Abstract
Modern urban societies generate tremendous amounts of hazardous wastes, including toxic organics and metals. Toxic metals harm plants and pose a risk to human health; examples of them are copper (Cu), zinc (Zn), palladium (Pb), and cadmium (Cd). Wetland plants are excellent for the ecological restoration of toxic metal-affected environments. Phragmites australis (common reed) belongs to the family Poaceae and is a broadly distributed wetland grass that is native to Bahrain, Europe, and America. P. australis shows a high content of chlorophyll. This study aimed to assess percentages of water, chlorophyll, and toxic metal content using acetone extraction; the calculation of the concentrations was performed according to the equations proposed by Lichtenthaler and the percentage of water content was calculated. After the metal exposure, the reed plants were digested, and their total mineral analysis was accomplished by atomic absorption spectroscopy; statistical analysis was conducted by Statistical Package for the Social Sciences (SPSS) version 21. The results revealed that the immature stage showed the highest chlorophyll a (mean 1641.5 (µg/g)) carotenoids (mean 359.75 (µg/g)) and total chlorophyll (mean 2183.93 (µg/g)), and the mature flowering stem had the highest chlorophyll b (mean 676.45 (µg/g)). The mature flowering stem stage showed the highest Pb (mg/L) and Cd (mg/L) values; on the other hand, Cu was the highest in the fully elongated non-flowering stage (0.108 mg/L), and the highest Zn content was found in the immature stage (mean 2.083). Owing to its growth in contaminated environments, P. australis can be considered a potential source of phytonutrients; higher concentrations were mostly available in the immature and mature flowering stages, with a favorable immature stage. The use of such marginal wetland plants may be very useful in reducing the pollution burden of urban built environments. These plants offer a green and sustainable solution for the disposal of waste from urban areas. Hence, further planning and execution of such a green solution are pivotal for creating environmental sustainability.
1. Introduction
Phragmites australis (P. australis), also known as the common reed, is a type of C3 plant belonging to the family Poaceae in the order Poales of the plant kingdom. The genus is widely distributed as a wetlands grass, having a wide prevalence in cold-temperate regions and mostly growing in the wetlands of the hot and moist tropics [1]. P. australis has the ability of prolific growth through vegetative means by the vigorous rhizomes and stolons present in the soil [2]. Such tremendous growth ability contributes to its cosmopolitan nature. Studies have confirmed that P. australis has high productivity under various climatic conditions and grows in soils with different pH levels, salinity, fertility, and textural classes [3,4]. Such prolific plant growth shown by P. australis is responsible for its occupation in many wetlands, due to which it acts as a pest and is difficult to eliminate. The main stem of common reeds can reach 10 m or more in height. Their dense growth can smother the marsh grasses that hold the soil in place. In marshes like these, Phragmites can choke out other plants by crowding them out of the light and water necessary for their growth [5].
P. australis is a widely distributed wetlands plant genus worldwide. The plant is a Poaceous grass (The Plant List 2010) which has tremendous biomass and net primary production; it is not only native to Bahrain but is widespread in Europe and America [6]. It was first introduced by European colonists in North America as an ornamental plant from Europe. The plant possesses features that are very useful in phytoremediation; these include luxuriant biomass, an excessive ability to grow in varied habitats, an extensive root system, and a non-edible nature, and it can be employed in bioenergy production after the uptake of environmental contaminants. Other uses of the common reed include its use as industrial thatching material in Europe, where thatching is the most common traditional usage of the common reed [5]. Winter-harvested reeds that have been naturally dried are appropriate. The stem base (butt) should be included since it is the toughest and most decay-resistant component of the plant [2]. Owing to its growth characteristics, it is safely declared that the plant possesses a wide ecological amplitude [7].
Due to the ecological characteristics of the common reed, the plant has been extensively employed in water purification [7] because it can absorb various inorganic and organic pollutants. P. australis acts as a water filter plant in two ways. The first is the bacterial activity in the rhizosphere that is triggered by the reed stalks through oxygen transport to the roots through the aerenchyma [8]. Nitrogen is eliminated mainly by ammonification nitrification coupled with de-nitrification, ammonia volatilization, and cation exchange for ammonium, while phosphorus is removed by chemical adsorption and biological transformation [5]. Second, the growing aerial parts of common reeds remove pollutants through excessive transpiration pull and tend to accumulate the pollutants in the aerial stems and leaves. The nutrients and pollutant accumulation in the lower part of P. australis plant roots leaves 10–20% of the nutrients, and the majority of the pollutants are transported to the aerial parts during the fast growing season in July or August for the next growing season [6]. On the other hand, this accumulation leads to beneficial side effects, such as the removal of secondary pollutants caused by both plant decomposition and humification [2,9]. It has already been suggested that there is a need to explore the biochemistry and physiology of the common reed to cope with environmental pollutants. It is a well-established fact that there are three major metabolic pathways in green plants, namely C3, C4, and CAM (Crassulacean Acid Metabolism). The common reed has various ecotypes that have both C3 and C4 physiological pathways [7]; it is likely that the ecological success of this species depends on its varied physiological pathways (C3 or C4), which enable the common reed to grow at a fast speed under different climatic conditions.
Photosynthetic pigments (chlorophyll) are responsible for the green color of a plant [9]. They play an essential role in photosynthesis. Chlorophyll and other accessory pigments of green plants capture energy from light and transfer it to reaction centers to accomplish light reactions [10]. Various chlorophyll molecules include chlorophyll a and b, xanthophylls, and carotenoids. Chlorophyll is also known as green blood due to the similarity of the structures of chlorophyll a and b to the structure of hemoglobin, which is mostly present in mammals’ blood. The difference between their structures is in their central atom, where chlorophyll a and b have magnesium and hemoglobin has iron [10]. Recently, chlorophylls have been a focus of investigations for their physiological impact on human health in the maintenance and prevention of chronic diseases [3]. Chlorophylls and their derivatives have been extensively studied for their beneficial biological properties [11]. Chlorophylls have beneficial effects on inflammation, oxidation mechanisms, wound healing, and the production of calcium oxalate crystals, among other things [12]. In in vitro and in vivo studies, chlorophyll and its natural and industrial derivatives have shown antioxidant functions, antimutagenic activity, the regulation of xenobiotic metabolizing enzymes, and the activation of apoptotic events in cancer cell lines [13]. Furthermore, they have the capacity to induce mammalian phase 2 proteins [14], which shield cells from oxidants and electrophiles. In animals, the protective properties of chlorophyll and its water-soluble salts (chlorophyllin) against the effects of carcinogens, such as aflatoxin, have also been reported [4,15], In addition, chlorophyll-enriched food was proposed as a viable chemoprevention strategy [3].
Heavy metals include essential metals, such as Fe, Cu, and Zn, which play a role in metabolism, and non-essential metals that do not have physiological roles, such as Cd, Pb, Al, etc. Heavy metal toxicity can be considered abiotic oxidative stress. Non-essential metals are toxic even at low levels, while essential metals can also become toxic when they exceed the threshold limit of tolerance. Heavy metal toxicity in plants is thought to be caused by four different pathways. These include the following: (I) heavy metals can resemble nutrient cations, which causes absorption competition on the root surface (for example, Cd competes with P and Zn); (II) heavy metals directly interact with the sulfhydryl group (-SH) of functional proteins, disrupting their structure and function and making them inactive; (III) the removal of essential cations from particular binding sites results in a loss of function; and (IV) the development of reactive oxygen species (ROS), which destroy macromolecules. The toxic effects of heavy metals on plant growth are analogous when they exceed the allowable limits, regardless of their essential or non-essential nature for plant growth. Some general effects include chlorosis, low growth and biomass accumulation, low photosynthesis, reproductive suppression, altered water balance and nutrient assimilation, senescence, and eventually plant death [16]. Regardless of the role of the absorbed metal, an injurious impact on plants and may pose a risk to human health because of their tenacity in nature.
A valuable review was conducted [7] that collected information on the use of P. australis as a phytoremediator of contaminated wetlands. They concluded that the phytoremediation ability of the common reed was better when the plant was grown in environments with limited water content (such as soils). The plant was able to increase its biomass because of high pollutant uptake, and it was shown to possess developed mycorrhiza, enhanced photosynthesis, and thus increased growth. Thus, the authors anticipated that contaminated soils may have some effects on the photosynthetic pigments of the common reed.
The present work aimed to explore the impacts of toxic metal-contaminated soils (Cu, Zn, Cd, and Pb) on the photosynthetic machinery of the common reed, namely chlorophyll a and b and carotenoid levels. The present findings may serve to supply reference points for the extraction of chlorophyll from P. australis grown in contaminated sites.
2. Material and Methods
2.1. Plant Samples
The study was conducted at the University of Bahrain, where P. australis plants were harvested during the winter (January 2020) from wetlands in the vicinity of the University of Bahrain (26°02′48.0″ N 50°30′19.0″ E). After collection, the plants were brought to the laboratory and cleaned to remove any adhered soil and organic debris. They were cut into pieces with an average length of 30 cm containing appropriate buds for their germination. The cut plant samples were allowed to grow in a small tub containing Hoagland solution [17]. After the suitable growth at an optimal temperature, plants with uniform size, number of branches, and leaves were selected for further study. Ten plant samples from each stage of P. australis were separated in the biology department; four stages were determined according to the length of the shoot in centimeters (cm): immature (50 cm), rapidly elongating (100 cm), fully elongated non-flowering (200 cm), and mature flowering stems (200 cm). The shoot length of the sample was measured in cm. The weight of the shoot was measured in milligrams (mg). Plant growth was analyzed by taking the weight of the growing leaves of P. australis separately for each growth stage. Counting down from the apex, the 4th leaf was used as set (A) (when the weight was not enough, an adjustment was made by taking the upper leaves); the 5th leaf was used as set (B) (if the weight was not high enough to adjust from the lower leaves) for the analysis. The rest of the shoot was used in the percentage of water content and toxic metal measurement by atomic absorption spectrophotometry.
2.2. Percent Water Content
The sample (shoot) was cut into segments to fit the analytical balance for the measurement of fresh weight. Samples were placed in a paper bag and were placed in the oven at 60 °C for 72 h. After drying, the dry weight was measured with an analytical balance. The percent water content was then calculated with the following equation:
% Water Content = (fresh weight − dry weight)/fresh weight
The clustered column was performed for the average of each stage.
2.3. Toxic Metal Analyses
A properly dried and powdered plant sample (0.5 g) was added to MARS tubes, followed by the addition of 10 mL of aqua regia. The sample was microwave-digested by a MARS 6 iWave. The digested plant material was then filtered through a grade 1 filter paper. The digested material was retransferred to a graduated cylinder, and distilled water was added to make the volume 50 mL. A standard solution (50 mL) was prepared for each element from a 1000 ppm (μg/g) (0, 5, 10, 15, 25) stock solution. The standard solution was placed in an Atomic Absorption Spectroscopy (AAS) instrument, allowing it to plot a linear curve that had a coefficient of correlation between 0.8 and 0.9. The digested samples were placed in the AAS device to determine the concentrations of Cu, Zn, Pb, and Cd in the diluted sample at a wavelength of 324.8 nm with a slit width of 0.5 nm; zinc was analyzed at 213.9 nm with a slit width of 1.0 nm; Pb was analyzed with a 405.8 wavelength (nm), a 0.2 slit (nm); the wavelength for Cd was 326.1 (nm) and the slit was 0.2 (nm). All samples used an air–acetylene flame. The metal concentrations were measured by taking the average of three readings and comparing them with the standard curve expressed by , which equaled the ppm.
2.4. Total Chlorophyll Content
- The chlorophyll content was measured by acetone extraction, and the calculation of the concentration was determined according to the equations proposed by Lichtenthaler [18]. The percent water content was estimated by the formula mentioned in Section 2.2. The data obtained were statistically analyzed by Statistical Package (IBM Corp, Armonk, NY, USA) for the Social Sciences (SPSS) version 21.
- The fresh weight of sets A and B (0.05 g) was measured using an analytical balance and transferred to a pestle; then, 2 mL of acetone was added with the addition of 0.01 g of sea sand acid and (0.01 g) MgCO3. The mixture was ground using a mortar until it was dry (powder), and then the ground powder was transferred to a plastic centrifuge test tube. Using a micropipette, 1 mL of 80% acetone was added to the pestle, homogenized, and then transferred to a centrifuge tube, and the process was repeated until the pestle was clear; then, the volume was adjusted to 3 mL by adding 80% acetone. The sample was centrifuged at a speed of 5000 rpm for 15 min at 4 °C; after that, the supernatant was collected in a UV–Vis tube, and absorbance was determined by a UV–Vis spectrophotometer at 470, 646.8, 663.2, and 750 nm.
- The calculation of the chlorophyll concentration was performed according to the equations proposed by Lichtenthaler [18]:
Chl. a (µg/mL) = 12.21 (A663.2) − 2.81 (A646.8)
Chl. b (µg/mL) = 20.13 (A646.8) − 5.03(A663.2)
Total Chl. (µg/mL) = 17.32 (A646.8) + 7.18 (A663.2)
The concentration was converted from µg/mL to µg/g fresh weight:
2.5. Statistical Analysis
The differences in the contents of chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, and % water content during various developmental stages were analyzed by one-way ANOVA with Statistical Package for the Social Sciences (SPSS) version 21 (statistical significance was set at p < 0.05; a significance level of 0.01 was deemed in order to indicate the significance level). Correlations among chlorophyll and the metals absorbed from the wastewater were also analyzed on the basis of the linear regression model, and the columns were clustered by Office 365 Excel.
3. Results
3.1. Water and Chlorophyll Content
The immature stage showed the highest photosynthetic pigments (chlorophyll a, carotenoids, and total chlorophyll), followed by the mature flowering stem, which showed quite similar results, with the highest chlorophyll b observed after it was fully elongated during the non-flowering stage and the lowest chlorophyll observed in the rapidly elongating stage (Table 1 and Figure 1).

Table 1.
The mean estimated concentrations of chlorophyll a (Chl. a) and chlorophyll b (Chl. b), carotenoids, the total chlorophyll contents (total Chl.), and the percent water content.
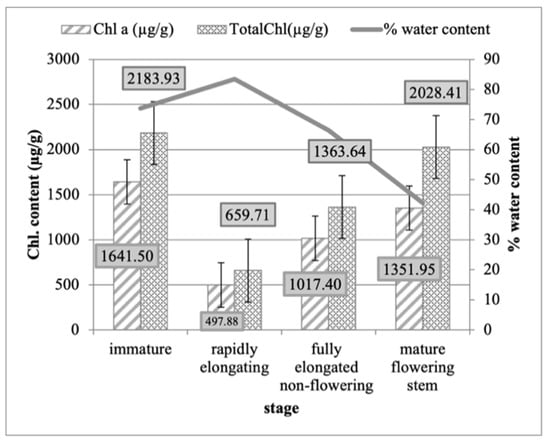
Figure 1.
The mean chlorophyll a (Chl. a) and total chlorophyll contents (Total Chl.) (µg/g) concentrations during each developmental stage of P. australis (immature, rapidly elongating, fully elongated non-flowering, and mature.
The immature stage was found to possess the highest chlorophyll a concentration, at 1641.5 (µg/g); carotenoid content, at 359.75 (µg/g); and total chlorophyll content, at 2183.93 (µg/g) (Figure 2). The observations were followed by the mature flowering stem, which showed quite similar results, but with the highest chlorophyll b content of 676.45 (µg/g) after it was fully elongated during the non-flowering stage. The plants were shown to have the lowest total chlorophyll content during the rapid elongation stage, containing a chlorophyll a value of 497.88 (µg/g), a chlorophyll b value of 161.82 (µg/g), a carotenoid value of 187.22 (µg/g), and a total chlorophyll value of 659.71 (µg/g).
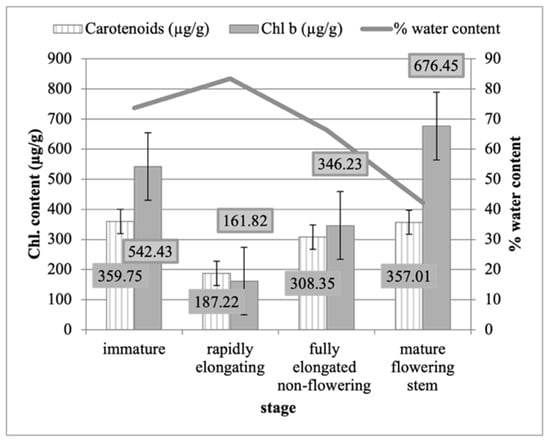
Figure 2.
Means chlorophyll b (Chl. b) and carotenoid contents (µg/g) in each developmental stage of P. australis (immature, rapidly elongating, and fully elongated.
3.2. Toxic Metal Contents
The atomic absorption spectroscopy standard curve (calibration) for the elements (Cu (I), Zn (II), Cd (III), and Pb (IV)) based on absorbance versus concentration was performed by AAS (Table 2).

Table 2.
The mean of various toxic metal contents for Cu (mg/L), Zn (mg/L), Pb (mg/L), and Cd (mg/L) during the developmental stage of P. australis plants (immature, rapidly elongating, fully elongated non-flowering, and mature flowering stem).
The mature flowering stem stage showed the highest Pb (mg/L) and Cd (mg/L) values; on the other hand, the highest Cu concentration was noted in fully elongated non-flowering plants (mean 0.1084 mg/L), and the highest Zn content was found in the immature stage (mean 2.083 mg/L) (Figure 3).
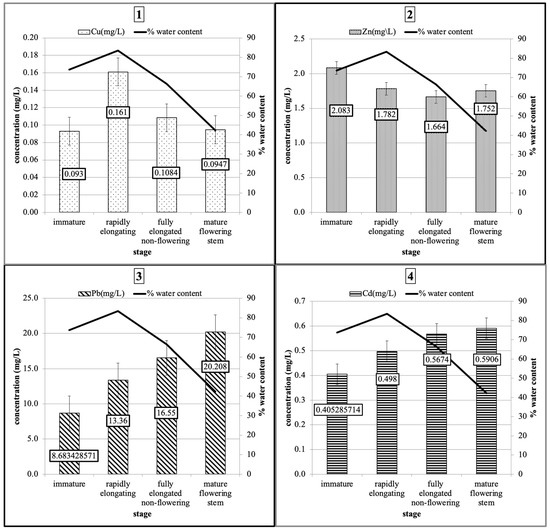
Figure 3.
Mean concentrations of (1) Cu, (2) Zn, (3) Pb, and (4) Cd (mg/L) at various developmental stages of P. australis (immature, rapidly elongating, fully elongated non-flowering, and mature flowering stem) with the mean % water content.
3.3. Statistical Analysis
The p-values compared various P. australis plants for their statistical significance level regarding the mean values of parameters such as water content, chlorophyll a (Chl. a), and chlorophyll b (Chl. b). The p-values of the statistical differences between plants in carotenoid levels (0.065) were more than 0.05, which implied that the values were not significantly different.
There was a significantly high negative correlation between shoot length and water content percentage (−0.705). The water content percentage in the shoots had a negative correlation with total chlorophyll (−0.448) and chlorophyll b (−0.67). Cu had a positive correlation with the water content percentage (0.487) and a weak negative correlation with total chlorophyll (−0.440), chlorophyll a (−0.395), and chlorophyll b (−0.497) (Appendix A Figure A1 and Figure A2); toxic metals were found to have a significantly high positive correlation with shoot length for Pb (0.872) and Cd (0.547) (Appendix A Figure A3).
4. Discussion
The present study analyzed various photosynthetic pigments in common reed plants after they were grown under toxic metal concentrations in their growth medium. It was observed that P. australis leaves displayed a significant difference, with the highest chlorophyll a during the immature stage, the highest chlorophyll b in the mature flowering stage, and the highest carotenoids in the immature stage. Moreover, such observations were in accordance with the previous studies in the literature, as the content of chlorophyll a was between 525 and 1025 µg/g, chlorophyll b ranged from 500 to 2250 µg/g, and total chlorophyll was between 161 and 208 µg/g [19]. However, the variance between the pigment concentrations at various growth stages differed from earlier studies in which the rapidly elongated stage had the maximum total chlorophyll content [5]. A negative association was discovered between the percent water content and chlorophyll a and b, where the lowest concentration of total chlorophyll was noted for the rapidly elongating stage, showing an unexpected relationship unlike that in the literature. A study reported an increase in chlorophyll a, chlorophyll b, and total chlorophyll contents of reeds with increasing strength of wastewater [5]. The difference between the chlorophyll content of various stages of common reeds could be due to many factors, such as the uptake of toxic metals from the water source and the atmosphere [5]. In the present study, the toxic metal and chlorophyll content showed a negative correlation in P. australis. The immature stage showed the highest chlorophyll a, carotenoids, and total chlorophyll, along with the lowest Cu, Pb, and Cd. However, the mature flowering stem showed a similar concentration of chlorophyll a, carotenoids, and total chlorophyll; moreover, it had the highest chlorophyll b, along with the highest Pb and Cd.
In comparison with the highest levels of Cu in the rapidly elongating stage (0.161), Zn in the immature stage (2.083), Pb in the mature flowering stem (20.208), Cd in the mature flowering stem (0.591), and the concentrations of toxic metals in a study by Vaičekonytė [6] were lower for Cu (1.362) and Zn (14.8) and higher for Pb (0.825) and Cd (0.029). According to a study by Staszewski [20], all concentrations of toxic metals during the four stages were lower than the toxic levels in the plant, which were as follows: for Cu, >30 mg/kg, for Zn, >100 mg/kg, for Pb, >30 mg/kg, and for Cd, >5 mg/kg.
Nonetheless, Cu had a negative correlation with chlorophyll a, b, total chlorophyll concentration, and percent water content. Reed plants behaved as Cd accumulators, as the plants accumulated various non-toxic levels of Cd in all stages [9]. In a comparison with the percent water content, there was a significant negative correlation between chlorophyll and Chl. B, which resulted from the water contents [5,11]. It has been shown that heavy metal accumulation is responsible for a decreased water content in plants and displays a negative effect on the total chlorophyll concentration [12]. However, this was not observed in our study. Considering the strong correlation between shoot length and the heavy metals Cd and Pb, a hypothesis was developed stating that the lower water content of the P. australis plant at a lower metal contamination level created stress, due to which they produced more chlorophyll [21,22,23,24].
Although Cu and Zn are essential minerals and nutrients for the proper growth and development of plants, Cu is essential for chlorophyll development, and Zn plays an important role in energy production during the light reactions of photosynthesis. However, higher concentrations of toxic metals, such as Cu, may lead to the development of toxicity symptoms causing stunted or reduced growth and chlorophyll contents. The heavy metal Pb can cause many problems when absorbed by the plants, as not all plants can absorb Pb; however, the result of this study proved that P. australis can grow in the presence of toxic metals and accumulate it in the shoots. Thus, the common reed acted as an accumulator of various metals in the present investigation [21,22,23,24,25,26].
The use of such a marginal wetlands plant may be very useful in reducing the pollution burden of the urban built environments [27,28,29,30]. These plants offer a green and sustainable solution for the disposal of waste from urban areas. Hence, further planning and execution of such a green solution are pivotal for creating environmental sustainability.
5. Conclusions
- The present investigation of the effect of selected toxic metal concentrations on the chlorophyll contents of the common reed generated some useful information. The common reed was found to accumulate significant amounts of toxic metals in its biomass. The photosynthetic machinery and plant growth were not affected by metal accumulation. Thus, the common reed (Phragmites australis) can be considered a potential source of phytonutrients, including chlorophyll a and b and carotenoids. Higher concentrations of total chloroplast pigments are mostly available in the immature and mature flowering stages.
- The immature stage of the stems of the plant can be favored as a source of phytonutrients. However, chloroplast pigment extraction from the common reed for human and animal consumption should be treated with caution to avoid any harmful side effects resulting from contamination with other hazardous compounds that accumulate in the plant tissues, as it can be a water filtering tool for toxic metals.
- The use of such marginal wetlands plants may be very useful in reducing the pollution burden of urban built environments. These plants offer a green, sustainable solution for the disposal of waste from urban areas.
Author Contributions
Conceptualization, S.P. and Q.M.; methodology, S.P.; software, S.P.; validation, Q.M., S.P. and Z.A.A.-Q.; formal analysis, Q.M.; investigation, Z.A.A.-Q.; resources, S.P.; data curation, Z.A.A.-Q.; writing—original draft preparation, Z.A.A.-Q.; writing—review and editing, Q.M.; visualization, Q.M.; supervision, S.P.; project administration, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
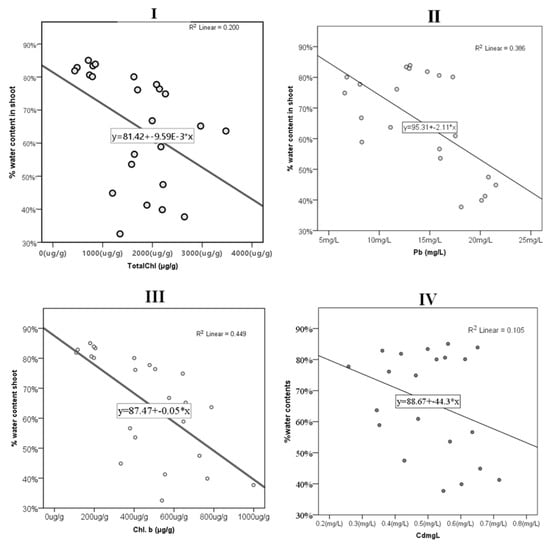
Figure A1.
The correlation between percent water content in P. australis and (I) total chlorophyll content (Total Chl. (μg/g)), (II) Pb (mg/L), (III) chlorophyll b (Total Chl. (μg/g)), and (IV) Cd (mg/L) and their best-fit line, equation line, and value.
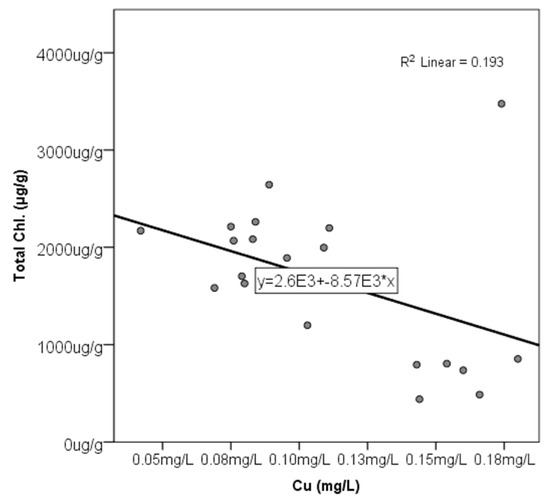
Figure A2.
The correlation between total chlorophyll content (Total Chl. (μg/g)) in P. australis and Cu concentration (mg/L), with the line equation for the best-fit line and value.
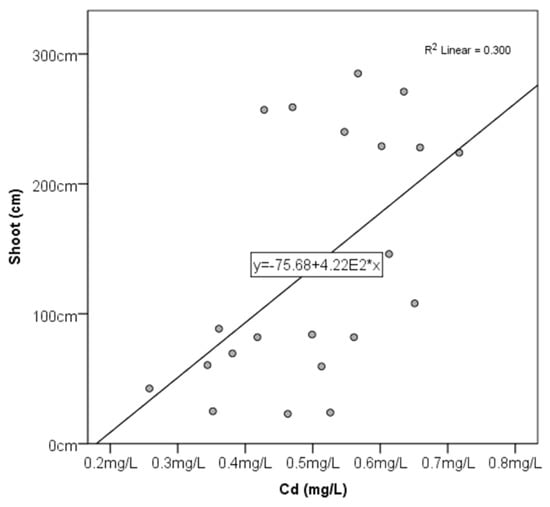
Figure A3.
The correlation between the shoot (cm) of P. australis and the Cd concentration (mg/L) and the best-fit line, line equation, and value.
References
- Antonielli, M.; Pasqualini, S.; Batini, P.; Ederli, L.; Massacci, A.; Loreto, F. Physiological and anatomical characterisation of Phragmites australis leaves. Aquat. Bot. 2002, 72, 55–66. [Google Scholar] [CrossRef]
- Menéndez, M. Decomposition of the common reed Phragmites australis in a Mediterranean stream pond. Arch. Hydrobiol. 2005, 163, 101–115. [Google Scholar] [CrossRef]
- Parker, C. Phragmites australis (common reed). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Srivastava, J.; Kalra, J.; Naraian, R. Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef]
- Wichmann, S.; Köbbing, J.F. Common reed for thatching—A first review of the European market. Ind. Crops Prod. 2015, 77, 1063–1073. [Google Scholar] [CrossRef]
- Sarafraz, S.; Mohammad, T.A.; Norr, M.J.; Laight, A. Wastewater treatment using horizontal subsurface flow constructed wetland. Am. J. Environ. Sci. 2009, 5, 99–105. Available online: http://www.scipub.org/fulltext/ajes/ajes5199-105.pdf (accessed on 22 November 2022).
- Milke, J.; Gałczyńska, M.; Wróbel, J. The Importance of Biological and Ecological Properties of Phragmites australis (Cav.) Trin. Ex Steud., in Phytoremendiation of Aquatic Ecosystems—The Review. Water 2020, 12, 1770. [Google Scholar] [CrossRef]
- Kronbergs, E.; Kaktis, A.; Smits, M.; Nulle, I. Biomass condition for solid biofuel compositions. In Use of Bioenergy in the Baltic Sea Region, Proceedings of the 2nd International Baltic Bioenergy Conference, 2–4 November 2006; Barz, M., Ahlhaus, M., Eds.; Fachhochschule: Stralsund, Germany, 2006; pp. 83–92. [Google Scholar]
- Hedelin, B. The Effect of Reed Harvesting on the Phosphorus Budget of Lake Wuliangsuhai, P.R. of China. Master’s Thesis, Royal Institute of Technology (KTH), Stockholm, Sweden, 2001. [Google Scholar]
- Huhta, A. Decorative or Outrageous—The significance of the Common Reed (Phragmites australis) on water quality. Comments Turku Univ. Appl. Sci. 2009, 48, 1–33. [Google Scholar]
- Kumar, S.; Kumar, R.; Pal, A.; Chopra, D.S. Enzymes. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 335–358. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, J.; Mihai, D.M.; Washington, I. Light-harvesting chlorophyll pigments enable mammalian mitochondria to capture photonic energy and produce ATP. J. Cell Sci. 2014, 127, 388–399. [Google Scholar] [CrossRef]
- And, H.; Jones, T. Haems and chlorophylls: Comparison of function and formation. J. Med. Genet. 1980, 17, 1–14. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1048480/pdf/jmedgene00123-0008.pdf (accessed on 5 March 2023).
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Nakata, P.A. CALCIUM OXALATE IN PLANTS: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method of Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 32. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Staszewski, T.; Łukasik, W.; Kubiesa, P. Contamination of Polish national parks with heavy metals. Environ. Monit. Assess. 2011, 184, 4597–4608. Available online: https://www.researchgate.net/publication/51571985_Contamination_of_Polish_national_parks_with_heavy_metals (accessed on 25 May 2021). [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Egner, P.A.; Kensler, T.W.; Talalay, P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis 2005, 26, 1247–1255. [Google Scholar] [CrossRef]
- Jubert, C.; Mata, J.; Bench, G.; Dashwood, R.; Pereira, C.; Tracewell, W.; Turteltaub, K.; Williams, D.; Bailey, G. Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B(1) pharmacokinetics in human volunteers. Cancer Prev. Res. 2009, 2, 1015–1022. [Google Scholar] [CrossRef]
- Bragato, C.; Brix, H.; Malagoli, M. Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environ. Pollut. 2006, 144, 967–975. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Kaszás, L.; Kovács, Z.; Nagy, E.; Elhawat, N.; Abdalla, N.; Domokos-Szabolcsy, E. Jerusalem artichoke (Helianthus tuberosus L.) as a potential chlorophyll source for humans and animals nutrition. Environ. Biodivers. Soil Secur. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Lippert, I.; Rolletschek, H.; Kohl, J.G. Photosynthetic pigments and efficiencies of two Phragmites australis stands in different nitrogen availabilities. Aquat. Bot. 2001, 69, 359–365. [Google Scholar] [CrossRef]
- Su, F.; Dong, L.; Li, H.; Wang, T. Influence of Paper Mill Wastewater on Reed Chlorophyll Content and Biomass. Phys. Chem. Earth 2018, 108, 13–18. [Google Scholar] [CrossRef]
- Vaičekonytė, R.; Kiviat, E.; Nsenga, F.; Ostfeld, A. An exploration of common reed (Phragmites australis) bioenergy potential in North America. Mires Peat 2014, 13, 1–9. [Google Scholar]
- Gjorgieva Ackova, D. Heavy metals and their general toxicity for plants. Plant Sci. Today 2018, 5, 14–18. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).