Beyond Boundaries—Genetic Implications of Urbanisation and Isolation in Eastern Grey Kangaroos (Macropus giganteus)
Abstract
1. Introduction
- (i)
- Compare genetic characteristics of urban and non-urban kangaroo populations across multiple regions on the east coast of Australia;
- (ii)
- Assess the influence that habitat characteristics and isolation have on the genetics of these populations.
2. Materials and Methods
2.1. Study Sites
2.2. Habitat Classification
2.3. Immediate Habitat Characteristics
2.4. Surrounding Habitat Characteristics
2.5. Genetic Sample Collection
2.6. Scat Sampling
2.7. Tissue Sampling
2.8. Genetic Analysis
2.8.1. DNA Extraction
2.8.2. Reduced-Representation (SNP) Data and Filtering
2.9. Statistical Analysis
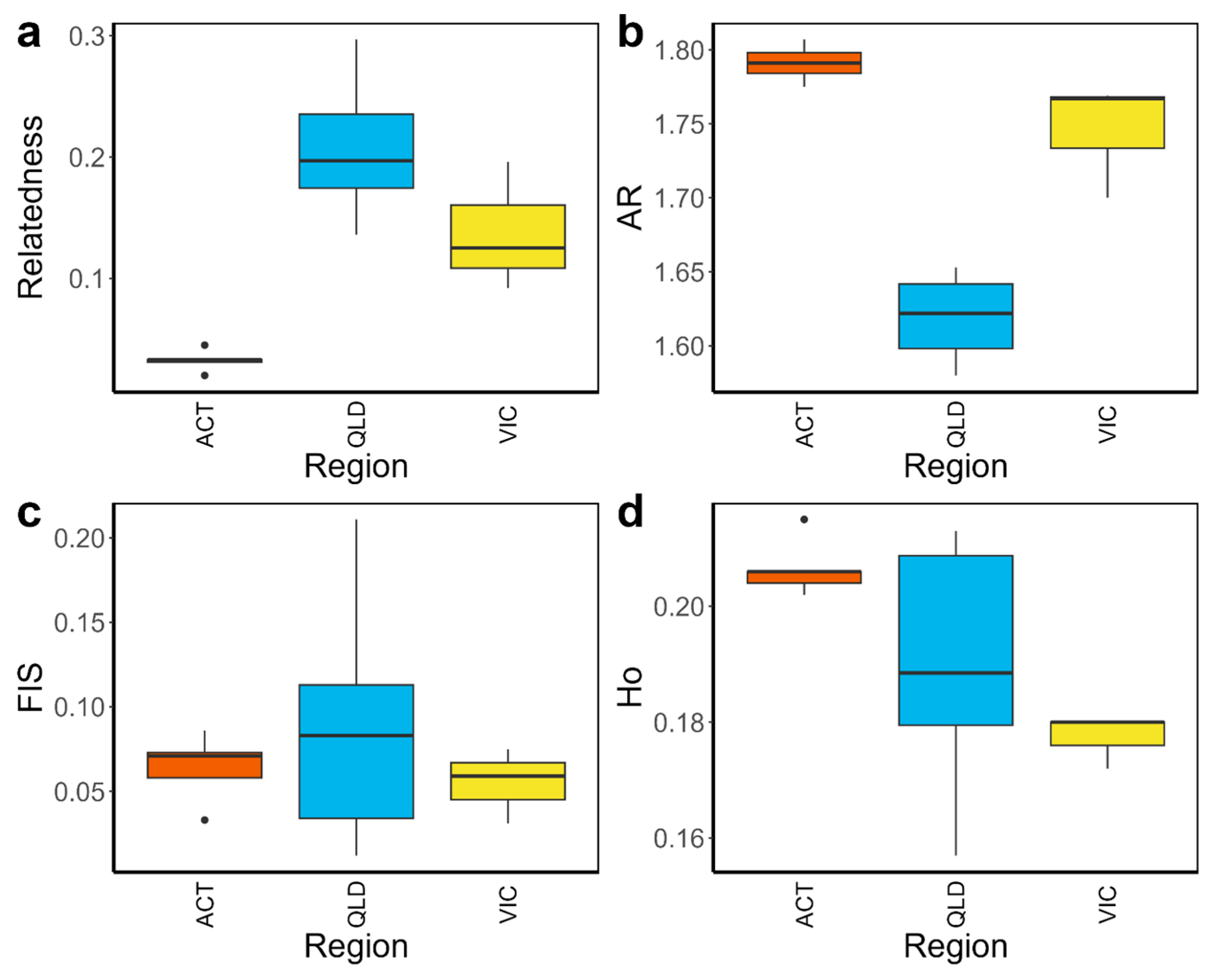
3. Results
3.1. Genetic Comparisons of Urban and Non-Urban Kangaroo Populations
3.2. Influence of Habitat Characteristics
3.2.1. Immediate Habitat
3.2.2. Surrounding Habitat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economics and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420); United Nations Department of Economics and Social Affairs, Population Division: New York, NY, USA, 2019; p. 41. [Google Scholar]
- Barnosky, A.D.; Hadly, E.A.; Bascompte, J.; Berlow, E.L.; Brown, J.H.; Fortelius, M.; Getz, W.M.; Harte, J.; Hastings, A.; Marquet, P.A.; et al. Approaching a state shift in Earth’s biosphere. Nature 2012, 486, 52–58. [Google Scholar] [CrossRef]
- Young, H.S.; McCauley, D.J.; Galetti, M.; Dirzo, R. Patterns, Causes, and Consequences of Anthropocene Defaunation. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 333–358. [Google Scholar] [CrossRef]
- Lino, A.; Fonseca, C.; Rojas, D.; Fischer, E.; Pereira, M.J.R. A meta-analysis of the effects of habitat loss and fragmentation on genetic diversity in mammals. Mamm. Biol. 2019, 94, 69–76. [Google Scholar] [CrossRef]
- Ramp, D.; Ben-Ami, D. The effect of road-based fatalities on the viability of a peri-urban swamp wallaby population. J. Wildl. Manag. 2006, 70, 1615–1624. [Google Scholar] [CrossRef]
- Taylor-Brown, A.; Booth, R.; Gillett, A.; Mealy, E.; Ogbourne, S.M.; Polkinghorne, A.; Conroy, G.C.; Bhadauria, T. The impact of human activities on Australian wildlife. PLoS ONE 2019, 14, e0206958. [Google Scholar] [CrossRef]
- Lowe, W.H.; Allendorf, F.W. What can genetics tell us about population connectivity? Mol. Ecol. 2010, 19, 3038–3051. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Lourenço, A.; Álvarez, D.; Wang, I.J.; Velo-Antón, G. Trapped within the city: Integrating demography, time since isolation and population-specific traits to assess the genetic effects of urbanization. Mol. Ecol. 2017, 26, 1498–1514. [Google Scholar] [CrossRef]
- Lancaster, M.L.; Cooper, S.J.B.; Carthew, S.M. Genetic consequences of forest fragmentation by agricultural land in an arboreal marsupial. Landsc. Ecol. 2016, 31, 655–667. [Google Scholar] [CrossRef]
- Ross-Gillespie, A.; O’RIain, M.J.; Keller, L.F. Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution 2007, 61, 2268–2273. [Google Scholar] [CrossRef]
- Seymour, A.M.; Montgomery, M.E.; Costello, B.H.; Ihle, S.; Johnsson, G.; John, B.S.; Taggart, D.; Houlden, B.A. High effective inbreeding coefficients correlate with morphological abnormalities in populations of South Australian koalas (Phascolarctos cinereus). Anim. Conserv. 2001, 4, 211–219. [Google Scholar] [CrossRef]
- Crnokrak, P.; Roff, D.A. Inbreeding depression in the wild. Heredity 1999, 83, 260–270. [Google Scholar] [CrossRef]
- Escoda, L.; González-Esteban, J.; Gómez, A.; Castresana, J. Using relatedness networks to infer contemporary dispersal: Application to the endangered mammal Galemys pyrenaicus. Mol. Ecol. 2017, 26, 3343–3357. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.W. Urban wildlife ecology and conservation:A brief history of the discipline. Urban Ecosyst. 2005, 8, 139–156. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Munshi-South, J.; Zolnik, C.P.; Harris, S.E. Population genomics of the Anthropocene: Urbanization is negatively associated with genome-wide variation in white-footed mouse populations. Evol. Appl. 2016, 9, 546–564. [Google Scholar] [CrossRef]
- Holyoak, M.; Leibold, M.A.; Holt, R.D. Metacommunities: Spatial Dynamics and Ecological Communities; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Fraser, D.L.; Ironside, K.; Wayne, R.K.; Boydston, E.E. Connectivity of mule deer (Odocoileus hemionus) populations in a highly fragmented urban landscape. Landsc. Ecol. 2019, 34, 1097–1115. [Google Scholar] [CrossRef]
- Garrott, R.A. Effective management of free-ranging ungulate populations using contraception. Wildl. Soc. Bull. 1995, 23, 445–452. [Google Scholar]
- Hepenstrick, D.; Thiel, D.; Holderegger, R.; Gugerli, F. Genetic discontinuities in roe deer (Capreolus capreolus) coincide with fenced transportation infrastructure. Basic Appl. Ecol. 2012, 13, 631–638. [Google Scholar] [CrossRef]
- Lecis, R.; Dondina, O.; Orioli, V.; Biosa, D.; Canu, A.; Fabbri, G.; Iacolina, L.; Cossu, A.; Bani, L.; Apollonio, M.; et al. Main roads and land cover shaped the genetic structure of a Mediterranean island wild boar population. Ecol. Evol. 2022, 12, e8804. [Google Scholar] [CrossRef]
- Saito, M.; Koike, F.; Mettke-Hofmann, C. Distribution of Wild Mammal Assemblages along an Urban–Rural–Forest Landscape Gradient in Warm-Temperate East Asia. PLoS ONE 2013, 8, e65464. [Google Scholar] [CrossRef]
- Epps, C.W.; Palsbøll, P.J.; Wehausen, J.D.; Roderick, G.K.; Ramey, R.R.; McCullough, D.R. Highways block gene flow and cause a rapid decline in genetic diversity of desert bighorn sheep. Ecol. Lett. 2005, 8, 1029–1038. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Data by Region: South East Queensland. 2025. Available online: https://dbr.abs.gov.au/ (accessed on 31 July 2024).
- Lawton, A.; Morrison, N. The loss of peri-urban agricultural land and the state-local tensions in managing its demise: The case of Greater Western Sydney, Australia. Land Use Policy 2022, 120, 106265. [Google Scholar] [CrossRef]
- Herbert, C.A.; Snape, M.A.; Wimpenny, C.E.; Coulson, G. Kangaroos in peri-urban areas: A fool’s paradise? Ecol. Manag. Restor. 2021, 22 (Suppl. S1), 167–175. [Google Scholar] [CrossRef]
- Hill, G. A Study of habitat preferences in the grey kangaroo. Wildl. Res. 1981, 8, 245–254. [Google Scholar] [CrossRef]
- Moore, B.D.; Coulson, G.; Way, S. Habitat selection by adult female eastern grey kangaroos. Wildl. Res. 2002, 29, 439–445. [Google Scholar] [CrossRef]
- Jaremovic, R.; Croft, D. Social organization of eastern grey kangaroos in southeastern New South Wales. II. Associations within mixed groups. Mammalia 1991, 55, 543–554. [Google Scholar] [CrossRef]
- Coghlan, B.A.; Seddon, J.M.; Best, E.C.; Thomson, V.A.; Goldizen, A.W. Evidence of male-biased dispersal in eastern grey kangaroos (Macropus giganteus). Aust. J. Zool. 2017, 64, 360–369. [Google Scholar] [CrossRef]
- Kirsch, J.; Poole, W. Taxonomy and distribution of the grey kangaroos, Macropus giganteus Shaw and Macropus fuliginosus (Desmarest), and their subspecies (Marsupialia: Macropodidae). Aust. J. Zool. 1972, 20, 315–339. [Google Scholar] [CrossRef]
- Coulson, G.; Cripps, J.K.; Wilson, M.E. Hopping down the main street: Eastern grey kangaroos at home in an urban matrix. Animals 2014, 4, 272–291. [Google Scholar] [CrossRef]
- Brunton, E.A.; Srivastava, S.K.; Burnett, S. Spatial ecology of an urban eastern grey kangaroo (Macropus giganteus) population: Local decline driven by kangaroo–vehicle collisions. Wildl. Res. 2018, 45, 685–695. [Google Scholar] [CrossRef]
- Brunton, E.; Brunton, A.; Hohwieler, K.; Ogbourne, S.; Conroy, G. Spatial genetic structure and gene flow of the eastern grey kangaroo (Macropus giganteus), in a rapidly urbanising landscape. Glob. Ecol. Conserv. 2022, 38, e02273. [Google Scholar] [CrossRef]
- Queensland Government. State of the Environment Summary Report; Department of Environment and Science: Brisbane, Australia, 2020. [Google Scholar]
- Bureau of Meteorology. Monthly Climate Statistics—Canberra City. 2024. Available online: https://www.bom.gov.au/climate/averages/tables/cw_070282.shtml (accessed on 1 August 2024).
- Australian Capital Territory Government. Canberra Nature Park Reserve Management Plan; ACT Government: Canberra, Australia, 2021. [Google Scholar]
- Victorian Government. Data VIC—Open Data Portal. 2022. Available online: https://www.data.vic.gov.au/ (accessed on 9 March 2023).
- Queensland Government. Queensland Spatial Catalogue. 2022. Available online: https://qldspatial.information.qld.gov.au/catalogue/custom/index.page (accessed on 9 March 2023).
- Australian Capital Territory Government. Data ACT—Open Data Portal. 2018. Available online: https://www.data.act.gov.au/ (accessed on 9 March 2023).
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Uszynski, G.; et al. Diversity Arrays Technology: A Generic Genome Profiling Technology on Open Platforms. In Data Production and Analysis in Population Genomics: Methods and Protocols; Pompanon, F., Bonin, A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 67–89. [Google Scholar]
- Pew, J.; Muir, P.H.; Wang, J.; Frasier, T.R. related: An R package for analysing pairwise relatedness from codominant molecular markers. Mol. Ecol. Resour. 2015, 15, 557–561. [Google Scholar] [CrossRef]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. dartr: An r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Mangel, M. The Ecological Detective: Confronting Models with Data (MPB-28); Princeton University Press: Princeton, NJ, USA, 1997; Available online: http://www.jstor.org/stable/j.ctt24hqnx (accessed on 18 March 2023).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- Anderson, D.R.; Burnham, K.P.; Gould, W.R.; Cheery, S. Concerns about finding effects that are actually spurious. Wildl. Soc. Bull. 2001, 29, 311–316. [Google Scholar]
- Johnson, D.H. The insignificance of statistical significance testing. J. Wildl. Manag. 1999, 63, 763. [Google Scholar] [CrossRef]
- Taylor, R.J. Foraging in the Eastern Grey Kangaroo and the Wallaroo. J. Anim. Ecol. 1984, 53, 65–74. [Google Scholar] [CrossRef]
- Carter, A.J.; Macdonald, S.L.; Thomson, V.A.; Goldizen, A.W. Structured association patterns and their energetic benefits in female eastern grey kangaroos, Macropus giganteus. Animal Behaviour. 2009, 77, 839–846. [Google Scholar] [CrossRef]
- Best, E.C.; Dwyer, R.G.; Seddon, J.M.; Goldizen, A.W. Associations are more strongly correlated with space use than kinship in female eastern grey kangaroos. Anim. Behav. 2014, 89, 1–10. [Google Scholar] [CrossRef]
- Best, E.C.; Seddon, J.M.; Dwyer, R.G.; Goldizen, A.W. Social preference influences female community structure in a population of wild eastern grey kangaroos. Anim. Behav. 2013, 86, 1031–1040. [Google Scholar] [CrossRef]
- Montana, L.; King, W.J.; Coulson, G.; Garant, D.; Festa-Bianchet, M. Large eastern grey kangaroo males are dominant but do not monopolize matings. Behav. Ecol. Sociobiol. 2022, 76, 78. [Google Scholar] [CrossRef]
- Henderson, T.; Vernes, K.; Körtner, G.; Rajaratnam, R. Using GPS Technology to Understand Spatial and Temporal Activity of Kangaroos in a Peri-Urban Environment. Animals 2018, 8, 97. [Google Scholar] [CrossRef]
- Jarman, P.; Taylor, R. Ranging of Eastern Grey Kangaroos and Wallaroos on a New England Pastoral Property. Wildl. Res. 1983, 10, 33–38. [Google Scholar] [CrossRef]
- Neaves, L.E.; Roberts, M.W.; Herbert, C.A.; Eldridge, M.D.B. Limited sex bias in the fine-scale spatial genetic structure of the eastern grey kangaroo and its relationship to habitat. Aust. J. Zool. 2017, 65, 33. [Google Scholar] [CrossRef]
- King, W.J.; Garant, D.; Festa-Bianchet, M. Mother–offspring distances reflect sex differences in fine-scale genetic structure of eastern grey kangaroos. Ecol. Evol. 2015, 5, 2084–2094. [Google Scholar] [CrossRef]
- Coghlan, B.A.; Goldizen, A.W.; Thomson, V.A.; Seddon, J.M.; Janke, A. Phylogeography of eastern grey kangaroos, Macropus giganteus, suggests a mesic refugium in eastern Australia. PLoS ONE 2015, 10, e0128160. [Google Scholar] [CrossRef] [PubMed]
- Brunton, E.A.; Srivastava, S.K.; Schoeman, D.S.; Burnett, S. Quantifying trends and predictors of decline in eastern grey kangaroo (Macropus giganteus) populations in a rapidly urbanising landscape. Pac. Conserv. Biol. 2018, 24, 63–73. [Google Scholar] [CrossRef]
- Garner, A.; Rachlow, J.L.; Hicks, J.F. Patterns of Genetic Diversity and Its Loss in Mammalian Populations. Conserv. Biol. 2005, 19, 1215–1221. [Google Scholar] [CrossRef]
- Bond, A.R.F.; Jones, D.N. Roads and macropods: Interactions and implications. Aust. Mammal. 2014, 36, 14. [Google Scholar] [CrossRef]
- Ramp, D. Roads as drivers of change for macropodids. In Macropods: The Biology of Kangaroos, Wallabies, Rat-Kangaroos; Coulson, G., Eldridge, M., Eds.; CSIRO: Melbourne, Australia, 2010; p. 279. [Google Scholar]
- Urbanek, C.; Conroy, G.; Leon, J.X.; Hohwieler, K.; Brunton, E. Roo-tine Obstacles: Uncovering the spatio-genetic barriers for Eastern Grey Kangaroos (Macropus giganteus) in an urbanising landscape. Landsc. Ecol. 2025, 40, 58. [Google Scholar] [CrossRef]
- Tilman, D.; May, R.M.; Lehman, C.L.; Nowak, M.A. Habitat destruction and the extinction debt. Nature 1994, 371, 65–66. [Google Scholar] [CrossRef]
- Landguth, E.L.; Cushman, S.A.; Schwartz, M.K.; McKelvey, K.S.; Murphy, M.; Luikart, G. Quantifying the lag time to detect barriers in landscape genetics. Mol. Ecol. 2010, 19, 4179–4191. [Google Scholar] [CrossRef] [PubMed]
- Hohwieler, K.R.; de Villiers, D.L.; Cristescu, R.H.; Frere, C.H. Genetic erosion detected in a specialist mammal living in a fast-developing environment. Conserv. Sci. Pract. 2022, 4, e12738. [Google Scholar] [CrossRef]
- Hufschmid, J.; Beveridge, I.; Coulson, G.; Gould, J. Bone fluoride concentrations of eastern grey kangaroos (Macropus giganteus) resident near an aluminium smelter in south-eastern Australia. Ecotoxicology 2011, 20, 1378–1387. [Google Scholar] [CrossRef]
- Coulson, G.; Wilson, M.E. Implementing implants: Delivery efficiency, contraceptive efficacy and population outcomes in two overabundant kangaroo populations. Wildl. Res. 2023, 51, WR22170. [Google Scholar] [CrossRef]

| Site | Region | Habitat | Ho | AR | FIS | RC |
|---|---|---|---|---|---|---|
| Buderim | QLD | urban | 0.188 | 1.617 | 0.092 | 0.241 |
| Mt Coolum | QLD | urban | 0.157 | 1.580 | 0.211 | 0.188 |
| Sippy Downs | QLD | urban | 0.211 | 1.653 | 0.029 | 0.189 |
| Tewantin | QLD | urban | 0.205 | 1.596 | 0.046 | 0.218 |
| Twin Waters | QLD | urban | 0.179 | 1.587 | 0.075 | 0.242 |
| Anglesea | VIC | urban | 0.18 | 1.769 | 0.059 | 0.092 |
| Farrer Ridge | ACT | urban | 0.215 | 1.791 | 0.033 | 0.031 |
| Gold Creek | ACT | urban | 0.206 | 1.798 | 0.073 | 0.033 |
| South Lawson | ACT | urban | 0.204 | 1.807 | 0.086 | 0.02 |
| Weston Park | ACT | urban | 0.206 | 1.784 | 0.058 | 0.033 |
| North Lawson | ACT | urban | 0.202 | 1.775 | 0.071 | 0.045 |
| Mean (Standard Deviation) | 0.196 (0.018) | 1.705 (0.097) | 0.076 (0.049) | 0.121 (0.094) | ||
| Chevallum | QLD | Non-urban | 0.189 | 1.651 | 0.126 | 0.167 |
| Landsborough | QLD | Non-urban | 0.210 | 1.642 | 0.038 | 0.205 |
| Ninderry | QLD | Non-urban | 0.173 | 1.627 | 0.171 | 0.136 |
| Weyba | QLD | Non-urban | 0.213 | 1.641 | 0.012 | 0.172 |
| Conondale | QLD | Non-urban | 0.181 | 1.605 | 0.091 | 0.297 |
| Portland | VIC | Non-urban | 0.172 | 1.7 | 0.031 | 0.196 |
| Serendip | VIC | Non-urban | 0.18 | 1.767 | 0.075 | 0.125 |
| Mean (Standard Deviation) | 0.188 (0.017) | 1.662 (0.055) | 0.078 (0.057) | 0.185 (0.057) |
| Variable | Estimate | Standard Error | t Value | p Value |
|---|---|---|---|---|
| Model 1—RC | ||||
| Region QLD | 1.871 | 0.249 | 7.502 | <0.001 |
| Region VIC | 1.434 | 0.242 | 5.927 | <0.001 |
| Model 2—AR | ||||
| Region QLD | 0.116 | 0.014 | −8.110 | <0.001 |
| Model 3—HO | ||||
| Boundary—Open | 0.267 | 0.115 | 2.312 | 0.046 |
| Restriction (%) | 0.003 | 0.001 | 2.290 | 0.048 |
| Region QLD | −0.196 | 0.063 | −3.125 | 0.012 |
| Region VIC | −0.166 | 0.061 | −2.731 | 0.023 |
| Model 4—FIS | ||||
| Boundary—Open | −1.653 | 0.835 | −1.980 | 0.0791 * |
| Variable | Estimate | Standard Error | t Value | p Value |
|---|---|---|---|---|
| Model 1—RC | ||||
| Region QLD | 2.255 | 0.323 | 6.981 | <0.001 |
| Region VIC | 1.719 | 0.295 | 5.831 | <0.001 |
| Model 2—AR | ||||
| Region QLD | −0.118 | 0.019 | −6.178 | <0.001 |
| Region VIC | −0.044 | 0.017 | −2.527 | <0.001 |
| Model 3—HO | ||||
| Access—Yes | 0.128 | 0.043 | 2.968 | 0.013 |
| Region VIC | −0.202 | 0.074 | −2.730 | 0.020 |
| Model 4—FIS | ||||
| Access—Yes | −0.925 | 0.328 | −2.818 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunton, E.; Levengood, A.; Brunton, A.; Clarke, N.; Coulson, G.; Wimpenny, C.; Conroy, G. Beyond Boundaries—Genetic Implications of Urbanisation and Isolation in Eastern Grey Kangaroos (Macropus giganteus). Urban Sci. 2025, 9, 257. https://doi.org/10.3390/urbansci9070257
Brunton E, Levengood A, Brunton A, Clarke N, Coulson G, Wimpenny C, Conroy G. Beyond Boundaries—Genetic Implications of Urbanisation and Isolation in Eastern Grey Kangaroos (Macropus giganteus). Urban Science. 2025; 9(7):257. https://doi.org/10.3390/urbansci9070257
Chicago/Turabian StyleBrunton, Elizabeth, Alexis Levengood, Aaron Brunton, Neil Clarke, Graeme Coulson, Claire Wimpenny, and Gabriel Conroy. 2025. "Beyond Boundaries—Genetic Implications of Urbanisation and Isolation in Eastern Grey Kangaroos (Macropus giganteus)" Urban Science 9, no. 7: 257. https://doi.org/10.3390/urbansci9070257
APA StyleBrunton, E., Levengood, A., Brunton, A., Clarke, N., Coulson, G., Wimpenny, C., & Conroy, G. (2025). Beyond Boundaries—Genetic Implications of Urbanisation and Isolation in Eastern Grey Kangaroos (Macropus giganteus). Urban Science, 9(7), 257. https://doi.org/10.3390/urbansci9070257






