821 [80–1318] 1.0 | 570 [161–1025] 1.0 | 500 |
| Metformin | 865 [bdl–3230] 0.67 | 1521 [bdl–4057] 0.69 | 518 [bdl–4145] 0.57 | 920 [bdl–3179] 0.57 | 160,000 |
| Sulfamethoxazole | 167 [5–2159] 1.0 | 294 [bdl–1067] 0.92 | 409 [bdl–1922] 0.93 | 99 [38–1786] 1.0 | 600 |
| Valsartan | 247 [89–481] 1.0 | 328 [109–1506] 1.0 | 246 [81–578] 1.0 | 262 [109–1326] 1.0 | 560,000 |
| Acesulfame | 2543 [1107–10,577] 1.0 | 15,087 [4769–22,707] 1.0 | 13,331 [2072–22,188] 1.0 | 8950 [2466–21,567] 1.0 | 72,400 |
| Caffeine | 867 [202–2832] 1.0 | 4804 [1569–6771] 1.0 | 4538 [685–9180] 1.0 | 4713 [1357–11,556] 1.0 | 1200 |
| Sucralose | 954 [bdl–3663] 0.75 | 6842 [1891–38,335] 1.0 | 4445 [1085–11,239] 1.0 | 3119 [bdl–7017] 1.0 | 29,700 |
| 4-Nitrophenol | 21 [bdl–735] 0.67 | 296 [33–5055] 1.0 | 990 [bdl–18–5169] 1.0 | 209 [bdl–8727] 0.86 | 5000 |
| TBEP | 158 [bdl–2069] 0.83 | 101 [bdl–342] 0.85 | 81 [bdl–2864] 0.71 | 189 [bdl–3223] 0.86 | 44,800 |
| Tolyltriazole | 7 [bdl–71] 0.58 | 140 [bdl–2191] 0.85 | 90 [bdl–1235] 0.93 | 84 [bdl–1242] 0.86 | 8000 (m) |
| 2,4-D | bdl [bdl–180] 0.33 | 28 [bdl–519] 0.54 | bdl [bdl–1303] 0.29 | 28 [bdl–808] 0.64 | 600 |
| Atrazine | 17 [bdl–105] 0.75 | 42 [21–147] 1.0 | 50 [19–109] 1.0 | 45 [bdl–240] 0.93 | 600 |
| Fenobucarb | bdl [bdl–56] 0.17 | 20 [bdl–294] 0.54 | 25 [bdl–1159] 0.57 | bdl [bdl–411] 0.36 | 2130 |
| P-10 | P-41 | P-39 | PNEC | |
|---|---|---|---|---|
| Sample Number | 13 | 13 | 13 | |
| Atenolol | 54 [bdl–416] 0.92 | 61 [24–110] 1.0 | bdl | 150,000 |
| Carbamazepine | 14 [bdl–59] 0.85 | 19 [bdl–39] 0.92 | 15 [bdl–37] 0.92 | 2000 |
| Estrone | 6 [bdl–14] 0.69 | 6 [bdl–15] 0.54 | bdl [bdl–9] 0.15 | <1 |
| Metoprolol | 194 [45–592] 1.0 | 123 [29–263] 1.0 | 8 [bdl–26] 0.62 | 8600 |
| Diclofenac | 41 [27–274] 1.0 | 34 [bdl–83] 0.77 | bdl [bdl–15] 0.38 | 40 |
| Ibuprofen | 262 [141–1727] 1.0 | 257 [78–558] 1.0 | bdl [bdl–39] 0.31 | 11 |
| Naproxen | 55 [bdl–192] 0.69 | 74 [bdl–171] 0.85 | bdl [bdl–27] 0.46 | 1700 |
| Diphenhydramine | 37 [bdl–525] 0.85 | 33 [bdl–173] 0.69 | bdl | 990 |
| Acetaminophen | 533 [bdl–3595] 0.92 | 511 [bdl–891] 0.85 | bdl [bdl–245] 0.15 | 46,000 |
| Fexofenadine | 1177 [378–8883] 1.0 | 1530 [574–6445] 1.0 | 471 [107–1415] 1.0 | 200,000 |
| Gabapentin | 3624 [1292–12,287] 1.0 | 3076 [1045–6541] 1.0 | 1763 [bdl–3719] 0.92 | 1,000,000 |
| Gemfibrozil | 294 [201–2008] 1.0 | 372 [123–584] 1.0 | 48 [35–158] 1.0 | 500 |
| Metformin | 771 [bdl–19,977] 0.69 | 1460 [bdl–6815] 0.69 | bdl [bdl–493] 0.46 | 160,000 |
| Sulfamethoxazole | 276 [59–3223] 1.0 | 187 [95–456] 1.0 | 14 [bdl–75] 0.69 | 600 |
| Valsartan | 201 [bdl–1086] 0.92 | 510 [bdl–861] 0.92 | 53 [bdl–121] 0.69 | 560,000 |
| Acesulfame | 4751 [2735–21,469] 1.0 | 4629 [2001–7779] 1.0 | 611 [403–1342] 1.0 | 72,400 |
| Caffeine | 2220 [1004–5750] 1.0 | 2526 [1207–3682] 1.0 | 278 [102–909] 1.0 | 1200 |
| Sucralose | 1216 [bdl–10,695] 0.85 | 1665 [bdl–10,358] 0.92 | 861 [bdl–2556] 0.85 | 29,700 |
| 4-Nitrophenol | 30 [bdl–1681] 0.85 | 84 [5–1189] 1.0 | bdl [bdl–53] 0.38 | 5000 |
| TBEP | 645 [bdl–2265] 0.85 | 1649 [bdl–3950] 0.85 | 214 [bdl–636] 0.85 | 44,800 |
| Tolyltriazole | 15 [bdl–681] 0.77 | 60 [17–278] 1.0 | 63 [bdl–143] 0.85 | 8000 (m) |
| 2,4-D | 36 [bdl–115] 0.69 | 55 [bdl–408] 0.85 | 74 [15–1712] 1.0 | 600 |
| Atrazine | 41 [16–105] 1.0 | 53 [25–157] 1.0 | 40 [15–136] 1.0 | 600 |
| Fenobucarb | bdl [bdl–110] 0.38 | bdl [bdl–62] 0.31 | bdl [bdl–493] 0.38 | 2130 |
| Compound | Samples Enriched * | Median ERF | ERF Range | Comment |
|---|---|---|---|---|
| Acesulfame | 100% | 13 | 5 to 590 | Constant |
| Gemfibrozil | 100% | 11 | 4 to 31 | Constant |
| Fexofenadine | 92% | 72 | 11 to 692 | Near-constant |
| TBEP | 85% | 21 | 2 to 60 | Near-constant |
| Caffeine | 77% | 4 | 2 to 7 | Frequent |
| Sucralose | 62% | 70 | 20 to 273 | Episodic |
| Sulfamethoxazole | 62% | 6 | <2 to 31 | Episodic |
| 2,4-D | 62% | 7 | <2 to 18 | Episodic |
| Tolyltriazole | 54% | 10 | 3 to 23 | Episodic |
| Fenobucarb | 54% | 8 | 3 to 32 | Episodic |
| EPC | Mae Kha Canal (MK) | Other Urban Canals (OU) | Peri-Urban Canals (PU) | Rural-Remote (RR) | EF (OU vs. PU) | EF (OU vs. RR) |
|---|---|---|---|---|---|---|
| ng/L | ng/L | ng/L | ng/L | - | - | |
| Acesulfame | 13,221 | 3275 | 257 | 45 | 13 | 73 |
| Ibuprofen | 634 | 84 | bdl | bdl | 34+ | 34+ |
| Gemfibrozil | 648 | 138 | 8 | bdl | 16 | 55+ |
| TBEP | 112 | 171 | bdl | 10 | 23+ | 16 |
| Caffeine | 4619 | 598 | 213 | 122 | 3 | 5 |
| Metformin | 915 | 401 | 157 | 50 | 3 | 8+ |
| Sucralose | 4083 | 1970 | bdl | bdl | 394+ | 394+ |
| 4-Nitrophenol | 441 | 72 | bdl | bdl | 19+ | 29+ |
| Tolyltriazole | bdl | 7 | bdl | bdl | 3+ | 3+ |
| Fexofenadine | 1019 | 175 | 19 | bdl | 9 | 70+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegler, A.D.; Lee, T.H.Y.; Srinuansom, K.; Boonta, T.; Promya, J.; Webster, R.D. Canals, Contaminants, and Connections: Exploring the Urban Exposome in a Tropical River System. Urban Sci. 2025, 9, 302. https://doi.org/10.3390/urbansci9080302
Ziegler AD, Lee THY, Srinuansom K, Boonta T, Promya J, Webster RD. Canals, Contaminants, and Connections: Exploring the Urban Exposome in a Tropical River System. Urban Science. 2025; 9(8):302. https://doi.org/10.3390/urbansci9080302
Chicago/Turabian StyleZiegler, Alan D., Theodora H. Y. Lee, Khajornkiat Srinuansom, Teppitag Boonta, Jongkon Promya, and Richard D. Webster. 2025. "Canals, Contaminants, and Connections: Exploring the Urban Exposome in a Tropical River System" Urban Science 9, no. 8: 302. https://doi.org/10.3390/urbansci9080302
APA StyleZiegler, A. D., Lee, T. H. Y., Srinuansom, K., Boonta, T., Promya, J., & Webster, R. D. (2025). Canals, Contaminants, and Connections: Exploring the Urban Exposome in a Tropical River System. Urban Science, 9(8), 302. https://doi.org/10.3390/urbansci9080302






