Abstract
Urban streams often suffer from poor water quality, in part due to nutrient pollution, especially in highly developed areas. Poor water quality, driven by high concentrations of nitrate and phosphate entering waterways from runoff, wastewater, and stormwater systems, contributes to urban stream syndrome. This study evaluates the long-term performance of a floating wetland (FW) system installed in a canal of the North Branch of the Chicago River near Goose Island, an area heavily impacted by urban runoff. From 2018 to 2023, surface and subsurface water samples were collected upstream and downstream of a 90 m2 FW system and analyzed for nitrate as nitrogen (NO3-N) and phosphate (PO43−) using ion chromatography. A paired t-test and two-way ANOVA revealed statistically significant reductions (p < 0.001) in NO3-N (mean: 1.31 mg/L surface, 1.02 mg/L at 0.3 m) and PO43− (mean: 0.64 mg/L surface, 0.57 mg/L at 0.3 m) between waters entering and exiting the FW, with no significant seasonal differences in removal efficiency. These results highlight the FW’s consistent, year-round nutrient mitigation performance driven by plant uptake and microbial processes. Over the five-year period of the study, the FW served as a means of improving the water quality, delivering a sustainable, low-maintenance solution for urban stream management with broader implications for ecological resilience and water quality enhancement.
1. Introduction
Urban stream syndrome describes the suite of ecological changes that occur in urban streams as a result of urbanization, a process that involves the conversion of natural landscapes into urban areas, significantly impacting the ecological health of streams [1,2,3]. Typical symptoms include elevated nitrogen and phosphorus levels, warmer water temperatures, low dissolved oxygen, elevated concentrations of suspended sediments and heavy metals, and the loss of aquatic habitats. These impacts lead to less biodiversity, a shift toward pollution tolerant taxa, and problems with water flow [4,5,6]. Many urban streams around the world show these signs [1], but the effects can be very different depending on the area and factors such as the type of stormwater infrastructure, the stream’s physiology, climate, and geology [5,7].
The increase in impervious surfaces that comes with urban development is one of the main causes of urban stream syndrome [8]. Reduced infiltration and higher surface runoff during precipitation events are associated with impervious surfaces, which leads to more water entering streams draining urban areas [9]. The runoff can carry nutrients and pollutants, increasing the contaminant load entering the receiving stream and negatively impacting aquatic ecosystems [1].
Direct discharge of effluent from both industrial processes and domestic wastewater into streams can negatively impact the stream [10], as some of these wastes are discharged into urban streams during overflows. All these respective inputs can lower water quality, harm aquatic habitats, and decrease biodiversity, affecting the stream ecosystem [11].
Nutrients are essential in supporting the growth and functionality of aquatic organisms. However, the overabundance of nutrients when discharged into aquatic ecosystems can lead to the excessive growth of algae. Algal blooms can have significant ecological, economic, and health impacts [12]. Some algal species produce toxins that can be harmful to aquatic organisms, animals, and humans [13,14]. The decomposition of the algal blooms consumes oxygen in the water, creating low-oxygen (hypoxic) or even oxygen-free (anoxic) conditions that can be harmful to aquatic organisms and ecosystems [15,16]. This chain of events is part of a process called eutrophication, which ultimately leads to the decline of aquatic ecosystems and a loss of biodiversity [17].
Nitrogen exists in various forms in water, ranging from organic nitrogen to ammonium, nitrite, and nitrate. In aquatic systems, three main processes help remove nitrogen from the water column. In natural systems such as lakes, rivers, and wetlands, denitrification stands out as the most effective, offering a permanent solution [18]. The denitrification process, which relies on bacteria to convert nitrate into nitrous oxide and nitrogen gas released into the atmosphere, thrives under low-oxygen or anaerobic conditions typically found in wetland sediments and plant root zones [19,20]. Sedimentation is another nitrogen removal pathway. Sedimentation occurs when nitrogen attached to organic material and suspended particles settle to the floor of the water body and over time are buried, becoming unavailable for use by plants or microorganisms. Direct plant uptake can also occur as the plants can absorb dissolved nitrogen through their roots, incorporating it into their tissues and storing it as organic matter [8,18,19].
In freshwater ecosystems, the availability of nutrients, particularly phosphorus, can influence biological activity [21]. Phosphorus is often a limiting nutrient in freshwater, which means that it is often in short supply and its lack of availability can limit the growth of algae and aquatic plants. Hence, even small increase in the availability of phosphorus can substantially increase ecosystem productivity, potentially resulting in algal blooms [22,23,24]. Microorganisms are responsible for a significant portion of the phosphorus cycling process. These organisms convert organic phosphorus into orthophosphate, which is a form that is easily absorbed by organisms such as plants, algae, and bacteria [8]. Phosphorus can also bond to wetland sediments or form compounds that do not dissolve with metals like iron or calcium; both pathways bind phosphorus in less bioavailable forms [25].
Floating wetlands (FWs) are eco-friendly systems that use natural processes including denitrification, sedimentation, and plant uptake to remediate polluted water [26]. Constructed from recycled plastic, foam, or natural fibers, FWs are buoyant and help plants flourish without soil. The plant roots extend down through the substrate, forming thick networks that hold the plants in place and give microbes and aquatic creatures places where they can thrive [27,28].
Both the plant roots and microorganisms around the root zone of the plants of a FW are important for reducing nitrogen and phosphorus concentration into forms that plants can use [8,29]. Macrophytes are aquatic plants that get their nutrients directly from the water column [26,30]. Macrophytes are very important in floating wetlands because their large root systems help remove contaminants including nutrients, metals, fertilizers, and organic waste. The plants store the nutrients they take in their biomass, which may then be harvested to permanently remove those nutrients [31,32]. Microbial biofilms that grow on the roots of macrophytes improve floating wetlands pollutant removal capacity. These biofilms, which are made up of bacteria, fungi, algae, and protozoa, are very important for nutrient uptake and removal processes [33]. Biofilms are responsible for important biochemical processes in nitrate and phosphate reduction. In addition to lowering the concentrations of nutrients, FWs provide shade to keep the water temperature stable and reduce sunlight, which stops algae from growing.
Hydraulic design is important in the efficiency of floating wetland treatment. The important parameter in hydraulic design includes hydraulic retention time (HRT), hydraulic loading rate (HLR), and surface coverage ratio [34]. HRT refers to how long the water interacts with the plant root and microbial communities [35]. Longer HRT has been shown to improve FW nutrient removal. In a constructed wetland, Ewemoje et al. [36] showed that longer hydraulic retention times from 3 to 7 days improved removal efficiencies of nutrients such as phosphorus, orthophosphate (PO4), ammonium nitrogen (NH4+-N), and chemical oxygen demand (COD) in a vertical flow constructed wetland system. Optimal removal rates were observed at 7 days HRT, achieving up to 89.1% for phosphorus and 92.0% for COD. Reference [37] showed that increasing the hydraulic retention time from 2 to 6 days increased the removal of ammonium nitrogen (NH4+-N) and orthophosphate (PO43−-P). The best performance occurred at a 6-day HRT, achieving up to 80% removal of NH4+-N and 55% for PO43−-P. Both studies highlight the importance of HRT, but in urban streams, the HRT is much lower than in the traditional wetlands studied.
Hydraulic loading rate (HLR) is the volume of water passing through the system per unit surface area [38] HLRs have an impact on FW performance. Higher HLRs reduces the hydraulic retention time and alter microbial community composition and function, which affects nutrient removal efficiencies [39]. Using integrated vertical-flow constructed wetlands, Chang et al. [40] showed that the hydraulic loading rate impacted nitrate removal. A high HLR of 250 mm/day led to a relatively low nitrate removal efficiency. This was due to the reduced retention time, limiting the opportunity for nitrate to be removed.
In ponded waters and laboratory settings, AFWs have been shown to improve water quality [26,29,41,42,43,44], with a small number examining AFWs in open water systems [6,45,46]. Peterson et al. [6] conducted a pilot study between 29 April 2018, and 19 November 2019, on a small FW on the Chicago River and observed the concentrations of nitrate as nitrogen (NO3-N) were significantly lower downstream of the FW than they were upstream and Rome et al. [46] demonstrated the absorption of nutrients by plants. Both studies suggest plants play an important role in nutrient removal. For the year, [6] witnessed that the concentrations of NO3-N varied between the spring and fall upstream and downstream of FWs, suggesting that the ability to remove nutrients may change between the growth and dormant seasons.
While Peterson et al. [6] suggested that FWs could be used as a long-term solution to solving nutrient pollution in urban streams, the one-year study was limited in duration examining only one growing season. This study builds on their work using a larger five-year dataset (2018–2023) to address two key research questions. Do FWs lower concentrations of nitrate as nitrogen ([NO3-N]) and phosphate ([PO43−]) in urban streams? Do FWs exhibit seasonal differences in nutrient reduction performance between growing and dormant seasons?
2. Materials and Methods
2.1. Study Site
The study location is a side canal on the North Branch of the Chicago River, adjacent to Goose Island in Chicago, IL, USA (Figure 1). Situated in a highly urbanized environment, this modified waterway experiences anthropogenic stressors characteristic of urban stream syndrome, including elevated nutrient loads, heavy metal contamination, and other pollutants. Two combined sewer overflow outfalls near the northern (upgradient) end and one at the southern (downgradient) end, which can discharge during heavy rain, highlight these stressors. Extensive impermeable urban landscapes drain into the canal, contributing to sediment load and nutrient pollution. The canal spans 24–37 m in width and varies in depth from 1 m at the northern end to 2.5 m at the southern end [47]. Within the North Branch, the water flows from the northwest to the southeast, rejoining the main branch approximately 1.5 km south. Mean discharge within the canal is 0.88 m3/s based on 67 years of USGS data [6].
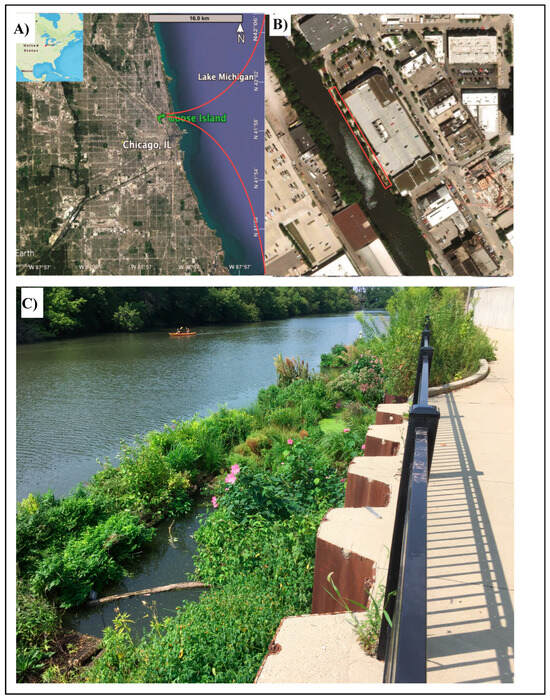
Figure 1.
Location of the FW along (A) North Branch Canal adjacent to (B) Goose Island, Chicago, Illinois. The study area in red rectangle, with the inset illustrating the garden (3 m × 30 m) in relation to the river (37 m × 30 m). Images obtained from Google Earth. (C) FW in August 2018.
The study site features a FW system along the eastern bank, installed in 2017. Comprising interconnecting HDPE pontoons and flexible planting rows wrapped in coconut husk as a buoyant scaffolding, the 150 m2 FW are overall approximately 50 m in length and between 1 m and 3 m in width. Initially planted with around 1500 plugs representing over 50 native plant species at a density of 10 plants per m2, the system allows rapid, sustained growth and generally can achieve up to 30 plants per m2. The FW system was planted with support for ecological health as the primary goal of maintaining NGO, Urban Rivers Inc. Since the initial FW installation in 2017, the species representing the most sustained biomass include Hibiscus moscheutos, Juncus effusus, Justicia americana, Eutrochium maculatum, and Eupatorium perfoliatum. Other species which were not planted but volunteer onto the FWs include the native Impatiens capensis and Eupatorium serotonium, and non-native Mentha and Lycopus sp. Relatively little maintenance of the FW systems has been performed throughout the study period, restricted to occasional non-native plant removal, and replacement of <10% of the planting area, typically with Juncus effusus or Carex sp. Dead plant material is generally left on the FWs to encourage overwintering insects and promote soil development where possible, and occasional light freezing of the surface layer of the canal can occur during winter.
2.2. Data Gathering and Sampling Protocol
Weekly water sampling and analysis were carried out following the method described by [6]. Upstream and downstream of the FW, sampling occurred at two depths; surface water (0.0 m) and 0.3 m below the surface to capture the root zone where nutrient uptake by FW macrophytes and microbial processes are most active (Figure 2). Sampling beyond the root zone was not necessary to capture the vertical effects of the system as depths below the root zone are less affected by the root activity. The paired sampling was conducted under similar hydrologic conditions to ensure that any changes in nutrient levels were caused by the floating wetland, not by natural changes in the water.
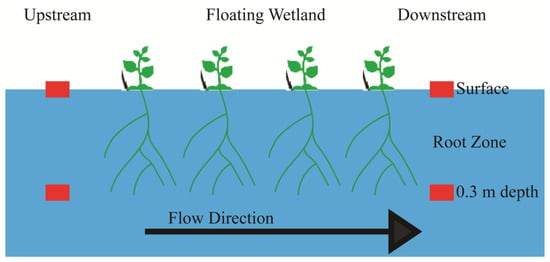
Figure 2.
Samples and in situ measurements were collected upstream and downstream of the FW at the surface water and 0.3 m below the surface. The red rectangles indicate points of sample collection.
From each depth, in situ measurements of dissolved oxygen (DO) (mg/L), temperature (°C), and specific conductance (SpC) (μS/cm) were recorded using a YSI-85 (YSI Incorporated, Yellow Springs, OH, USA). Water samples collected using a horizontal sampler were passed through a 0.4-μm membrane filter into acid washed 30 mL sample bottles. All samples were frozen prior to analysis for major anions. A Dionex ICS-1100 Ion Chromatograph (Dionex, Sunnyvale, CA, USA) quantified chloride (Cl−), nitrate as nitrogen (NO3-N), phosphate (PO43−), and sulfate (SO42−) following US EPA method 300.1 [48]. Employing a quality assurance (QA) and quality control (QC) protocol in which blanks, duplicates, and replicates were analyzed along with the samples, the analytical error was less than 3%. The dataset included water samples gathered between 29 April 2018, and 19 November 2019 [49] and samples collected from 20 November 2019, to 16 December 2023, resulting in a comprehensive 69 month record.
2.3. Statistical Analysis
Paired t-tests evaluated whether the FW reduced NO3-N and PO43− concentrations, comparing upstream and downstream samples from the same sampling event and depth. Each nutrient and depth combination were analyzed separately, NO3-N surface (n = 116), NO3-N 0.3 m (n = 109), PO43− surface (n = 117), and PO43− 0.3 m (n = 104), with additional tests by season (growing, dormant) to explore temporal patterns. The growing season was defined by April–September when the plants were growing (active), while the dormant season, October–March, represented the time when the plants were dormant (inactive). Tests were conducted at a 95% confidence level (α = 0.05), with the null hypothesis (H0) stating no significant difference between upstream and downstream concentrations, and the alternative hypothesis (H1) positing that upstream concentrations exceed downstream ones (one-tailed). This method leveraged the dependency of paired samples to assess FW nutrient removal effectiveness.
A two-way ANOVA was conducted to examine how nutrient reduction effectiveness by the FW varied by season. The analysis evaluated nutrient concentrations using two factors, Location (upstream, downstream) and Season (growing, dormant) and their interaction (Location × Season). This approach tested whether the reduction (upstream-downstream difference) varied between growing and dormant seasons across all data for each nutrient and depth combination, NO3-N surface (n = 232), NO3-N 0.3 m (n = 218), PO43− surface (n = 234), and PO43− 0.3 m (n = 208). The ANOVA was conducted at α = 0.05, with the null hypothesis (H0) for the interaction term asserting no seasonal difference in reduction effectiveness, and the alternative hypothesis (H1) indicating a difference. Assumptions of normality and homogeneity of variance were assessed (Shapiro–Wilk and Levene’s tests); despite some violations (e.g., non-normal residuals, p < 0.05), a Box–Cox power transformation was applied to improve normality, with the transformed concentrations used in the ANOVA.
3. Results
The dataset comprises 104–117 paired sampling events, depending on nutrient and depth, collected upstream and downstream of the FW. The data span both growing (April–September) and dormant (October–March) seasons, allowing for an analysis of seasonal variation in the FW performance. Summary statistics for NO3-N and PO43− concentrations are provided in Table 1, detailing mean upstream and downstream values, mean differences, and sample sizes across all seasons at both surface and 0.3 m depth.

Table 1.
Summary statistics and the results of paired t-tests for NO3-N and PO43− concentrations (mg/L) at each depth and season.
3.1. Nitrate as Nitrogen (NO3-N)
Concentrations of NO3-N in the upstream waters were greater than the concentrations in the downstream waters (Figure 3; Table 1), resulting in a loss of NO3-N as the waters passed under the FW (Figure 4). At the surface depth (n = 116 paired events), the overall analysis revealed a statistically significant reduction in NO3-N concentrations downstream of the FW (mean upstream = 5.77 mg/L, mean downstream = 4.45 mg/L, mean difference = 1.31 mg/L, t(115) = 6.95, p < 0.001). During the growing season (n = 69), the reduction was significant (mean upstream = 4.94 mg/L, mean downstream = 3.82 mg/L, mean difference = 1.11 mg/L, t(68) = 4.84, p < 0.001). Similarly, in the dormant season (n = 47), a significant decrease was observed (mean upstream = 6.98 mg/L, mean downstream = 5.37 mg/L, mean difference = 1.61 mg/L, t(46) = 5.02, p < 0.001) (Figure 3a).
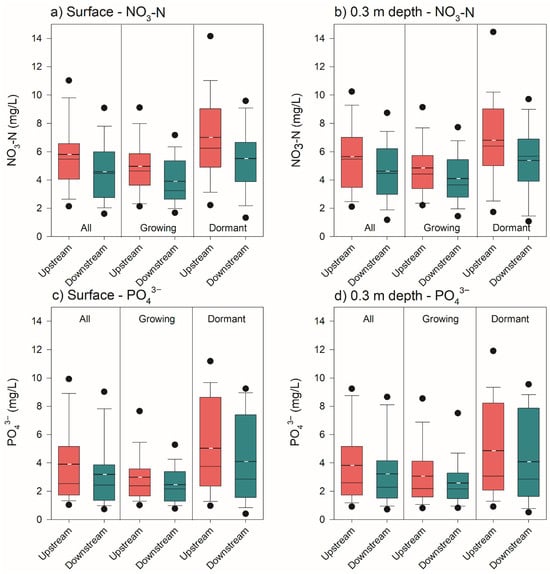
Figure 3.
Boxplot model of the nitrate as nitrogen (NO3-N) and phosphate (PO43−) concentrations in the surface waters (a,c) and at the 0.3 m depth (b,d) upstream and downstream of the wetlands for all periods, the growing season and the dormant seasons. The ends of the boxes represent the 25th and 75th percentiles with the solid line at the median and the dashed line at the mean; the error bars depict the 10th and 90th percentiles and the points (black dots) represent the 5th and 95th percentiles.
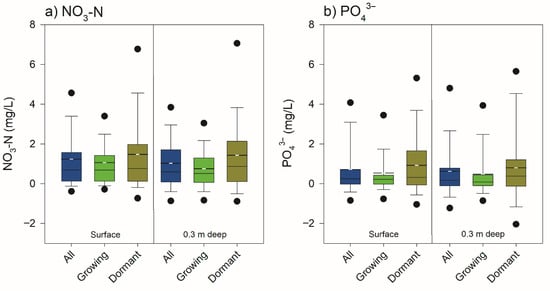
Figure 4.
Boxplot model of the difference between upstream and downstream (a) nitrate as nitrogen (NO3-N) and (b) phosphate (PO43−) concentrations in the surface waters and at the 0.3 m depth for all periods, the growing season and the dormant seasons. The ends of the boxes represent the 25th and 75th percentiles with the solid line at the median and the dashed line at the mean; the error bars depict the 10th and 90th percentiles and the points (black dots) represent the 5th and 95th percentiles.
At the 0.3 m depth (n = 109 paired events), the overall reduction was significant (mean upstream = 5.63 mg/L, mean downstream = 4.61 mg/L, mean difference = 1.02 mg/L, t(108) = 6.72, p < 0.001). In the growing season (n = 64), downstream concentrations were significantly lower (mean upstream = 4.81 mg/L, mean downstream = 4.07 mg/L, mean difference = 0.75 mg/L, t(63) = 5.49, p < 0.001). The dormant season (n = 45) also showed a significant reduction (mean upstream = 6.81 mg/L, mean downstream = 5.39 mg/L, mean difference = 1.42 mg/L, t(44) = 4.62, p < 0.001) (Figure 3b).
3.2. Phosphate (PO43−)
Phosphate behaved similarly to NO3-N (Figure 3 and Figure 4; Table 1). At the surface depth (n = 117 paired events), a significant overall reduction was observed (mean upstream = 3.90 mg/L, mean downstream = 3.26 mg/L, mean difference = 0.64 mg/L, t(116) = 4.04, p < 0.001). In the growing season (n = 67), a significant decrease was observed (mean upstream = 3.00 mg/L, mean downstream = 2.48 mg/L, mean difference = 0.52 mg/L, t(66) = 2.55, p = 0.007), and in the dormant season (n = 50), it remained significant (mean upstream = 5.12 mg/L, mean downstream = 4.31 mg/L, mean difference = 0.81 mg/L, t(49) = 3.18, p = 0.001) (Figure 3c).
At the 0.3 m depth (n = 104 paired events), the overall reduction was significant (mean upstream = 3.84 mg/L, mean downstream = 3.27 mg/L, mean difference = 0.57 mg/L, t(103) = 3.47, p < 0.001). During the growing season (n = 62), a significant decrease occurred (mean upstream = 2.98 mg/L, mean downstream = 2.55 mg/L, mean difference = 0.43 mg/L, t(61) = 2.56, p = 0.006). In the dormant season (n = 42), the reduction was significant (mean upstream = 5.09 mg/L, mean downstream = 4.33 mg/L, mean difference = 0.77 mg/L, t(41) = 2.40, p = 0.011) (Figure 3d).
3.3. Seasonal Differences in Nutrient Reduction
A total of 232 observations at the surface and 218 at 0.3 m depth for NO3-N and a total of 234 observations at the surface and 208 at 0.3 m depth for PO43− formed the basis for seasonal comparison. The mean nutrient concentrations tended to be higher in the dormant season, compared to the growing season (Figure 3; Table 1). This background provides the context for the ANOVA analysis that follows, which evaluated whether the FW’s nutrient reduction performance varied significantly between seasons. The two-way ANOVA tested the effects of Location (upstream vs. downstream), Season (growing vs. dormant), and their interaction on nutrient concentrations. The results of this analysis are summarized in Table 2. No significant Location–Season interaction was recorded.

Table 2.
Summary statistics for ANOVA result.
For NO3-N at the surface (n = 232 observations, 116 pairs), initial residuals were non-normal (Shapiro–Wilk W = 0.94, p < 0.001) and variances unequal (Levene’s F(3, 228) = 3.923, p = 0.009). A Box–Cox transformation (lambda = 0.30) improved normality (W = 0.99, p = 0.160), meeting the assumption. The Levene’s test, which checks if variances across groups are equal, showed significant improvement after applying the Box–Cox transformation (F(3, 228) = 0.722, p = 0.540), indicating equal variances. ANOVA on transformed concentrations showed a significant Location effect (F(1, 228) = 17.289, p < 0.001), indicating overall reduction, a significant Season effect (F(1, 228) = 26.235, p < 0.001), reflecting higher dormant concentrations, but no significant Location–Season interaction (F(1, 228) = 0.028, p = 0.867). This suggests consistent reduction effectiveness across seasons (overall mean difference = 1.31 mg/L; growing: 1.11 mg/L; dormant: 1.61 mg/L) (Figure 5a).
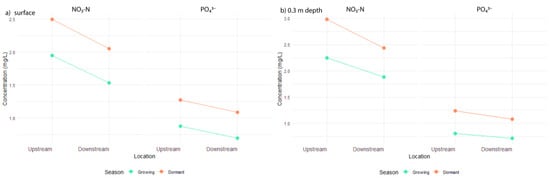
Figure 5.
Interaction plot of mean NO3-N and Phosphate (PO43−) concentrations at the (a) surface and (b) at 0.3 m depth, showing a significant reduction from Upstream and higher Dormant season concentrations, with no significant Location–Season interaction.
For NO3-N at the 0.3 m depth (n = 218 observations, 109 pairs), initial residuals were non-normal (Shapiro–Wilk W = 0.97, p < 0.001) and variances were unequal (Levene’s F(3, 214) = 4.05, p = 0.008). A Box–Cox transformation (lambda = 0.48) improved normality (W = 0.99, p = 0.219), meeting the assumption. The Levene’s test, which checks if variances across groups are equal, showed significant improvement after applying the Box–Cox transformation (F(3, 214) = 1.383, p = 0.25), indicating equal variances. ANOVA on transformed concentrations showed a significant Location effect (F(1, 214) = 9.88, p = 0.002), indicating overall reduction, a significant Season effect (F(1, 214) = 20.70, p < 0.001), reflecting higher dormant concentrations, and no significant Location–Season interaction (F(1, 214) = 0.43, p = 0.512). Overall mean difference = 1.02 mg/L; growing: 0.75 mg/L; dormant: 1.42 mg/L (Figure 5b).
For PO43− at the surface (n = 234 observations, 117 pairs), initial residuals were non-normal (Shapiro–Wilk W = 0.94, p < 0.001) and variances were unequal (Levene’s F(3, 230) = 14.69, p < 0.001). A Box–Cox transformation (lambda = −0.08) improved normality (W = 0.99, p = 0.020). The Levene’s test, which checks if variances across groups are equal, showed some improvement after applying the Box–Cox transformation, though variances remained unequal (F(3, 230) = 5.45, p = 0.001). ANOVA on transformed concentrations showed a significant Location effect (F(1, 230) = 4.93, p = 0.027), indicating overall reduction, a significant Season effect (F(1, 230) = 22.30, p < 0.001), reflecting higher dormant concentrations, and no significant Location–Season interaction (F(1, 230) = 0.00, p = 0.978). Overall mean difference = 0.64 mg/L; growing: 0.52 mg/L; dormant: 0.81 mg/L (Figure 5a).
For PO43− at the 0.3 m depth (n = 208 observations, 104 pairs), initial residuals were non-normal (Shapiro–Wilk W = 0.93, p < 0.001) and variances were unequal (Levene’s F(3, 204) = 9.66, p < 0.001). A Box–Cox transformation (lambda = −0.10) improved normality (W = 0.98, p = 0.008). The Levene’s test, which checks if variances across groups are equal, showed only slight improvement after applying the Box–Cox transformation (F(3, 204) = 2.61, p = 0.053), though variances remained unequal. ANOVA on transformed concentrations showed no significant Location effect (F(1, 204) = 1.81, p = 0.181), indicating no overall reduction, a significant Season effect (F(1, 204) = 19.35, p < 0.001), reflecting higher dormant concentrations, and no significant Location–Season interaction (F(1, 204) = 0.15, p = 0.702). Overall mean difference = 0.57 mg/L; growing: 0.43 mg/L; dormant: 0.77 mg/L (Figure 5b).
3.4. Environmental Parameters
Dissolved oxygen and temperature were also analyzed to help interpret what was happening within the study. Table 3 presents the DO and temperature measurements collected upstream and downstream of the floating wetland at surface and 0.3 m depths from 2018 to 2023, across growing (April–September) and dormant (October–March) seasons.

Table 3.
Summary statistics for Dissolved Oxygen (DO) and Temperature.
3.5. Dissolved Oxygen (DO)
During the growing season, DO ranged from 2.03 to 8.54 mg/L at the free water surface and from 1.94 to 5.51 mg/L at 0.3 m depth, while, During the dormant season, DO ranged from 3.12 to 8.4 mg/L at the free water surface and from 3.23 to 7.64 mg/L at 0.3 m depth. At the surface depth (n = 61 paired events), the overall analysis revealed no statistically significant difference in DO concentrations downstream of the FW (mean upstream = 4.90 mg/L, mean downstream = 4.77 mg/L, mean difference = 0.135 mg/L, t(60) = 1.996, p = 0.050). During the growing season (n = 31), the difference was not significant (mean upstream = 4.04 mg/L, mean downstream = 3.91 mg/L, mean difference = 0.125 mg/L, t(30) = 1.349, p = 0.187). Similarly, in the dormant season (n = 30), no significant difference was observed (mean upstream = 5.80 mg/L, mean downstream = 5.65 mg/L, mean difference = 0.146 mg/L, t(29) = 1.449, p = 0.158) (Figure 6).
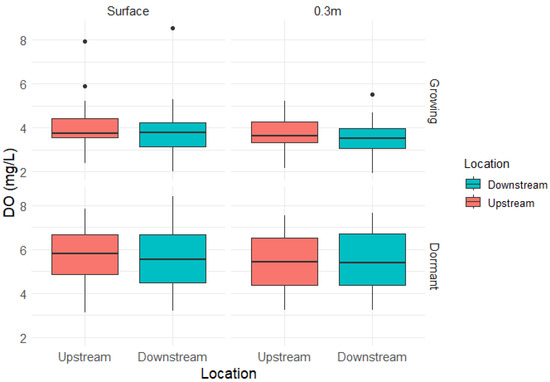
Figure 6.
Boxplot model of the dissolved oxygen (DO) concentrations in the surface and 0.3 m depth upstream and downstream of the wetland during the Growing and Dormant seasons. For both seasons, the downstream DO concentrations were not statistically different from the upstream concentrations. The black dots represent outliers.
At the 0.3 m depth (n = 51 paired events), the overall analysis revealed no statistically significant difference in DO concentrations downstream of the FW (mean upstream = 4.61 mg/L, mean downstream = 4.49 mg/L, mean difference = 0.124 mg/L, t(50) = 1.939, p = 0.058). During the growing season (n = 26), the difference was not significant (mean upstream = 3.70 mg/L, mean downstream = 3.56 mg/L, mean difference = 0.135 mg/L, t(25) = 1.322, p = 0.198). Similarly, in the dormant season (n = 25), no significant difference was observed (mean upstream = 5.43 mg/L, mean downstream = 5.32 mg/L, mean difference = 0.112 mg/L, t(24) = 1.447, p = 0.161).
3.6. Temperature
Water temperatures at the surface ranged from 11.6 to 26.5 °C in the growing season and 1.9 to 20.5 °C in the dormant season, with similar ranges at 0.3 m depth. At the surface depth (n = 61 paired events), the overall analysis revealed no statistically significant difference in temperature downstream of the FW (mean upstream = 15.35 °C, mean downstream = 15.33 °C, mean difference = 0.013 °C, t(60) = 0.484, p = 0.630). During the growing season (n = 31), the difference was not significant (mean upstream = 20.88 °C, mean downstream = 20.83 °C, mean difference = 0.045 °C, t(30) = 1.039, p = 0.307). Similarly, in the dormant season (n = 30), no significant difference was observed (mean upstream = 9.63 °C, mean downstream = 9.65 °C, mean difference = −0.02 °C, t(29) = −0.633, p = 0.532) (Figure 7).
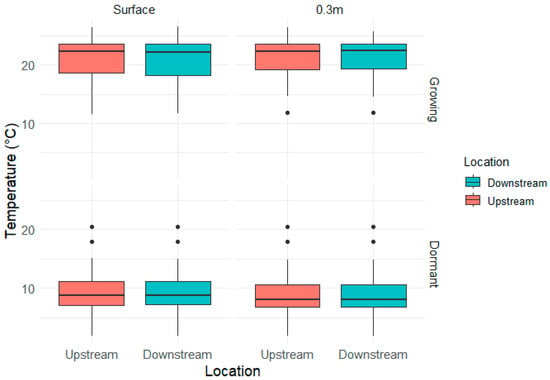
Figure 7.
Boxplot model of the temperature in the surface and 0.3 m upstream and downstream of the wetlands during the Growing and Dormant seasons. For both seasons, the downstream temperatures were not statistically different from the upstream temperatures.
At the 0.3 m depth (n = 51 paired events), the overall analysis revealed no statistically significant difference in temperature downstream of the FW (mean upstream = 15.40 °C, mean downstream = 15.39 °C, mean difference = 0.006 °C, t(50) = 0.233, p = 0.817). During the growing season (n = 26), the difference was not significant (mean upstream = 21.30 °C, mean downstream = 21.30 °C, mean difference = 0.000 °C, t(25) = −1.0×10−14, p = 1.000). Similarly, in the dormant season (n = 25), no significant difference was observed (mean upstream = 9.3 °C, mean downstream = 9.29 °C, mean difference = 0.012 °C, t(24) = 0.721, p = 0.478).
4. Discussion
During the period of study, the FW in the North Branch Canal of the Chicago River confirmed the findings of Peterson et al. [6], verifying the long-term effectiveness of the FW as an intervention for reducing nitrate as nitrogen (NO3-N) and phosphate (PO43−) concentration. The decrease in nutrient concentrations downstream of the FW highlighted its performance across seasons at both surface and 0.3 m sampling depths. The consistent FW performance was particularly significant given the lack of significant interaction between location (upstream vs. downstream) and season (growing vs. dormant) in the two-way ANOVA analyses, indicating that the FW maintained its nutrient removal capacity year-round, despite fluctuations in environmental conditions.
Floating wetlands enhance nutrient removal by supporting microbial transformations within the root zones and plant uptake. Processes such as nitrification, denitrification, phosphorus adsorption, critical for nutrient removal in floating wetlands are highly sensitive to environmental parameters, particularly dissolved oxygen (DO) and temperature [50]. Nitrification, the oxidation of ammonia to nitrate, requires aerobic conditions with DO levels typically above 2 mg/L and thrives at temperatures between 20 and 30 °C, while denitrification, converting nitrate to nitrogen gas, occurs under low-oxygen or anoxic conditions (DO < 1 mg/L) and is optimal at 15–25 °C [19,50]. Phosphorus adsorption and microbial assimilation are less dependent on DO but are enhanced by temperatures above 15 °C, which boost microbial activity [25]. Data from this study indicate that DO levels in the Chicago River’s North Branch Canal, ranging from 3.63 to 5.72 mg/L across seasons (Table 3), support nitrification in the bulk water column, particularly during the growing season (mean DO: 3.98 mg/L surface, 3.63 mg/L 0.3 m).
Nitrate concentrations were significantly reduced with statistically meaningful differences observed at both the surface and 0.3 m depths across growing and dormant seasons. Nitrate is primarily removed through plant uptake and microbial denitrification. While work in similar systems, FW in flowing water, is limited, studies have shown that denitrification is a key microbial process in removing nitrate in similar systems, i.e., wetlands [51,52], constructed wetlands [40], riparian treatment systems [53,54]. The reduction in nitrate during the dormant season suggests that denitrification is the main removal mechanism. During the growing season, warmer temperatures, and enhanced photosynthetic activity likely stimulated the assimilation of nitrate by macrophytes and their associated biofilms [55]. The dense root networks of the native aquatic vegetation extended into the water column, intercepting nutrients directly and providing a rich substrate for microbial colonization. Although mean dissolved oxygen (DO) levels ranged from 3.63 to 5.72 mg/L across all seasons, and denitrification is typically associated with low-oxygen environments, the dense and complex structure of biofilms can generate localized anoxic zones within the root zone, where oxygen diffusion is limited. Within these microenvironments, denitrifying bacteria are able to thrive and reduce nitrate to nitrogen gas, effectively removing it from the water column [56]. This phenomenon allows denitrification to proceed despite the surrounding water being well-oxygenated, highlighting the importance of root-associated microbial communities in sustaining nitrate attenuation throughout the year.
The dormant season exhibited even greater nitrate reductions, coinciding with a seasonal rise in input concentrations. Despite the reduction in plant nutrient uptake during colder months, the persistence of microbial activity appears sufficient to maintain the system’s treatment effectiveness. This aligns with observations from other cold-environment wetland systems, where denitrification continues under low-temperature regimes, albeit at slower kinetics [19]. The stable dissolved oxygen (DO) levels measured in both seasons suggest that while the bulk water remained oxygenated, conditions within the biofilms could have been conducive to continued microbial nitrate processing. Although direct measurements of biofilm conditions were not conducted, the dense root networks and microbial communities likely created localized anoxic microsites, potentially supporting denitrification despite the oxygenated surrounding water [56].
Phosphate reduction at both the free water surface and 0.3 m depth was significant but not as significant as the nitrate reduction. Unlike nitrate, which can be permanently removed via denitrification, phosphate removal relies more on biological uptake and sedimentation and lacks a gaseous phase for removal [57]. In FWs, phosphate is removed through plant uptake, microbial assimilation, adsorption to sediments or root surfaces, and co-precipitation with metal ions [58]. The relatively small but significant reductions as observed by the result, especially during the dormant season, are likely to reflect a balance of these processes. FW promotes the settling of suspended solids and algae beneath the mats by shading the water surface and reducing turbulence [59]. The rhizosphere also plays a key role for sediment trapping and nutrient aggregation [40]. Sediment trapping not only helps phosphate removal but also prevents resuspension of nutrient-rich particles.
In terms of seasonal differences for how well the floating wetland reduced nutrient concentration during the growing and dormant season, no significant variation was observed. The concentration of nutrients was, however, higher during the dormant season. The high concentration of nutrients during the dormant season could be likely due to reduced plant uptake and higher surface runoff from rain and snowmelt. The wetland consistently reduced nutrient concentrations during the growing and dormant season. The plant species and their growth cycles play a role in nutrient removal efficiency. Plants have varying nutrient uptake capacity, which can be linked to their growth rate and biomass production [60].
The ability of the wetland to lower nutrient concentrations during both the growing season and the dormant season can be seen from the result obtained in the two-way ANOVA analysis, where no significant interaction between the two seasons and location were observed. The year-round performance of the wetland is likely attributed to the microbial activities occurring in the wetland as well as passive mechanisms such as adsorption and sedimentation, especially during the dormant season, when plant uptake is reduced.
Coverage ratio of FW can significantly affect nutrient removal. Higher coverage helps promote processes like denitrification and nitrogen uptake by plants, leading to better overall water treatment performance [61]. Pavlineri et al. [29] showed a strong correlation between vegetation coverage and nutrient (ammonium-nitrogen (NH4-N) and total phosphorus (TP)) reduction. This shows how important coverage ratio is in the design of FW. It is important to note that microbial activity and pollutant removal efficiency are sensitive to these operational parameters. The hydraulic parameters can influence the microbial community structure and nutrient removal processes [38].
The roots of these plants extend down through the substrate, forming thick networks that hold the plants in place and give microbes and macroinvertebrate communities places where they can thrive [27,28]. Microbial biofilms that grow on the roots of macrophytes improve floating wetlands pollutant removal capacity. These biofilms, which are made up of bacteria, fungi, algae, and protozoa, are very important for nutrient uptake and removal processes [33]. According to Sartori et al. [62] macroinvertebrates are usually among the first animals to colonize new wetlands. Their populations and diversity also increase quickly over time. However, how much FW improves aquatic communities depends on the specific environment, existing habitats and the local conditions [63]. FW also provides shade to keep the water temperature stable and reduce sunlight. This lowers chlorophyll-a concentrations and overall primary productivity, helping prevent algal blooms and keeping oxygen levels in balance [64].
Limitations and Future Research
While this study provides valuable long-term evidence of floating wetland performance in an urban canal environment, there are several limitations to the study. The floating wetland occupied only one side of the canal, and therefore, did not intercept the entire cross-section of flow. The results reflect only the section of the water column that passed under the wetland. Sampling was performed to effectively capture the water surface to the root-zone layer but not the deeper water column where additional processes may occur. While the HRT and HLR were not known, the stream had a historic flow rate of 0.88 m3/s, suggesting a short HRT and a higher HLR. Despite not knowing these numbers, the data highlight the utility of the FWs to remove nutrients. Future work should focus on measuring hydrodynamic parameters such as retention time and flow variability for mass-balance calculation. More sampling depths and locations across the channel should be performed to capture vertical and lateral variations in treatment effects.
5. Conclusions
The floating wetland installed in the North Branch Canal of the Chicago River was effective in lowering the concentration of nutrients (nitrate as nitrogen and phosphate) in the waters passing underneath the wetland. The reduction of NO3-N and PO43− occurred during both the growing and the dormant season. The floating wetland demonstrated potential as a long-term solution for nutrient pollution and maintaining water quality. Although there were not significant differences in average dissolved oxygen or temperature between upstream and downstream locations, both factors stayed within a range that could support microbial life, which likely supported consistent nutrient removal. Despite not altering the DO and temperature, the FW reduced nutrient concentrations, lessening the symptoms of urban stream syndrome.
The study shows that FW is a practical and sustainable way to improve water quality. FWs are scalable and can fit easily into stormwater system, canal, and retention pond designs. Floating wetlands are adaptable to various scales and settings, from small units to larger systems along urban waterways and agricultural settings; see [65,66] for examples and costs.
Floating wetlands are cost-effective, relying primarily on natural ecological processes with minimal mechanical input or maintenance. Over the five years, the wetlands consistently reduced nutrient levels with no significant drop in performance, showing that they are not only reliable but also inexpensive to maintain over time. Incorporating FW into urban water management can bring both environmental and social benefits. Beyond cleaning the water, these systems enhance biodiversity and add green spaces. They align closely with sustainability goals. Still, widespread use would benefit from further research, particularly on optimizing design, choosing the best plant species for different regions, and evaluating full life-cycle costs to guide effective adoption across varied urban settings.
Overall, the five-year duration of this study provides robust evidence of the floating wetland’s durability and reliability, offering a sustainable, low-maintenance solution for urban stream management with broader implications for ecological resilience and water quality enhancement.
Author Contributions
Conceptualization, D.C. and E.W.P., methodology, D.C. and E.W.P.; formal analysis, D.C. and E.W.P.; investigation, D.C., E.W.P. and P.N.; data curation, E.W.P.; writing—original draft preparation, D.C.; writing—review and editing, D.C., E.W.P. and P.N.; visualization, D.C. and E.W.P.; supervision, E.W.P.; project administration, E.W.P.; funding acquisition, E.W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Illinois Water Resources Center, grant number 079901-17703.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. (The data are not publicly available at this time due to data embargo restrictions).
Acknowledgments
The authors wish to thank Urban Rivers volunteers, especially Jon Meisenbach for reliable water sample collection throughout the study. The authors additionally appreciate the contributions of three anonymous reviewers for their comments and suggestions that have improved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FW | Floating Wetland |
| DO | Dissolved Oxygen |
| NO3-N | Nitrate as Nitrogen |
| PO43− | Phosphate |
| Cl− | Chloride |
| SO42− | Sulfate |
References
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Lisi, P.J.; Childress, E.S.; Gagne, R.B.; Hain, E.F.; Lamphere, B.A.; Walter, R.P.; Hogan, J.D.; Gilliam, J.F.; Blum, M.J.; McIntyre, P.B. Overcoming urban stream syndrome: Trophic flexibility confers resilience in a Hawaiian stream fish. Freshw. Biol. 2018, 63, 492–502. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Clinton, S.; Jefferson, A.; Manda, A.; McMillan, S. Urbanization Effects on Watershed Hydrology and In-Stream Processes in the Southern United States. Water 2010, 2, 605–648. [Google Scholar] [CrossRef]
- Paul, M.J.; Meyer, J.L. Streams in the Urban Landscape. Annu. Rev. Ecol. Evol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Komínková, D. The Urban Stream Syndrome—A Mini-Review. Open Environ. Biol. Monit. J. 2012, 5, 24–29. [Google Scholar] [CrossRef][Green Version]
- Peterson, E.W.; Nicodemus, P.; Spooner, E.; Heath, A. The Effectiveness of an Artificial Floating Wetland to Remove Nutrients in an Urban Stream: A Pilot-Study in the Chicago River, Chicago, IL USA. Hydrology 2021, 8, 115. [Google Scholar] [CrossRef]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global perspectives on the urban stream syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef]
- Bi, R.; Zhou, C.; Jia, Y.; Wang, S.; Li, P.; Reichwaldt, E.S.; Liu, W. Giving waterbodies the treatment they need: A critical review of the application of constructed floating wetlands. J. Environ. Manag. 2019, 238, 484–498. [Google Scholar] [CrossRef]
- Maxwell, B.; Winter, D.; Birgand, F. Floating treatment wetland retrofit in a stormwater wet pond provides limited water quality improvements. Ecol. Eng. 2020, 149, 105784. [Google Scholar] [CrossRef]
- Iloms, E.; Ololade, O.O.; Ogola, H.J.O.; Selvarajan, R. Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1096. [Google Scholar] [CrossRef]
- House, M.A.; Ellis, J.B.; Herricks, E.E.; Hvitved-Jacobsen, T.; Seager, J.; Lijklema, L.; Aalderink, H.; Clifforde, I.T. Urban Drainage—Impacts on Receiving Water Quality. Water Sci. Technol. 1993, 27, 117–158. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Roelke, D.; Greene, R.M. Health and Ecological Impacts of Harmful Algal Blooms: Risk Assessment Needs. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1329–1345. [Google Scholar] [CrossRef]
- Kumar, R. Toxic Algae and Effects of Algal Poisoning in Animals and Human Beings. World J. Environ. Biosci. 2021, 10, 9–12. [Google Scholar] [CrossRef]
- Noga, E.J. Toxic Algae, Fish Kills and Fish Disease. Fish Pathol. 1998, 33, 337–342. [Google Scholar] [CrossRef]
- Harrison, P.J.; Piontkovski, S.; Al-Hashmi, K. Understanding how physical-biological coupling influences harmful algal blooms, low oxygen and fish kills in the Sea of Oman and the Western Arabian Sea. Mar. Pollut. Bull. 2017, 114, 25–34. [Google Scholar] [CrossRef]
- Misra, A.K.; Tiwari, P.K.; Chandra, P. Modeling the Control of Algal Bloom in a Lake by Applying Some External Efforts with Time Delay. Differ. Equ. Dyn. Syst. 2021, 29, 539–568. [Google Scholar] [CrossRef]
- Bai, S.; Wang, M. Nitrogen and Phosphorus Removal from Eutrophic Surface Water by Constructed Multi-Media Floating Islands. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–5. [Google Scholar]
- Saunders, D.L.; Kalff, J. Nitrogen retention in wetlands, lakes and rivers. Hydrobiologia 2001, 443, 205–212. [Google Scholar] [CrossRef]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Peck, E.K.; Inamdar, S.; Sherman, M.; Hripto, J.; Peipoch, M.; Gold, A.J.; Addy, K. Nitrogen Sinks or Sources? Denitrification and Nitrogen Removal Potential in Riparian Legacy Sediment Terraces Affected by Milldams. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG007004. [Google Scholar] [CrossRef]
- Vass, K.K.; Wangeneo, A.; Samanta, S.; Adhikari, S.; Muralidhar, M. Phosphorus dynamics, eutrophication and fisheries in the aquatic ecosystems in India. Curr. Sci. 2015, 108, 1306–1314. [Google Scholar]
- Chen, Y.; Chen, J.; Xia, R.; Li, W.; Zhang, Y.; Zhang, K.; Tong, S.; Jia, R.; Hu, Q.; Wang, L.; et al. Phosphorus—The main limiting factor in riverine ecosystems in China. Sci. Total Environ. 2023, 870, 161613. [Google Scholar] [CrossRef] [PubMed]
- Correll, D.L. Phosphorus: A rate limiting nutrient in surface waters. Poult. Sci. 1999, 78, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Fogg, G.E. Phosphorus in primary aquatic plants. Water Res. 1973, 7, 77–91. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kadlec, R.H.; Flaig, E.; Gale, P.M. Phosphorus Retention in Streams and Wetlands: A Review. Crit. Rev. Environ. Sci. Technol. 1999, 29, 83–146. [Google Scholar] [CrossRef]
- Headley, T.R.; Tanner, C.C. Constructed Wetlands with Floating Emergent Macrophytes: An Innovative Stormwater Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Al Lami, M.H.; Whelan, M.J.; Boom, A.; Harper, D.M. Mechanistic Understanding of Nitrogen Behaviour in Floating Treatment Wetlands: Abatement of Ammonia Flux. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012093. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating Wetlands: A Sustainable Tool for Wastewater Treatment. CLEAN—Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Pavlineri, N.; Skoulikidis, N.T.; Tsihrintzis, V.A. Constructed Floating Wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chem. Eng. J. 2017, 308, 1120–1132. [Google Scholar] [CrossRef]
- Brix, H. Wastewater treatment in constructed wetlands: System design, removal processes, and treatment performance. In Constructed Wetlands for Water Quality Improvement; Moshiri, G.A., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 9–22. [Google Scholar]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.K.; Joner, E. Utilising the Synergy between Plants and Rhizosphere Microorganisms to Enhance Breakdown of Organic Pollutants in the Environment. Environ. Sci. Pollut. Res. 2005, 12, 34–48. [Google Scholar] [CrossRef]
- Osem, Y.; Chen, Y.; Levinson, D.; Hadar, Y. The effects of plant roots on microbial community structure in aerated wastewater-treatment reactors. Ecol. Eng. 2007, 29, 133–142. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Jannah, N.; Putra, M.S.T. Design of floating treatment wetland in PT Antam Tbk UBP Bauksit Kalimantan Barat. E3S Web Conf. 2024, 577, 01005. [Google Scholar] [CrossRef]
- da Silva, I.P.; da Costa, G.B.; Queluz, J.G.T.; Garcia, M.L. Effect of hydraulic retention time on chemical oxygen demand and total nitrogen removal in intermittently aerated constructed wetlands. Rev. Ambiente Água 2020, 15. [Google Scholar] [CrossRef]
- Ewemoje, O.; Sangodoyin, A.; Adegoke, A. On the effect of hydraulic retention time and loading rates on pollutant removal in a pilot scale wetland. J. Sustain. Dev. Stud. 2015, 8, 342–355. [Google Scholar]
- Hunter, R.G.; Combs, D.L.; George, D.B. Nitrogen, Phosphorous, and Organic Carbon Removal in Simulated Wetland Treatment Systems. Arch. Environ. Contam. Toxicol. 2001, 41, 274–281. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Xiang, X.; Zhou, L.; Zhang, P.; Wang, Q.; Li, Y.; Wei, J.; Li, L.; Cheng, S. Study on the impact of hydraulic loading rate (HLR) on removal of nitrogen under low C/N condition by modular moving bed constructed wetland (MMB-CW) system. Environ. Technol. Innov. 2024, 34, 103579. [Google Scholar] [CrossRef]
- Sheng, H.; Liu, Y.; Zhang, N.; Xia, J.; Wen, H.; Yu, K.; Chen, H.; Yao, Z. Evaluating the influence of hydraulic loading rate on functional genes associated with nutrient cycling in constructed wetlands. J. Water Process Eng. 2024, 59, 104998. [Google Scholar] [CrossRef]
- Chang, J.-J.; Wu, S.-Q.; Dai, Y.; Wu, Z.-B.; Liang, W. Nitrate removal from tail water by integrated vertical-flow constructed wetlands at a high hydraulic loading rate. Desalination Water Treat. 2013, 51, 6031–6037. [Google Scholar] [CrossRef]
- Winston, R.J.; Hunt, W.F.; Kennedy, S.G.; Merriman, L.S.; Chandler, J.; Brown, D. Evaluation of floating treatment wetlands as retrofits to existing stormwater retention ponds. Ecol. Eng. 2013, 54, 254–265. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman-Beck, E.A.; Winston, R.J.; Hunt, W.F.; Tanner, C.C. Implementation and Maintenance of Floating Treatment Wetlands for Urban Stormwater Management. J. Environ. Eng. 2015, 141, 04015030. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.-J.; Yu, Z.-L.; Sheng, G.-P.; Yu, H.-Q. Enhanced nitrogen and phosphorus removal from eutrophic lake water by Ipomoea aquatica with low-energy ion implantation. Water Res. 2009, 43, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Sample, D.J. Assessment of the nutrient removal effectiveness of floating treatment wetlands applied to urban retention ponds. J. Environ. Manag. 2014, 137, 23–35. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Phytoremediation to remove nutrients and improve eutrophic stormwaters using water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2010, 17, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Rome, M.; Happel, A.; Dahlenburg, C.; Nicodemus, P.; Schott, E.; Mueller, S.; Lovell, K.; Beighley, R.E. Application of floating wetlands for the improvement of degraded urban waters: Findings from three multi-year pilot-scale installations. Sci. Total Environ. 2023, 877, 162669. [Google Scholar] [CrossRef]
- Spooner, E.A. The Role of Floating Gardens to Alter the Water Quality of the Chicago River: Chicago, Il; Illinois State University: Normal, IL, USA, 2020. [Google Scholar]
- Hautman, D.P.; Munch, D.J. Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography. 1997. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-300.1.pdf (accessed on 1 February 2024).
- Peterson, E.W.; Nicodemus, P.; Spooner, E.A. Supporting Data—Floating Gardens, Chicago River, April 29, 2018 to November 19, 2019. In Faculty Publications—Geography, Geology, and the Environment, March 2021 ed.; Illinois State University: Normal, IL, USA, 2021; Volume 3, Available online: https://ir.library.illinoisstate.edu/fpgeo/3 (accessed on 6 November 2023).
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Maxwell, E.L.; Peterson, E.W.; O’Reilly, C.M. Enhanced nitrate reduction within a constructed wetland system: Nitrate removal within groundwater flow. Wetlands 2017, 37, 413–422. [Google Scholar] [CrossRef]
- Ackerman, J.R.; Peterson, E.W.; Van der Hoven, S.; Perry, W. Quantifying nutrient removal from groundwater seepage out of constructed wetlands receiving treated wastewater effluent. Environ. Earth Sci. 2015, 74, 1633–1645. [Google Scholar] [CrossRef]
- Miller, J.; Peterson, E.W.; Budikova, D. Diurnal and seasonal variation in nitrate-nitrogen concentrations of groundwater in a saturated buffer zone. Hydrogeol. J. 2019, 27, 1373–1387. [Google Scholar] [CrossRef]
- Sahad, A.; Peterson, E.W. Transport and fate of nitrate in a saturated buffer zone as assessed with a chloride tracer test. Environ. Eng. Geosci. 2024, 30, 161–171. [Google Scholar] [CrossRef]
- Castaldelli, G.; Soana, E.; Racchetti, E.; Vincenzi, F.; Fano, E.A.; Bartoli, M. Vegetated canals mitigate nitrogen surplus in agricultural watersheds. Agric. Ecosyst. Environ. 2015, 212, 253–262. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, S.; Wang, P.; Wang, C.; Guo, C.; Addo, F.G.; Li, Y. Responses of bacterial community structure and denitrifying bacteria in biofilm to submerged macrophytes and nitrate. Sci. Rep.-UK 2016, 6, 36178. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Norton, S. Removal Mechanisms in Constructed Wastewater Wetlands; Iowa State University: Ames, IA, USA, 2014; p. 13. [Google Scholar]
- Tanner, C.C.; Headley, T.R. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol. Eng. 2011, 37, 474–486. [Google Scholar] [CrossRef]
- Choudhury, M.I.; Nilsson, J.E.; Hylander, S.; Hauber, M.; Ehde, P.M.; Weisner, S.E.B.; Liess, A. Enhancing nitrogen removal through macrophyte harvest and installation of woodchips-based floating beds in surface-flow constructed wetlands. Chemosphere 2024, 359, 142284. [Google Scholar] [CrossRef] [PubMed]
- Abi Hanna, R.; Borne, K.E.; Andres, Y.; Gerente, C. Effect of floating treatment wetland coverage ratio and operating parameters on nitrogen removal: Toward design optimization. Water Sci. Technol. 2024, 89, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Sartori, L.; Canobbio, S.; Cabrini, R.; Fornaroli, R.; Mezzanotte, V. Macroinvertebrate assemblages and biodiversity levels: Ecological role of constructed wetlands and artificial ponds in a natural park. J. Limnol. 2015, 74, 335–345. [Google Scholar] [CrossRef]
- Chaffee, M.; Mittelstet, A.R.; Comfort, S.; Messer, T.; Uden, D.R.; McCoy, J. Context-Dependent Macroinvertebrate Responses to Prolonged Biological and Chemical Treatment in Urbanized Lentic Ecosystems. J. Nat. Resour. Agric. Ecosyst. 2024, 2, 213–225. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Ke, T.Y.; Lin, Y.L. Algal Growth Control Within Natural Water Purification Systems: Macrophyte Light Shading Effects. Water Air Soil Pollut. 2011, 214, 575–586. [Google Scholar] [CrossRef]
- Awad, J.; Walker, C.; Page, D.; Arslan, M.; White, S.A.; Lucke, T.; Beecham, S.; Winston, R.J.; Strosnider, W.H.J.; Nicodemus, P.; et al. Assessing the Costs of Constructed Floating Wetlands for the Treatment of Surface Waters and Wastewater. ACS EST Water 2025, 5, 4737–4747. [Google Scholar] [CrossRef]
- Bista, S.; Karki, B.K.; Maharjan, R. A Review Paper on Floating Treatment Wetlands: Working Principles and Applications for River Water Remediation. J. Adv. Coll. Eng. Manag. 2025, 10, 13–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).