Abstract
Along with exposure to parasites and other biological disease vectors, animal faeces can also contain heavy metals and metalloids. We quantified metals, metalloids, and non-metals in the faeces of capybara (Hydrochoerus hydrochaeris) that live in parks in the city of Campo Grande (Brazil). Quantification of metalloids was obtained after acid digestion using an inductively coupled plasma optical emission spectrometer. Higher mean concentrations in mg/kg of aluminium (Al) (140.322), arsenic (As) (0.010), cadmium (Cd) (1.042), chromium (Cr) (26.866), cobalt (Co) (1.946), copper (Cu) (50.764), lead (Pb) (8.762), manganese (Mn) (291.469), molybdenum (Mo) (3.634), nickel (Ni) (5.475), and zinc (Zn) (100.027) were quantified in samples of faeces of capybara that live on the banks of a lagoon that receives input from streams that cross the city. According to the risk assessment, potential risks to the health of children and adults may occur due to the presence of Al, As, Cd, Co, Cu, and Mn through involuntary oral ingestion of faeces, via inhalation and dermal contact. The hazard index (HI) due to oral ingestion was greater than 1 for children and adults. Therefore, we believe that faeces of H. hydrochaeris can be considered as a bioindicator of environmental pollution in urban parks.
Keywords:
anthropic area; biomonitoring; contamination; ecotourism; impact; pollutant; safety; savanna; toxicology; urban forest 1. Introduction
Contamination of soil and water with heavy metals and metalloids is associated with food production, industrial processes, pesticide and fertilizer production and use, residential sewage pollution, and mining [1,2,3]. The accumulation of heavy metals, along with leaching and surface runoff [4,5], can promote metal migration and affect soil fertility and product quality [6]. Such metals in soil are incorporated by plants, which in turn are ingested by animals. In addition to contaminated soil and water, humans and captive animals are also affected by the presence of heavy metals, whose harmful effects exceed bio-recommended limits [7]. Hence, heavy metal residues in animal excrement have recently received scientific attention due to potential pollution risks [8,9,10], and utility in acting as sentinels for environmental pollution [11]. Metal contamination is routinely observed in animals and humans living in or near contaminated areas [12,13,14,15].
The main sources of faecal pollution of human or animal origin (domestic or wild) that affect urban land surfaces are not specific. They are related to (i) the lack of sewage treatment; (ii) sewage, septic tank, and tank leaks; (iii) inadequate waste disposal; and (iv) open defecation of humans and other animals. Such sources are common in developing countries and cause a random dispersion of faeces in urban areas. The ingestion of dust and soil contaminated with faecal material from humans and domestic animals occurs mainly during childhood [16]. Floors or carpets act as collectors for dust that contains dust mites, bacteria, allergens, and faeces from shoes. Hence, ingesting or inhaling this material in urban areas can be a threat to human health [16,17]. From now on, the involuntary ingestion of faeces is referred to as “soil/faeces”.
The use of sentinels, biological indicators, may be used to assess potential health hazards in an environmental set [11] and are a valuable tool for evaluating possible health risks to wildlife and humans. In this sense, using non-invasive tissues, such as faeces, urine, nails, hair, feathers, and spines [18,19], is helpful in screening wildlife compared to traditional methods once they protect animals from stress, pain, and sacrifice. In addition, the samples are easy to acquire, transport, and stock, and allow several measurements in the same individuals [19].
Therefore, it is possible to monitor the health of mammals and infer the level of environmental pollution through animal excrement [20,21,22,23,24], since it is an important source of heavy metal(loid)s (Cu, Zn, Cr, Cd, Pb, and As) for the environment [7]. Furthermore, it is possible that contaminated faeces can produce impacts on human health, as they can pollute the water used for population supply [9]. In China, for example, the intensive production of poultry and pigs was considered one of the main environmental contaminants due to the presence of heavy metals in faeces [25,26,27].
Heavy metals present in soil, such as Cr, Cu, and Zn, can cause human health risks, such as neurological disorders, headaches, and liver disease [28,29]. Excessive Cu intake promotes liver damage and gastric problems, while Zn can affect high-density lipoprotein concentrations and alter the immune system [30]. In addition, high doses of Al, As, Cd, Cr, Ni, and Pb are known to cause cancer [10,31], and Pb and Mn can lead to Alzheimer’s disease and manganism [32]. Furthermore, the ingestion of food contaminated with multiple metals, such as Cd and Pb, can promote renal dysfunction [33].
Despite investigations using different animal species to determine the effect of contaminants on human health and the environment, some species have not yet been studied to verify whether they have a profile to be good indicators of pollution. In this sense, the capybara Hydrochoerus hydrochaeris Linnaeus, 1766 (Order Rodentia, Family Caviidae) can be an indicator of pollution due to the presence of heavy metal(loid)s in the environment. Although H. hydrochaeris is widely distributed in South America and commonly found in parks and biological reserves in Brazil, few studies have monitored the concentration of metals in blood plasma, fur, and excrement [25]. Therefore, characteristics such as large populations, proximity to humans, and the availability of large quantities of faeces (given that it is the world’s largest rodent) make this material viable as an indicator of environmental pollution.
Since there are no studies aimed at quantifying heavy metal(loid)s in H. hydrochaeris faeces to propose this animal as an indicator of pollution, carrying out studies with this purpose is adequate, especially because it is a non-invasive technique. Given the above, since biological materials are considered bioindicators of pollution due to the presence of metal(loid)s [7] and mammal faeces have been used as bioindicators [22,23,24,26], we hypothesize that H. hydrochaeris excrement may be a bioindicator of heavy metal contamination in wild mammals.
Therefore, we aimed to quantify aluminium (Al), arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), lead (Pb), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), phosphorus (P), selenium (Se), and zinc (Zn) in the faeces of capybaras living in urban parks in Central-West Brazil. Thus, it is expected to contribute to the levels of metal(loid) contamination in mammals, most of which are affected by the bioaccumulation process. Moreover, we assessed the human health risk due to the possibility of contact with faeces.
2. Materials and Methods
2.1. Sampling Sites
We selected four locations in the urban region of the city of Campo Grande, state of Mato Grosso do Sul (MS), Central-West Brazil (Figure 1), namely:
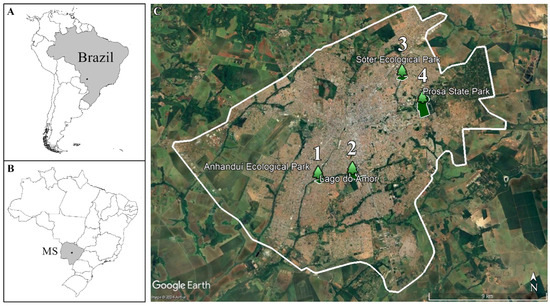
Figure 1.
Geographic location of Campo Grande/Brazil (A) Brazilian territory (B) State of Mato Grosso do Sul (MS), Brazilian Central-West (C) City of Campo Grande and sampling sites 1. Anhanduí Ecological Park; 2. Lago do Amor; 3. Sóter Ecological Park; and 4. Prosa State Park (Satellite image from Google Earth).
- (i)
- Anhanduí Ecological Park: located at the confluence of the Bandeira Stream with the Anhanduí River, the southern region of the city of Campo Grande (coordinates −20.50653, −54.64299).
- (ii)
- Sóter Ecological Park: this park is watered by the Sóter Stream, in the northeast region of the city of Campo Grande (coordinates −20.42934, −54.57655).
- (iii)
- Prosa State Park: this park is watered by the Desbarrancado and Joaquim Português streams. It is located on the Serra de Maracaju Plateau, in the eastern region of the city of Campo Grande (coordinates −20.45021, −54.56084).
- (iv)
- Lago do Amor: this is an artificial lagoon, supplied by the Cabaça and Bandeira streams, which form the Bandeira basin. It is located within the area of the Federal University of Mato Grosso do Sul (UFMS), southern region of the city of Campo Grande (coordinates −20.503133, −54.618797). This lagoon is influenced by human activity and receives waste/chemical products brought by streams that cross the city, and the faeces samples were collected on its margins.
2.2. Faeces Collection
The study was carried out with due permission and authorisation from the competent authorities, such as SEMADUR—Municipal Secretariat for the Environment and Urban Management (Anhanduí Ecological Park), FUNESP—National Sports Foundation/MS (Sóter Ecological Park), and IMASUL—Mato Grosso do Sul Environmental Institute (Prosa State Park). Three faecal samples were collected in a zigzag pattern in locations close to water bodies for each site. Samplings were harvested in September 2021, and samples with soil direct contact were avoided. A quantity of 550 g of faeces from each site was collected using a large 12.5 cm stainless-steel spoon and surgical gloves to avoid contamination. The stainless-steel spoon was washed with ultrapure water and 70% alcohol and surgical gloves were replaced between sample collections. The samples were collected in the morning, using the central faeces in a pile only, then immediately stored in a 10 × 14–80 PE zip-lock plastic bag. Afterwards, we transported samples to the Mineral Metabolism and Biomaterials Laboratory in the Faculty of Medicine of the UFMS for analysis.
2.3. Microwave-Assisted Digestion Procedure, Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES), Elemental Analysis, and Calibration Curves
The faeces were oven-dried at 40 °C for 10 h until reaching a constant weight. The total pool for each dried sample was crushed separately using an industrial blender with stainless steel blades to powder and then sieved (stainless steel sieve, particle size 200 μm). Approximately 0.25 g of powder from each excrement sample from the different locations were placed in Teflon DAP60® containers and 3.0 mL of HNO3 (65%, Merck, Darmstadt, Germany), 1.0 mL of high-purity water (18 MΩ cm, Milli-Q, Millipore, Bedford, MA, USA), and 2.0 mL of H2O2 (35%, Merck, Darmstadt, Germany). The digestion procedure was carried out using a microwave-assisted digester (Speedwave four, Berghof, Eningen, Germany) according to the program presented in Table 1.

Table 1.
Program for microwave digestion of faeces samples of H. hydrochaeris.
Chemical elements in animal excrement samples were quantified using inductively coupled plasma optical emission spectrometry (ICP-OES) (iCAP 6300 Duo, Thermo Fisher Scientific, Bremen, Germany). To quantify the metal(loid)s, we used an axial view with an operating power of 1250 W; sample flow rate = 0.35 L/min; plasma gas flow = 12 L/min; integration time = 5 s; stabilisation time = 20 s; and nebulizing pressure = 20 psi. Additionally, the following emission wavelengths were configured and used in ICP-OES for analysis of each of the metal(loid)s: Al 309.271 nm, As 189.042 nm, Cd 228.000 nm, Co 228.616 nm, Cr 267.716 nm, Cu 324.754 nm, Fe 259.940 nm, Mg 279.553 nm, Mn 257.610 nm, Mo 202.030 nm, Ni 221.647 nm, P 214.914 nm, Se 196.00 nm, Zn 213.856 nm, and Pb 220.353 nm.
We prepared standard solutions by diluting a standard multi-element stock solution (SpecSol, Quimlab, Jacareí, Brazil) containing 1000 mg/L of each metal(loid) (Al, As, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, P, Se, Zn, and Pb). For quantitative analysis of metal(loid)s, external calibration curves were constructed at five different concentrations in the range of 0.01–5.0 mg/L. Optimisation conditions were evaluated in terms of precision (per recall test) and limit of detection. The addition and recovery procedure was completed by enriching with 1 ppm from a 1000 ppm multi-element stock solution. The method had a recovery range of 87–110%. The limits of detection (LOD) were calculated as 3 times the standard deviation of the mean of the blank curve (SB) determinations divided by the slope of the calibration curve (Sp), that is 3 × SB/Sp [34]. In addition, the limits of quantification (LOQs) were calculated as follows: LOQ = 10 × SB/Sp. The LOD range of all metal(loid)s was 0.02–0.3 µg/L, and the LOQ range of all metalloids was 0.06–10 µg/L. The range of the correlation coefficient (R2) was between 0.9992 and 0.9995.
2.4. Calculation of Human Health Risk Assessment
Contact with faeces can occur through different routes, and the following are considered in this study: (i) hand-to-mouth contact, which represents the contact of dirty hands with the mouth, as can occur with children; hand-to-mouth contact with caregivers’ hands; object-to-mouth contact; ingestion of contaminated food and water; and accidental direct ingestion of faeces. This follows the hypothesis adopted by Kwong et al. (2020) [35] in their study who considered these routes of contamination or contact with faeces, especially children who may be more exposed due to the habit of playing in contact with the floor (Section 2.5). (ii) particulate inhalation via environmental contamination (Section 2.6); (iii) dermal exposure to metal(loid) contaminants (Section 2.7); and (iv) risk analysis due to contact oral, respiratory tract, and dermal contact (Section 2.8).
2.5. Calculation of Accidental Ingestion Dose of Soil/Faeces
The calculation of the dose of accidental ingestion of soil/faeces (Doral) was carried out using the following Equation (1):
where Cs (mg/kg) is the contaminant’s concentration in the soil/faeces. Therefore, in this study, we will consider that it corresponds to the concentration of metal(loid)s in the faeces of capybaras. In this case, ingestion can occur when a person contacts faeces with their hands and unintentionally puts it in their mouth, resulting in unintentional ingestion, and it will be considered 100% of the amount detected in the samples; IR (kg/day) is the rate of accidental soil/faeces ingestion for children (1000 mg/day) and adults (100 mg/day); EF is exposure to children and adults 350 days/year; CF is the conversion factor = 1.0 × 10−6; and AT = ED × 365 = 5 × 365 = 1825 days for children, and adults AT = 40 × 365 = 14,600 days. CF is conversion factor 1.0 × 10−6. AT = EF × ED corresponds to non-carcinogenic effects, but for carcinogenic effects AT = 365 × 70 [36]. BW (kg) is the individual’s body weight, in this case 32.9 kg for children aged 5 years and 70.7 kg for adults > 40 years (values adapted from Health Canada 2004 [37]).
2.6. Calculation of Inhalation Dose of Soil/Faeces Particles
The calculation of the inhalation dose of soil/faeces particles (Dinal) was performed using the following Equation (2):
where Cs (mg/kg) is the concentration of the contaminant in the soil/faeces, quantified in the faeces of H. hydrochaeris, which can be carried by the wind along with dust and inhaled; Par (μg/m3) is the concentration of particles in the air, with a value of 1.36 × 109 m3/kg assumed for typical conditions according to USEPA (1992) [38]; IRinal (m3/day) is the inhalation rate (7.6 m3/day for children and 20 m3/day for adults) [39]; and EF = exposure frequency of 350 days/year for children and adults. ED is the exposure duration, which is 5 years for children and 40 years for adults; and BW (weight, kg) is the individual’s body weight, 32.9 kg for children aged 5 years and 70.7 kg for adults aged 40 years (values adapted from Health Canada 2004 [37]). In Equation (2), AT is the average time for non-carcinogenic effects: 1825 days for children and 14,600 days for adults.
2.7. Calculation of Dermal Contact Dose with Soil/Faeces
The calculation of the dermal contact dose (Dosecont-derm) was performed using the following Equation (3):
where in Equation (2) [40], Cs (mg/kg) is the concentration of the contaminant in the soil/faeces quantified in capybara faeces, generally at the 90th percentile or maximum; SAH is the surface area of the hands (cm2), arms, and legs (assuming that only the hands, arms, and legs are exposed, 2800 cm2 for children, and 5700 cm2 for adults) [41]; and AFS corresponds to the Skin to Soil Adhesion Factor (0.2 mg/cm2 for children, 0.07 mg/cm2 for adults) [41]. EF is the frequency of exposure; for children and adults, it is 350 days/year. ABS is the Dermal Absorption Factor (dimensionless); here, we will consider it to be 0.001 for children and adults. ED is the exposure duration, considering 5 years and 40 years. BW (kg) is the individual’s body weight, 32.9 kg for children aged 5 years, and 70.7 kg for adults >40 years (values adapted from Health Canada 2004 [37]). CF is conversion factor 1.0 × 10−6. It was considered that AT = EF × ED for non-carcinogenic effects and AT = 365 × 70 for carcinogenic effects [36]. AT is 1825 days for children and 14,600 days for adults.
2.8. Target Hazard Quotient
The target hazard quotient (THQ) is the level of exposure to the toxic metal(loid) at which no adverse health effects are expected. THQ is calculated using the United States Environmental Protection Agency [42] equation, as shown in the following Equation (4).
In Equation (4), the subindex i = oral ingestion (1), inhalation (2), and dermal contact (3), where RfDi refers to the reference dose of non-carcinogenicity of the metal(loid) (mg/kg/day). In this study, the following oral reference (RfDoral) dose values will be considered: Al 1 mg/kg/day, As 3 × 10−4 mg/kg/day, Cd 1 × 10−4 mg/kg/day, Co 3 × 10−4 mg/kg/day, Cr 1.5 mg/kg/day, Cu 0.04 mg/kg/day, Fe 0.7 mg/kg/day, Mg ND (not determined), Mn 0.24 mg/kg/day; Mo 5 × 10−3 mg/kg/day, Ni 1.10 mg/kg/day, Zn 0.3 mg/kg/day [43], P ND (not determined), Pb 3 × 10−3 mg/kg/day [33]; RfDinhalation = Al 5 × 10−3 mg/kg/day, As 1.5 × 10−5 mg/kg/day, Cd 1 × 10−5 mg/kg/day, Co 6 × 10−6 mg/kg/day, Cr 2.86 × 10−5 mg/kg/day, Cu 0.04 mg/kg/day, Fe ND (not determined), Mg ND (not determined), Mn 5.0 × 10−5 mg/kg/day; Mo 2 × 10−3 mg/kg/day, Ni 2 × 10−5 mg/kg/day, Zn 0.3 mg/kg/day [43], P ND (not determined), Pb 2 × 10−4 mg/kg/day; RfDdermal = Al 1 mg/kg/day, As 3 × 10−4 mg/kg/day, Cd 1.25 × 10−5 mg/kg/day, Co 3 × 10−4 mg/kg/day, Cr 1.95 × 10−2 mg/kg/day, Cu 0.04 mg/kg/day, Fe 0.7 mg/kg/day, Mg ND (not determined), Mn 9.6 × 10−4 mg/kg/day; Mo 5 × 10−3 mg/kg/day, Ni 5.40 × 10−3 mg/kg/day, Zn 0.3 mg/kg/day [43], P ND (not determined), and Pb 0.04 mg/kg/day.
When THQ < 1, no non-carcinogenic health effects are expected. However, if THQ is >1, adverse health effects may occur. In addition, the sum of all THQs corresponds to the hazard index (HI) demonstrated in Equation (5). If the HI is >1, there is the potential for non-carcinogenic adverse health effects, where the THQ and HI represent a dimensionless value.
2.9. Statistical Analysis
We used One-way ANOVA to test if each elemental concentration differs in the different sampling sites in faeces samples. The significance of the differences between the means for the individual metal(loid) was considered at p < 0.05.
3. Results
3.1. Quantification of Metal(loid)s in Faeces
The concentrations of the metal(loid)s Al, As, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, P, Se, Zn, and Pb quantified in H. hydrochaeris faeces samples from Anhanduí Ecological Park, Prosa State Park, Sóter Ecological Park, and Lago do Amor are presented in Table 2. The concentration values of quantified metal(loid)s showed normal distribution. The chemical element P has not been determined, and Se is below the detection limit. Besides Mg, all other elements had significant differences between sampling sites (Table 2).

Table 2.
Concentration of metalloids quantified in H. hydrochaeris faeces (mg/kg).
When observing the order of concentration values for each site studied (Table 2), it is noted that the concentrations of metal(loid)s quantified in capybaras faeces at Anhanduí Ecological Park decrease in the following order: Fe 300.369 ± 0.811 > Mn 180.287 ± 1.980 > Al 124.488 ± 0.763 > Zn 87.495 ± 1.160 > Cu 29.946 ± 1.296 > Cr 20.275 ± 0.970 > Ni 4.014 ± 0.033 > Mg 2.470 ± 0.283 > Pb 2.077 ± 0.157 > Co 1.467 ± 0.130 > Mo 1.133 ± 0.107 > Cd 0.4193 ± 0.008 > As 2.760 ± 0.155 × 10−3 mg/kg. In capybara excrement from Prosa State Park, the concentrations of metal(loid)s decreased in the following order: Fe 291.713 ± 1.998 > Al 130.443 ± 2.179 > Mn 90.484 ± 1.152 > Zn 76.320 ± 2.100 > Cu 32.632 ± 0.843 > Cr 17.602 ± 0.827 > Ni 4.197 ± 0.330 > Pb 3.3115 ± 0.316 > Mg 2.08 ± 0.313 > Mo 1.560 ± 0.084 > Co 1.019 ± 0.0399 > Cd 0.940 ± 0.0407 > As 4.300 ± 0.267 × 10−3 mg/kg.
According to Table 2, the concentration of metal(loid)s quantified in capybaras faeces of the Sóter Ecological Park decreased as follows: Fe 295.074 ± 2.666 > Mn 281.015 ± 0.420 > Al 139.564 ± 0.669 > Zn 68.889 ± 0.829 > Cu 32.042 ± 2.042 > Cr 21.405 ± 0.970 > Pb 6.016 ± 0.679 > Ni 4.229 ± 0.230 > Mo 2.233 ± 0.289 > Mg 1.964 ± 0.043 > Co 1.776 ± 0.069 > Cd 0.568 ± 0.053 > As 3.691 ± 0.202 × 10−3 mg/kg. Regarding the concentrations of metal(loid)s in Lago do Amor, the metalloids decreased in the following order: Mn 291.469 ± 3.250 > Fe 290.366 ± 0.033 > Al 140.322 ±1.222 > Zn 100.027 ± 1.267 > Cu 50.764 ± 0.853 > Cr 26.866 ± 1.013 > Pb 8.762 ± 0.282 > Ni 5.475 ± 0.216 > Mo 3.634 ± 0.164 > Co 1.946 ± 0.840 > Mg 1.677 ± 0.506 > Cd 1.042 ± 0.302 > As 10.041 ± 0.193 × 10−3 mg/kg.
3.2. Human Health Risk Assessment
Daily doses of metal(loid)s via oral, inhalation, and dermal contact of children and adults with capybara faeces were compared to the Minimum Risk Levels (MRLs) established by the Agency for Toxic Substances and Disease Registry (ATSDR) [44] (Tables S1 and S2). These values consider daily human exposure to a hazardous substance in the environment that is unlikely to present an appreciable risk of non-carcinogenic adverse health effects during a specified exposure period. According to the ATSDR, acute exposure is defined as exposure lasting 1 to 14 days, intermediate exposure as lasting 15 to 364 days, and chronic exposure as lasting 1 year or more. No inhalation and dermal values exist for Al, As, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Zn, and Pb in substances established by ATSDR in units of mg/kg/day. Given the absence of inhalation and dermal data values established by ATSDR, the data in Tables S1 and S2 will be compared.
The oral ingestion values for Al considering children and adults (Tables S1 and S2) are lower than those established by MRLs/ATSDR for this metal in hazardous substances (oral: chronic duration 1 mg/kg/day), as well as being lower than the concentration of As (oral: acute 5 × 10−5 and chronic 3 × 10−3 mg/kg/day). Contrary to the values for oral faeces intake by children (Table S1) which are below those established by MRLs/ATSDR for Cd (oral: intermediate 5 × 10−4 and chronic 1 × 10−4 mg/kg/day), all Cd intake values for adults are higher than the proposed values (Table S2).
Regarding the ingestion of soil/faeces containing Co, the values for children (oral: chronic 1 × 10−2 mg/kg/day) (Table S1) and adults (oral: acute and intermediate 3 × 10−2) (Table S2) are below those established by the ATSDR. Regarding Cr, ingestion values in soil/faeces for children are below those established by ATSDR (oral: chronic 9 × 10−4, intermediate 5 × 10−3 mg/kg/day), while for adults, these values are higher than those established by the ATSDR orally. Cu intakes presented in soil/faeces for children are lower than the oral ingestion values considered by ATSDR (acute and chronic: 1 × 10−2 mg/kg/day). In contrast, ingestion of this metal in faecal samples from all adult sites is above the value predicted by ATSDR.
Oral ingestion values in soil/faeces samples by children are below those established by ATSDR for Mo (intermediate 6 × 10−2 mg/kg/day), Ni (intermediate 2 × 10−4, chronic 9 × 10−5 mg/kg/day), and Zn (intermediate and chronic 3 × 10−1 mg/kg/day). Furthermore, adult ingestion of Mo and Zn is lower than the intermediate and chronic oral ingestion values established by the ATSDR. The Ni presented a concentration above the intermediate (2 × 10−4 mg/kg/day) and chronic (9 × 10−5 mg/kg/day) oral ingestion values.
The target hazard quotient due to ingestion of faeces containing Al, As, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Zn, and Pb in all locations was below 1 for children (Table S3). In Anhanduí Ecological Park, Prosa State Park, Sóter Ecological Park, and Lago do Amor, the metalloids Cd, Co, and Cu exceeded the target hazard quotient THQ > 1 for adults. Meanwhile, Mn in Prosa State Park and Pb in Anhanduí Ecological Park did not exceed values above 1 (THQ values < 1) considering adult ingestion. Across all sites, THQ values were generally higher for adults than for children.
4. Discussion
Except for Mg, metal(loid) content varied between all sampling sites. We showed that capybara faeces are bioindicators of pollution and the detection of high concentrations of heavy metal(loid)s is a potential health risk for the population that frequents urban parks. The highest concentrations of metal(loid)s were quantified in samples from a population of capybaras living in a lagoon (Lago do Amor) that receives water from streams that cross the city. Based on the risk assessment, we detected that there is a potential risk to the health of children and adults, mainly due to the presence of Al, As, Cd, Co, Cu, and Mn.
The descending order of metal(loid)s within the same group and between groups are not the same. In this case, such differences in concentration values of metal(loid)s in animal faeces may be explained due to the different types of vegetation and sites. Individuals of H. hydrochaeris live in environments with riparian vegetation, as well as open areas and fields [45]. However, it is a herbivorous animal that feeds on grasses and aquatic vegetation, and can also feed on corn and sugar cane [46]. According to Borges and Colares (2007) [47], H. hydrochaeris shows opportunistic behaviour during winter and summer and more selective behaviour in spring and autumn. Thus, capybaras have a varied diet according to the time of year, and this can interfere with the concentrations of metalloids quantified in faeces. Therefore, the type of vegetation/food available in each location studied and the season can influence our findings.
The presence of H. hydrochaeris in ecological parks must be investigated, since vegetation, water, and even faeces contaminate the environment, increasing the presence and availability of heavy metal(loid)s and other toxic elements. Thus, quantifying the bioaccumulation and biomagnification of heavy metals in these mammals can be a tool to assist in assessing the health and quality of aquatic and terrestrial environments [48].
The highest concentrations of Al, As, Cd, Co, Cr, Cu, Mn, Mo, Ni, Zn, and Pb were quantified in the faeces of animals living in Lago do Amor (Table 2). This area has great human influence and can be considered a source of storage of chemical waste/products originating from or brought by the waters of streams that cross the city of Campo Grande, and which may be polluted due to the presence of sewage and waste.
Several heavy metals are common in our daily lives, and metal(loid)s such as Al, As, Cd, Cu, Co, Cr, Mo, Ni, and Pb are considered carcinogenic [10]. Aluminium causes neurodegenerative brain pathological changes and changes in cytoskeletal proteins, while cobalt prevents DNA repair [10]. Furthermore, Co and its salts are genotoxic in mammals, mainly due to oxidative damage to DNA. Chromium promotes mutagenesis, RNA polymerase, and DNA polymerase arrests, and alters gene expression. Copper is capable of inducing DNA strand breakage and causing base oxidation by oxygen free radicals and hydroxyl radicals, while manganese inhibits ATP synthesis in brain mitochondria at the glutamate/aspartate exchange site or at the complex II site, subject to the mitochondrial energy source. Nickel has a wide range of carcinogenic mechanisms that include transcription factors, production of free radicals, and controlled expression of specific genes [10].
Ramm (2015) reported capybara tissue contamination by metals [49]. In capybaras run over in Southern Brazil, Ag, Cd, Cu, Pb, and Zn were found in the liver, kidney, fat, and muscle, which is attributed to the use of pesticides and herbicides in the region [49]. Such animals are subject to the effects of bioaccumulation of contaminants, through ingestion of plants contaminated by metals, sediments, and water. It is worth mentioning that the time of year is a key factor that influences the concentration of metal(loid)s in the organs of these animals, which can be caused by the availability of food depending on the season [49].
When comparing the concentrations of metal(loid)s in the faeces of H. hydrochaeris with those of other mammals, the concentration of Cr measured in capybara excrement (Table 2) was higher than Cr measured in the excrement of other animals, such as red deer Cervus elaphus and roe deer Capreolus capreolus [50]. Furthermore, the concentration of Cu in capybara faeces was also higher than that quantified in 128 deer excrement samples (Cu 19.2 mg/kg). In contrast, the concentrations of Mo and Zn were lower than those found in deer excrement samples, where the average values were 5.2 and 23.1 mg/kg, respectively [50]. The metalloid arsenic was detected in seven samples of deer faeces, with an average of 6.9 mg/kg, presenting values above those quantified in capybara faeces (Table 2). Cadmium was quantified in a sample of faeces from C. elaphus (8 mg/kg) and C. capreolus (9 mg/kg) and had values above those obtained for H. hydrochaeris excrement (Table 2). Finally, only the faeces of capybaras studied in Lago do Amor present Pb concentration values lower than those quantified in deer excrement samples (average value of 8.2 mg/kg). It is worth highlighting that in the study by Hort et al. (2017) [50], some metal(loid)s such as Co, Ni, Se, Ag, and Hg were also analysed, but were not quantified in any sample (below the detection limit). In our study, we could not determine P. However, this element was quantified in five deer faecal samples with an average of 525.0 mg/kg across all sampling sites [50].
Our study considered that children and adults put their hands in their mouths, and unintentional ingestion of animal faeces containing metal(loid)s may occur. Nonetheless, for children, the daily doses of metal(loid)s did not surpass the MRLs proposed by the ATSDR for any metal(loid) [44] (Table S1), independent of the type of exposure (acute, intermediate, or chronic). For adults, Cd (intermediate and chronic), Cr (intermediate and chronic), Cu (acute and chronic), Ni (intermediate and chronic), and Zn (intermediate and chronic) were beyond the MRL accepted levels (Table S2). The MRLs are often used to determine if exposure to contaminated sites may cause adverse effects in a determined window of exposure (acute, intermediate, and chronic) [51]. In this sense, contact with these sites should be advised.
Metal(loid)s such as As, Cd, Cr, Hg, and Pb are the most common heavy metal(loid)s reported to induce human poisoning [10]. A study carried out in northeastern China showed that there is a significant level of heavy metal(loid)s content, such as As, Cd, Cr, Cu, Pb, and Zn, quantified in animal feed and faeces (a total of 104 feed and 118 manure samples animal) from different farms and herd sizes [52]. The bioaccumulation of these heavy metal(loid)s leads to a variety of toxic effects on various tissues and organs. The presence of metal(loid)s such as Cd, Co, and Cu in the faeces of H. hydrochaeris points to a potential health risk for users of parks or public sites intended for recreation and physical activities [8].
In addition to oral ingestion, inhalation of dust containing heavy metal(loid)s is another route of contamination [53,54,55]. Assessments of risks to human health due to exposure to high levels of As, Ba, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, V, and Zn in dust from parks and squares in China have shown that such sites are moderately polluted, exposing the population to possible contamination [55]. Additionally, particulate matter can be indirectly deposited in the oropharynx via mucociliary clearance and swallowing of saliva and mucus [56]. Therefore, it appears that there are possible risks to human health for users of public parks due to the inhalation of excrement from dust in the air.
Dermal absorption is also a subsequent health risk (Tables S3 and S4) since metal(loid)s such as Cd, Cu, Hg, Pb, and Zn, when in dermal contact through dust, can be harmful [40]. The concentrations of Al, As, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, and Zn in animal faeces, when compared with dermal contact for children and adults, are values below those for oral ingestion and inhalation. However, it is worth highlighting that there are no safe levels for metalloids such as As, Cd, Mo, Ni, and Pb, regardless of the route of exposure.
Besides the MRL levels, other tools help assess metal(loid) contamination risk. THQ values were below 1 for all individual metal(loid)s considering children, regardless of the exposure route in all sampling sites. However, for adults, elements such as Cd, Co, and Cu presented THQ values above 1 for all sampling sites for oral ingestion, while Mn surpassed THQ of 1 for oral exposure at the sampling points of Anhanduí Ecological Park, Sóter Ecological Park, and Lago do Amor. In addition, Pb oral exposure for adults had THQ above 1 at Prosa State Park, Sóter Ecological Park, and Lago do Amor (Tables S3 and S4). These values contribute to an HI above 1, considering the oral exposure route for adults at all sampling sites (Figure 2). No other form of exposure presented an HI above 1, and the HI was below 1 for children at all sampling sites, independent of the exposure route.
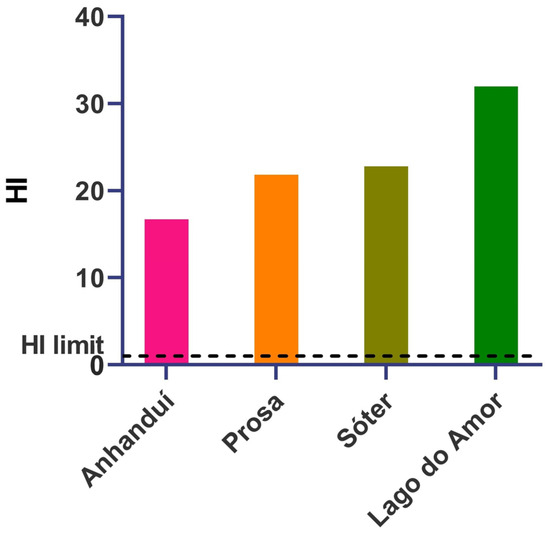
Figure 2.
Hazard index considering oral exposure for adults by contact with H. hydrochaeris faeces.
While children may be more susceptible to intoxication considering the higher intake relative to the body weight [57], adults are exposed for longer in the environments, which may explain why the THQ and HI values were above 1 for adults only.
Our results are limited only to H. hydrochaeris faecal samples, and we suggest further studies on soil and vegetation analysis and sampling in different seasons. In addition, it is also important to consider populations of H. hydrochaeris that live far from crops and urban regions, especially those that reside in threatened Brazilian biomes, such as Cerrado, Pantanal, and Atlantic Forest. This way, it will be possible to compare the values of the metal(loid)s between organs, tissues, and excrement.
Furthermore, future research should evaluate behaviours related to points of contact with animal faeces, animal faecal contamination with food, cultural animal faecal management behaviours, acute and chronic health risks associated with exposure to animal faeces, and factors that influence the concentrations and excretion rates of pathogens originating from animal faeces. It is also important to highlight that, for a more complete picture, it is necessary to evaluate the parasite load of capybara populations and the risk they pose to human health since they are animals with zoonotic potential affected by a wide range of diseases derived from protozoa and metazoa [58,59,60]. In addition, when considering faecal samples as a tool for biomonitoring, it is possible to detect and quantify metal(loid)s that enter the food chain of wild animals and are capable of bioaccumulation.
5. Conclusions
In summary, we believe that the faeces of H. hydrochaeris can be considered a bioindicator of environmental pollution. Higher concentrations of Al, As, Cd, Co, Cr, Cu, Mn, Mo, Ni, Pb, and Zn were quantified in the excrement of H. hydrochaeris population that live close to Lago do Amor since the lagoon receives water from streams that cross the city and is possibly polluted by human influence. Some metal(loid)s quantified in the faeces of these animals present higher concentrations than those found in other mammals.
Potential health risks to children and adults may occur due to the presence of Al, As, Cd, Co, Cu, and Mn through involuntary oral ingestion of faeces, inhalation, and dermal routes. The presence of heavy metal(loid)s in H. hydrochaeris excrement directly reflects the dietary profile of these animals. Therefore, studies must be carried out on the quantification of metal(loid)s in blood plasma, as well as in the vegetation on which animals feed in different seasons and sites.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/urbansci8040151/s1, Table S1: Daily dose of metalloids (mg/kg/day) via oral exposure (soil/faeces), inhalation and dermal contact of children due to contact with Hydrochoerus hydrochaeris faeces, compared with established Minimal Risk Levels (MRLs) by the Agency for Toxic Substances and Disease Registry [44]; Table S2: Daily dose of metalloids (mg/kg/day) via oral exposure (soil/faeces), inhalation and dermal contact of adult due to contact with Hydrochoerus hydrochaeris faeces, compared with established Minimal Risk Levels (MRLs) by the Agency for Toxic Substances and Disease Registry [44]; Table S3: Calculation of Target Hazard Quotient (THQ) and Hazard Index (HI) due to accidental soil ingestion, inhalation and dermal contact of children due to contact with Hydrochoerus hydrochaeris faeces; Table S4: Calculation of Target Hazard Quotient (THQ) and Hazard Index (HI) due to accidental soil ingestion, inhalation and dermal contact of adults due to contact with Hydrochoerus hydrochaeris faeces.
Author Contributions
Conceptualisation, F.Z.V.B., I.D.d.S. and V.A.d.N.; methodology, F.Z.V.B., I.D.d.S. and V.A.d.N.; formal analysis, F.Z.V.B., I.D.d.S. and V.A.d.N.; investigation, F.Z.V.B., I.D.d.S. and V.A.d.N.; resources, F.Z.V.B., I.D.d.S. and V.A.d.N.; data curation, F.Z.V.B., I.D.d.S. and V.A.d.N.; writing—original draft preparation, F.Z.V.B., I.D.d.S., D.A.Z.G., and V.A.d.N.; writing—review and editing, F.Z.V.B., I.D.d.S., D.A.Z.G., D.G.A., C.S.d.A.M., M.A.P.A., E.S.d.P.M. and V.A.d.N.; visualisation, D.A.Z.G., D.G.A., C.S.d.A.M., M.A.P.A., E.S.d.P.M. and V.A.d.N.; supervision, V.A.d.N.; project administration, V.A.d.N.; funding acquisition, V.A.d.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq—grant number 314551/2023-9), and the Brazilian Federal Agency Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES —finance code 001).
Data Availability Statement
All relevant materials and data are within the manuscript and available from the corresponding author on request.
Acknowledgments
The authors would like to thank the Federal University of Mato Grosso do Sul, Faculty of Medicine, for their scientific support, as well as the Municipal Secretariat for the Environment and Urban Management, the National Sports Foundation/MS, and the Mato Grosso do Sul Environmental Institute (IMASUL) for permission and authorisation to enter the parks to collect samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- Withanachchi, S.S.; Ghambashidze, G.; Kunchulia, I.; Urushadze, T.; Ploeger, A. Water quality in surface water: A preliminary assessment of heavy metal contamination of the Mashavera River, Georgia. Int. J. Environ. Res. Public Health 2018, 15, 621. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Guan, T.X.; He, H.B.; Zhang, X.D.; Bai, Z. Cu fractions, mobility and bioavailability in soil-wheat system after Cu-enriched livestock manure applications. Chemosphere 2011, 82, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Azeez, J.O.; Adekunle, I.O.; Atiku, O.O.; Akande, K.B.; Jamiu-Azeez, S.O. Effect of nine years of animal waste deposition on profile distribution of heavy metals in Abeokuta, South-western Nigeria and its implication for environmental quality. Waste Manag. 2009, 29, 2582–2586. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, H.L.; Schroder, J.L.; Udeigwe, T.K.; Zhang, Z.Q.; Dodla, S.K.; Stietiya, M.H. Reducing potential leaching of phosphorus, heavy metals, and fecal coliform from animal wastes using bauxite residues. Water Air Soil Pollut. 2011, 214, 241–252. [Google Scholar] [CrossRef]
- Bianco, K.; Albano, R.M.; Oliveira, S.S.A.; Nascimento, A.P.A.; Dos Santos, T.; Clementino, M.M. Possible health impacts due to animal and human fecal pollution in water intended for drinking water supply of Rio de Janeiro, Brazil. J. Water Supply Res. Technol.–AQUA 2020, 69, 70–84. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Li, W. Content of heavy metals in animal feeds and manures from farms of different scales in northeast China. Int. J. Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Amadi, C.N.; Frazzoli, C.; Orisakwe, O.E. Sentinel species for biomonitoring and biosurveillance of environmental heavy metals in Nigeria. J. Environ. Sci. Health C 2022, 38, 21–60. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, B.; Ribaric, L.C. Lead, cadmium and zinc in tissue of roe deer (Capreolus capreolus) near the lead smelter in the Koroska region (northern Slovenia). Bull. Environ. Contam. Toxicol. 2000, 64, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.N.; Gaston, G.; Brazzle, R.; Connell, A.F.; Audet, D.J. Deer exposed to exceptionally high concentrations of lead near the Continental Mine in Idaho, USA. Environ. Toxicol. Chem. 2007, 26, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.E.; Marra, P.P. The presence and impact of environmental lead in passerine birds along an urban to rural land use gradient. Arch. Environ. Contam. Toxicol. 2007, 53, 261–268. [Google Scholar] [CrossRef]
- Dzugan, M.; Zielinska, S.; Heclik, J.; Pieniazek, M.; Szostek, M. Evaluation of heavy metals environmental contamination based on their concentrations in tissues of wild pheasant (Phasianus colchicums L). J. Microbiol. Biotechnol. Food Sci. 2012, 2, 238–245. [Google Scholar]
- Rosas, I.; Amabile-Cuevas, C.F.; Calva, E.; Osornio-Vargas, A.R. Animal and human waste as components of urban dust pollution: Health implications, encyclopedia of environmental health. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 75–82. [Google Scholar]
- Penakalapati, G.; Swarthout, J.; Delahoy, M.J.; Mcaliley, L.; Wodnik, B.; Levy, K.; Freeman, M.C. Exposure to animal feces and human health: A systematic review and proposed research priorities. Environ. Sci. Technol. 2017, 51, 11537–11552. [Google Scholar] [CrossRef]
- Jota Baptista, C.; Seixas, F.; Gonzalo-Orden, J.M.; Oliveira, P.A. Biomonitoring metals and metalloids in wild mammals: Invasive versus non-invasive sampling. Environ. Sci. Pollut. Res. Int. 2022, 29, 18398–18407. [Google Scholar] [CrossRef]
- García-Muñoz, J.; Pérez-López, M.; Soler, F.; Míguez-Santiyán, M.P.; Martínez-Morcillo, S. Non-Invasive Samples for Biomonitoring Heavy Metals in Terrestrial Ecosystems. In Trace Metals in the Environment; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Reidinger, R.F., Jr. Factors Influencing Arizona Bat Population Levels. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1972. [Google Scholar]
- Way, C.A.; Schroder, R. Accumulation of lead and cadmium in wild population of the commensal rat, Rattus norvegicus. Arch. Environ. Contam. Toxicol. 1982, 11, 407–417. [Google Scholar] [CrossRef]
- Sileo, L.; Beyer, W.N. Heavy metals in white-tailed deer living near a zinc smelter in Pennsylvania. J. Wildl. Dis. 1985, 21, 289–296. [Google Scholar] [CrossRef]
- Gupta, V. Feces of captive wild mammal use as bio-indicator of heavy metal pollution in urban air. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2404–2411. [Google Scholar]
- Frossard, A.; Leite, F.L.G.; Silva, E.L.F.; Carneiro, M.T.W.D.; Júnior, J.L.R.; Gomes, L.C.; Endringer, D.C. The snake Bothrops jararaca (Squamata: Viperidae) is a suitable bioindicator of environmental exposure to cadmium: An experimental study. Ecol. Indic. 2019, 104, 166–171. [Google Scholar] [CrossRef]
- Cang, L.; Wang, Y.J.; Zhou, D.M.; Dong, Y.H. Heavy metals pollution in poultry and livestock feeds and manures under intensive farming in Jiangsu Province, China. J. Environ. Sci. 2004, 16, 371–374. [Google Scholar]
- Li, Y.X.; Li, W.; Wu, J.; Xu, L.C.; Su, Q.H.; Xiong, X. Contribution of additive Cu to its accummlation in pig feces: Study in Beijing and Fuxin of China. J. Environ. Sci. 2007, 5, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Li, Y.X.; Li, W.; Lin, C.Y.; Han, W. Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Res. Conserv. Recycl. 2010, 54, 985–990. [Google Scholar] [CrossRef]
- EPA/630/R-00/002; USEPA, 2000 Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. Risk Assessment Forum Technical Panel: Washington, DC, USA, 2000.
- Liu, Q.; Song, Y.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil–vegetable system: A multi-medium analysis. Sci. Total Environ. 2013, 463–464, 530–540. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.T.; Zhou, X.; Liu, L.; Gu, J.F.; Wang, W.L.; Zou, J.L.; Tian, T.; Peng, P.Q.; Liao, B.H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Cao, Y.; Chen, A.; Ren, M.; Ge, Y.; Yu, Z.; Wan, S.; Hu, A.; Bo, Q.; et al. Potential health risks of heavy metals in cultivated topsoil and grain, including correlations with human primary liver, lung and gastric cancer, in Anhui province, eastern China. Sci. Total Environ. 2014, 470–471, 340–347. [Google Scholar] [CrossRef]
- Obiora, S.C.; Chukwu, A.; Daveis, T.C. Heavy metals and health risk assessment of arable soils and food crops around Pb–Zn mining localities in Enyigba, southeastern Nigeria. J. Afr. Earth Sci. 2016, 116, 182–189. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.G.; Zhai, R.; Huang, Y.; Qiu, Y.; Liang, J. Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ. Int. 2005, 31, 784–790. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection: A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712a–724a. [Google Scholar]
- Kwong, L.H.; Ercumen, A.; Pickering, A.J.; Arsenault, J.E.; Islam, M.; Parvez, S.M.; Unicomb, L.; Rahman, M.; Davis, J.; Luby, S.P. Ingestion of fecal bacteria along multiple pathways by young children in rural Bangladesh participating in a cluster-randomized trial of water, sanitation, and hygiene interventions (WASH Benefits). Environ. Sci. Technol. 2020, 54, 13828–13838. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, L.; Wang, H.; Martín, J.D. Bioavailability and health risk of toxic heavy metals (As, Hg, Pb and Cd) in urban soils: A Monte Carlo simulation approach. Environ. Res. 2022, 214, 113772. [Google Scholar] [CrossRef]
- Health Canada 2004. Federal Contaminated Site Risk Assessment in Canada: Guidance on Human Health Preliminary Quantitative Risk Assessment (PQRA), Version 3.0. 2021. Available online: http://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/contaminated-sites/federal-contaminated-site-risk-assessment-canada-part-guidance-human-health-preliminary-quantitative-risk-assessment-pqra-version-2-0.html (accessed on 2 July 2023).
- USEPA (United States Environmental Protection Agency). Guidelines for Exposure Assessment U.S.; Environmental Protection Agency, Risk Assessment Forum: Washington, DC, USA, 1992.
- Van Den Berg, R. Human Exposure to Soil Contamination: A Qualitative and Quantitative Analysis towards Proposals for Human Toxicological Intervention Values; National Institute of Public Health and Environmental Protection (RIVM): Bilthoven, The Netherlands, 1995. [Google Scholar]
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Sci. Total Environ. 2010, 408, 726–733. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final, Office of Superfund Remediation and Technology Innovation U.S.; Environmental Protection Agency: Washington, DC, USA, 2004.
- USEPA (United States Environmental Protection Agency). Guidance Manual for Assessing Human Health Risks from Chemically Contaminated Fish and Shellfish; PTI Environmental Services: Bellevue, WA, USA, 1989. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Basic Information about Lead Air Pollution; Environmental Protection Agency: Washington, DC, USA, 2022.
- ATSDR (Agency for Toxic Substances and Disease Registry). Minimal Risk Levels in Draft Toxicological Profiles Are Provisional. 2023. Available online: http://wwwn.cdc.gov/TSP/MRLS/mrlsListing.aspx (accessed on 2 July 2023).
- Rocha, V.J.; Sekiama, M.L.; Gonçalves, D.D.; Sampieri, R.; Barbosa, G.P.; Dias, T.C.; Rossi, H.R.; Souza, P.F.P. Capivaras (Hydrochoerus hydrochaeris) e a presença do carrapato Amblyomma sculptum no campus da UFSCAR-Araras São Paulo. Cienc. Anim. Bras. 2017, 18, 1–15. [Google Scholar] [CrossRef]
- Ferraz, K.P.M.B.; Verdade, L.M. Ecologia comportamental da capivara: Bases biológicas para o manejo da espécie. In A Produção Animal na Visão dos Brasileiros; Sociedade Brasileira de Zootecnia: Piracicaba, Brazil, 2001; Volume 1, pp. 589–595. [Google Scholar]
- Borges, L.V.; Colares, I.G. Feeding habits of capybaras (Hydrochoerus hydrochaeris, Linnaeus 1766), in the ecological reserve of Taim (ESEC-Taim)—South of Brazil. Braz. Arch. Biol. Technol. 2007, 50, 409–416. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Horta, M.A.P.; Cunha, C.L.N. Avaliação das concentrações de metais pesados no sedimento, na água e nos órgãos de Nycticorax nycticorax (Garça-da-noite) na Baia de Sepetiba, RJ, Brasil. Rev. Gestão Costeira Integr. 2010, 10, 229–241. [Google Scholar] [CrossRef]
- Ramm, C.B. Contaminação por Metais nas Capivaras Hydrochaeris hydrochaeris no Sul do Brasil. Master’s Thesis, Federal University of Rio Grande, Porto Alegre, Brazil, 2015. [Google Scholar]
- Hort, J.; Mikolás, P.; Janiga, M. Heavy metals and other elements in faeces of wild ruminants in the area of paper mill industry. Oecologia Mont. 2017, 26, 56–62. [Google Scholar]
- Przybyla, J.; Buser, M.C.; Abadin, H.G.; Pohl, H.R. Evaluation of ATSDR’s MRL and EPA’s RfCs/RfDs: Similarities, Differences, and Rationales. J. Toxicol. Pharmacol. 2020, 4, 1–13. [Google Scholar]
- Hejna, M.; Onelli, E.; Moscatelli, A.; Bellotto, M.; Cristiani, C.; Stroppa, N.; Rossi, L. Heavy-metal phytoremediation from livestock wastewater and exploitation of exhausted biomass. Int. J. Environ. Res. Public Health 2021, 18, 2239. [Google Scholar] [CrossRef]
- Banerjee, A.D.K. Heavy metal levels and solid phase speciation in street dusts of Delhi, India. Environ. Pollut. 2003, 123, 95–105. [Google Scholar] [CrossRef]
- Ahmed, F.; Ishiga, H. Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmos. Environ. 2006, 40, 3835–3844. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Qinggeletu; Wu, Y. Health risks and contamination levels of heavy metals in dusts from parks and squares of an industrial city in semi-arid area of China. Int. J. Environ. Res. Public Health 2017, 14, 886. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Eengen, P.A.; Yazici, C.; Soberanes, S.; Hamanaka, R.B.; Nigdelioglu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- de Almeida, C.C.; Baião, D.S.; Rodrigues, P.A.; Saint’Pierre, T.D.; Hauser-Davis, R.A.; Leandro, K.C.; Paschoalin, V.M.F.; da Costa, M.P.; Conte-Junior, C.A. Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk. Int. J. Environ. Res. Public Health 2022, 19, 11178. [Google Scholar] [CrossRef]
- Souza, D.S.; Yang, S.G.N.S.; Alves, A.C.A.; Pontes, R.M.P.; Carvalho, C.C.D.; Soares, P.C.; Oliveira, J.B.O. Parasites and health status of free-ranging capybaras (Hydrochoerus hydrochaeris) in the Atlantic Forest and Caatinga biomes of Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 21, 100503. [Google Scholar] [CrossRef]
- Souza, S.L.P.; Benatti, H.R.; Luz, H.R.; Costa, F.B.; Pacheco, R.C.; Labruna, M.B. Endoparasites of capybaras (Hydrochoerus hydrochaeris) from anthropized and natural areas of Brazil. Braz. J. Vet. Parasitol. 2021, 30, e027420. [Google Scholar] [CrossRef]
- Uribe, M.; Hermosilla, C.; Rodríguez-Durán, A.; Vélez, J.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Cortés-Vecino, J.A. Parasites Circulating in Wild Synanthropic Capybaras (Hydrochoerus hydrochaeris): A One Health Approach. Pathogens 2021, 7, 1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).