Chitosan Nanoparticles Enhance Yield and Bioactive Compounds in Melon Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions and Plant Material
2.2. Chitosan Nanoparticles
2.3. Treatments and Experimental Design
2.4. Yield and Commercial Fruit Quality
2.5. Soluble Solids and Firmness
2.6. Fruit Weight Loss
2.7. Preparation of Extracts for Non-Enzymatic Antioxidants
2.8. Total Flavonoid Content
2.9. Total Phenolic Content
2.10. Antioxidant Capacity
2.11. Vitamin C
2.12. Statistical Data Analyses
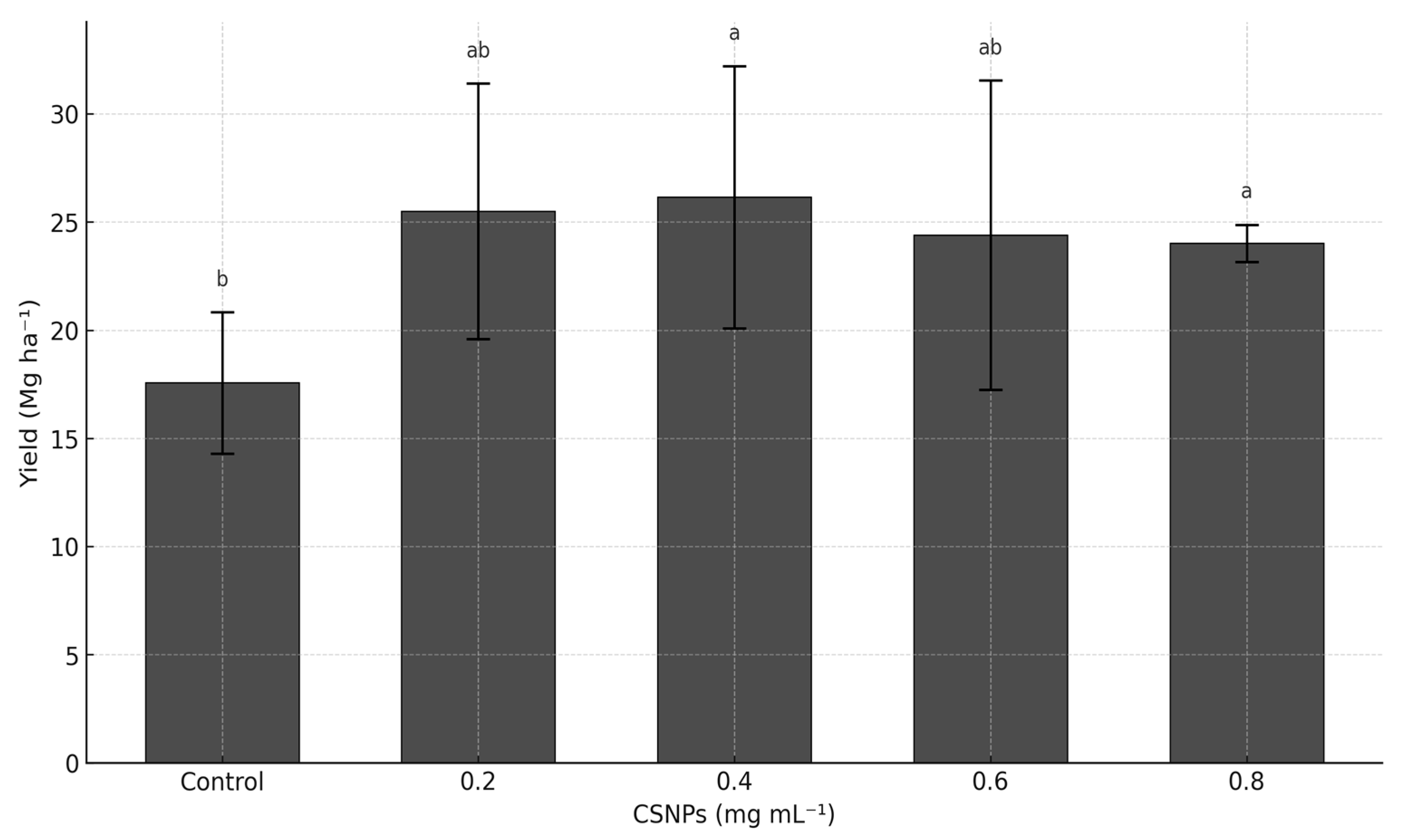
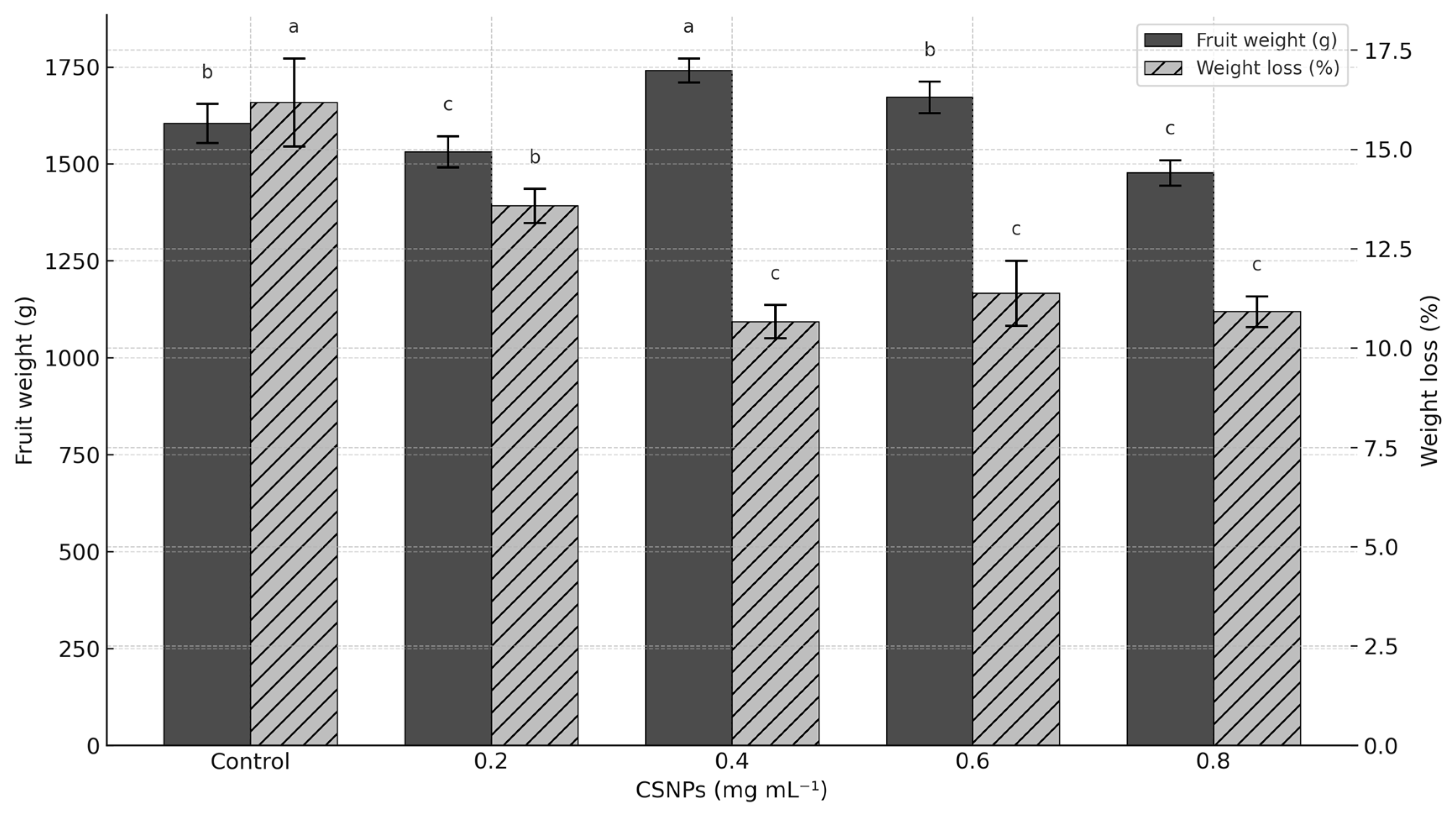
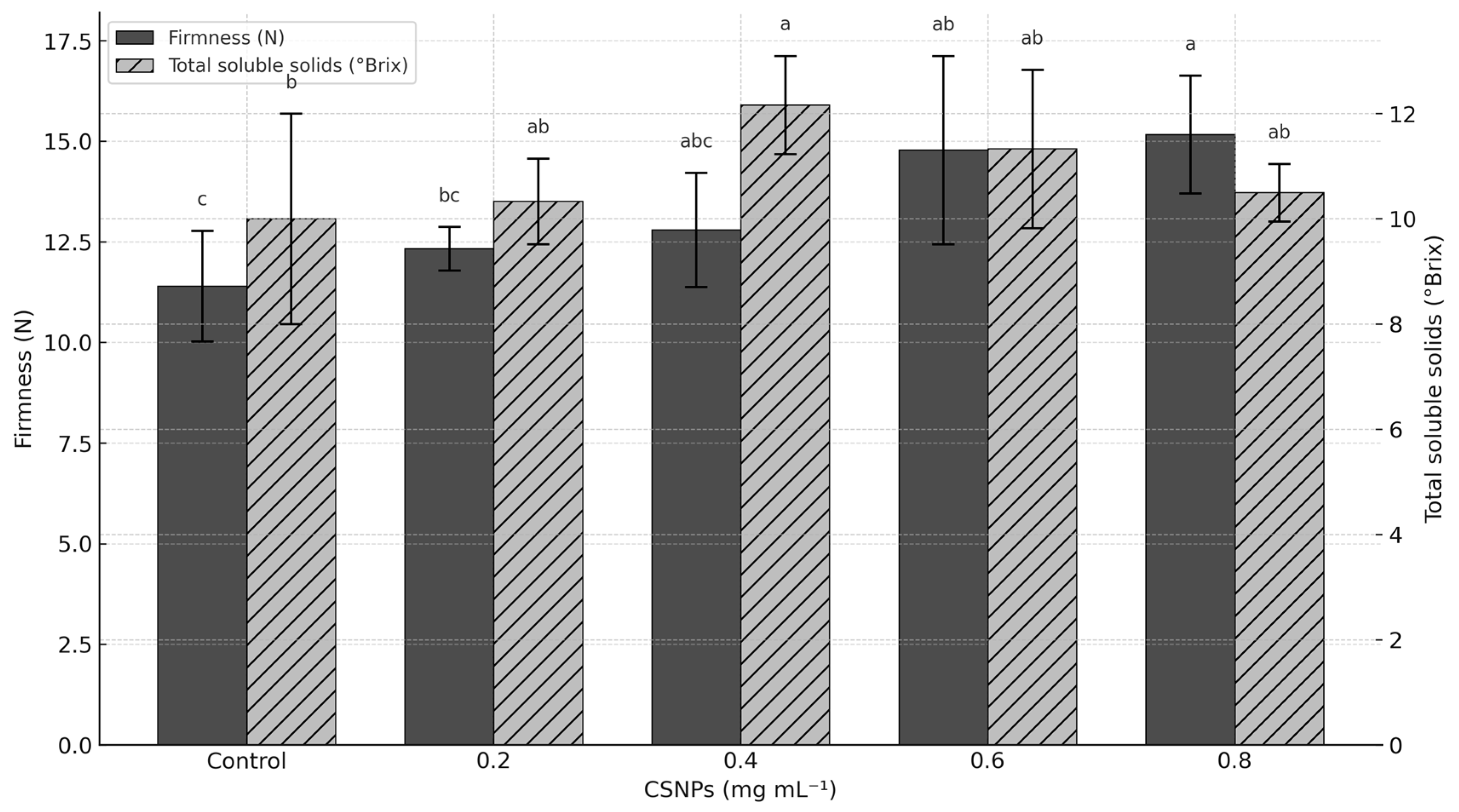
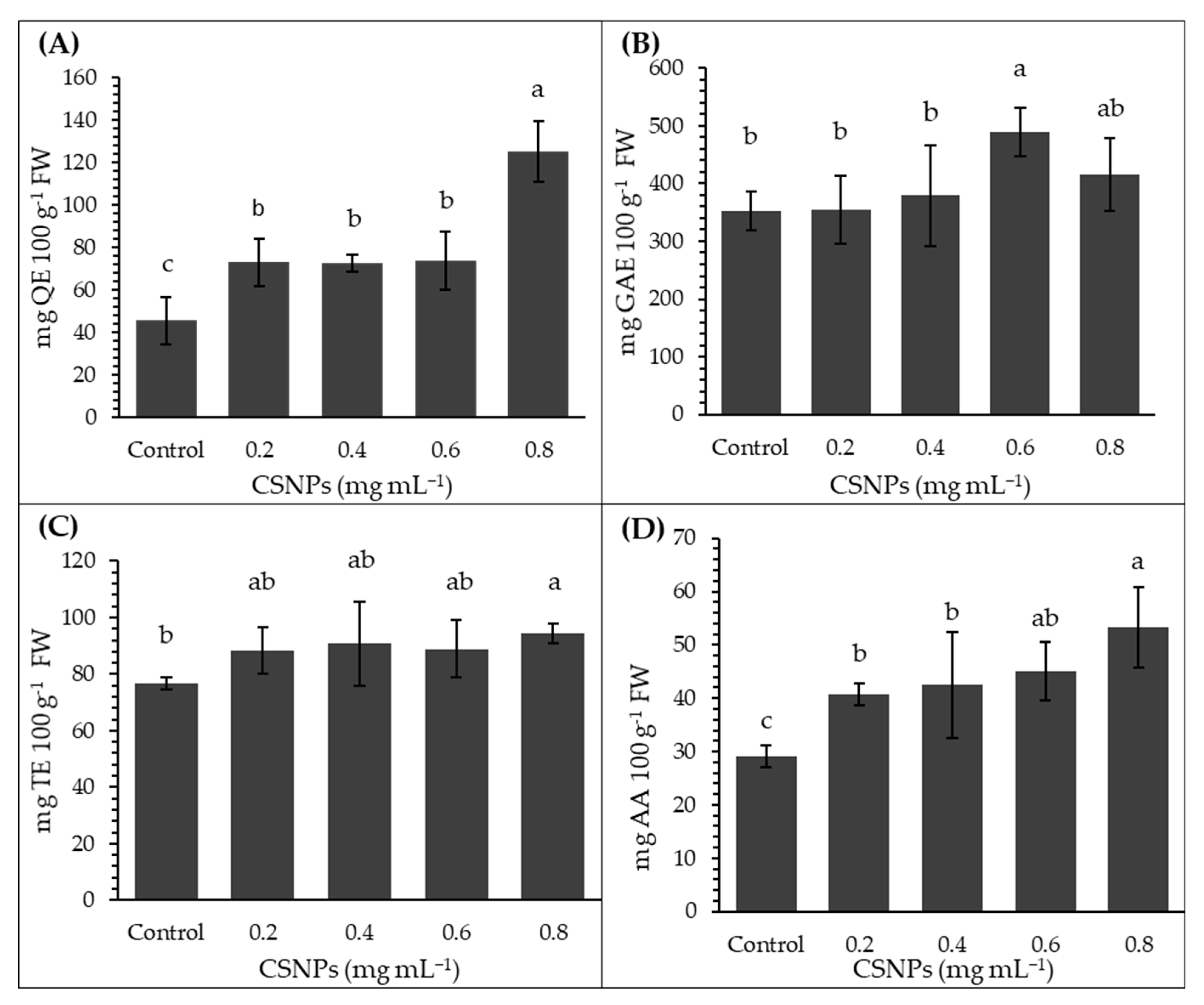
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burian, A.; Kremen, C.; Wu, J.S.-T.; Beckmann, M.; Bulling, M.; Garibaldi, L.A.; Krisztin, T.; Mehrabi, Z.; Ramankutty, N.; Seppelt, R. Biodiversity–Production Feedback Effects Lead to Intensification Traps in Agricultural Landscapes. Nat. Ecol. Evol. 2024, 8, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Swaine, M.; Bergna, A.; Oyserman, B.; Vasileiadis, S.; Karas, P.A.; Screpanti, C.; Karpouzas, D.G. Impact of Pesticides on Soil Health: Identification of Key Soil Microbial Indicators for Ecotoxicological Assessment Strategies through Meta-Analysis. FEMS Microbiol. Ecol. 2025, 101, fiaf052. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Sanghvi, G.; Yadav, M.; Padhiyar, H.; Christian, J.; Singh, V. Fate of Pesticides in Agricultural Runoff Treatment Systems: Occurrence, Impacts and Technological Progress. Environ. Res. 2023, 237, 117100. [Google Scholar] [CrossRef]
- Li, Y.; Zeleke, K.; Wang, B.; Liu, D.-L. A Review of Data for Compound Drought and Heatwave Stress Impacts on Crops: Current Progress, Knowledge Gaps, and Future Pathways. Plants 2025, 14, 2158. [Google Scholar] [CrossRef] [PubMed]
- Beyuo, J.; Sackey, L.N.A.; Yeboah, C.; Kayoung, P.Y.; Koudadje, D. The Implications of Pesticide Residue in Food Crops on Human Health: A Critical Review. Discov. Agric. 2024, 2, 123. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- De Groote, H.; Tessema, M.; Gameda, S.; Gunaratna, N.S. Soil Zinc, Serum Zinc, and the Potential for Agronomic Biofortification to Reduce Human Zinc Deficiency in Ethiopia. Sci. Rep. 2021, 11, 8770. [Google Scholar] [CrossRef]
- Ping, Y.; Cao, D.; Hu, J.; Lin, Y.; Dang, C.; Xue, D. The Application, Safety, and Challenge of Nanomaterials on Plant Growth and Stress Tolerance. Ind. Crops Prod. 2024, 222, 119691. [Google Scholar] [CrossRef]
- Ochoa, L.; Shrivastava, M.; Srivastava, S.; Cota-Ruiz, K.; Zhao, L.; White, J.C.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Nanomaterials for Managing Abiotic and Biotic Stress in the Soil–Plant System for Sustainable Agriculture. Environ. Sci. Nano 2025, 12, 1037–1058. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alabbosh, K.F.; Manan, S.; Khan, S.; Ahmad, F.; Ullah, M.W. Chitosan-Based Nanostructured Biomaterials: Synthesis, Properties, and Biomedical Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 79–99. [Google Scholar] [CrossRef]
- Benettayeb, A.; Seihoub, F.Z.; Pal, P.; Ghosh, S.; Usman, M.; Chia, C.H.; Usman, M.; Sillanpää, M. Chitosan Nanoparticles as Potential Nano-Sorbent for Removal of Toxic Environmental Pollutants. Nanomaterials 2023, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Y.; Yu, Z.; Du, L. Synthesis, Characterization of Chitosan/Tripolyphosphate Nanoparticles Loaded with 4-Chloro-2-Methylphenoxyacetate Sodium Salt and Its Herbicidal Activity against Bidens pilosa L. Sci. Rep. 2024, 14, 18754. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Ghazy, H.A.; Wissa, M.T.; Farroh, K.Y.; Wasonga, D.O.; Seleiman, M.F. Chitosan-Based NPK Nanostructure for Reducing Synthetic NPK Fertilizers and Improving Rice Productivity and Nutritional Indices. Front. Sustain. Food Syst. 2024, 8, 1464021. [Google Scholar] [CrossRef]
- Weng, J.; Durand, A.; Desobry, S. Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release. Pharmaceutics 2023, 15, 1455. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.-Á.; Aguilera-Aguirre, S.; Vega-Arreguín, J.; Acevedo-Hernández, G.; Tovar-Pérez, E.; Stoll, A.; Herrera-Estrella, L.; Chacón-López, A. Activation of the Phenylpropanoid Biosynthesis Pathway Reveals a Novel Action Mechanism of the Elicitor Effect of Chitosan on Avocado Fruit Epicarp. Food Res. Int. 2019, 121, 586–592. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan Nanoparticles Effectively Combat Salinity Stress by Enhancing Antioxidant Activity and Alkaloid Biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Alenazi, M.M.; El-Ebidy, A.M.; El-shehaby, O.A.; Seleiman, M.F.; Aldhuwaib, K.J.; Abdel-Aziz, H.M.M. Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants. Plants 2024, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Ishkeh, S.R.; Shirzad, H.; Asghari, M.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Effect of Chitosan Nanoemulsion on Enhancing the Phytochemical Contents, Health-Promoting Components, and Shelf Life of Raspberry (Rubus sanctus Schreber). Appl. Sci. 2021, 11, 2224. [Google Scholar] [CrossRef]
- Chun, S.-C.; Chandrasekaran, M. Chitosan and Chitosan Nanoparticles Induced Expression of Pathogenesis-Related Proteins Genes Enhances Biotic Stress Tolerance in Tomato. Int. J. Biol. Macromol. 2019, 125, 948–954. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Monir, G.A.; Hassan, M.S.S.; Ahmed, Y.; Refaat, M.H.; Ismail, I.A.; El-Garhy, H.A.S. Exogenously Applied Chitosan and Chitosan Nanoparticles Improved Apple Fruit Resistance to Blue Mold, Upregulated Defense-Related Genes Expression, and Maintained Fruit Quality. Horticulturae 2021, 7, 224. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Wang, X.; Liu, S.; Luo, L.; Zeng, Q.; Wu, Y.; Zeng, Y.; Yang, Z.; Sheng, G.; et al. Emerging Nanochitosan for Sustainable Agriculture. Int. J. Mol. Sci. 2024, 25, 12261. [Google Scholar] [CrossRef]
- Mancilla-Álvarez, E.; Serrano-Fuentes, M.K.; Fuentes-Torres, M.A.; Sánchez-Páez, R.; Bello-Bello, J.J. Chitosan Nanoparticles: An Alternative for In Vitro Multiplication of Sugarcane (Saccharum Spp.) in Semi-Automated Bioreactors. Plants 2025, 14, 1697. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Karimian, Z.; Mostafaei Dehnavi, M.; Samiei, L. Chitosan Nanoparticles Improve Physiological and Biochemical Responses of Salvia abrotanoides (Kar.) under Drought Stress. BMC Plant Biol. 2022, 22, 364. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, J.; Wang, H.; Mao, J.; Wu, L.; Li, J.; Wang, X. Variations of Physical Properties, Bioactive Phytochemicals, Antioxidant Capacities and PPO Activities of Cantaloupe Melon (Cucumis melo L.) Slices Subjected to Different Drying Methods. Front. Nutr. 2025, 12, 1548271. [Google Scholar] [CrossRef]
- Okcu, Z.; Yangılar, F. Quality Parameters and Antioxidant Activity, Phenolic Compounds, Sensory Properties of Functional Yogurt with Melon (Cucumis melo L.) Peel Powder. Turk. J. Agric. Food Sci. Technol. 2024, 12, 586–595. [Google Scholar] [CrossRef]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait Analysis of Fresh-Cut Cantaloupe Melon Enables Discrimination between Storage Times and Temperatures and Identifies Potential Markers for Quality Assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered Chitosan Based Nanomaterials: Bioactivities, Mechanisms and Perspectives in Plant Protection and Growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, S.; Preciado-Rangel, P.; Cabrera-De, M.; González-Morales, S.; Ortega-Ortiz, H. Chitosan Nanoparticles as Biostimulant in Lettuce (Lactuca sativa L.) Plants. Phyton 2024, 93, 777–787. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, X.; Deng, Y.; Xu, K.; Duan, X.; Wan, K.; Tang, X. Study on Characteristics and Lignification Mechanism of Postharvest Banana Fruit during Chilling Injury. Foods 2023, 12, 1097. [Google Scholar] [CrossRef]
- Castro-Cegrí, A.; Carvajal, F.; Osorio, S.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest Abscisic Acid Treatment Modulates the Primary Metabolism and the Biosynthesis of T-Zeatin and Riboflavin in Zucchini Fruit Exposed to Chilling Stress. Postharvest Biol. Technol. 2023, 204, 112457. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the Bioactive Compounds, Antioxidant Activity and Chemical Composition of Brazilian Blackberry, Red Raspberry, Strawberry, Blueberry and Sweet Cherry Fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Guillén-Enríquez, R.R.; Sánchez-Chávez, E.; Fortis-Hernández, M.; Márquez-Guerrero, S.Y.; Espinosa-Palomeque, B.; Preciado-Rangel, P. ZnO Nanoparticles Improve Bioactive Compounds, Enzymatic Activity and Zinc Concentration in Grapevine. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13377. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nielsen, S.S. Vitamin C Determination by Indophenol Method. In Nielsen’s Food Analysis Laboratory Manual; Ismail, B.P., Nielsen, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 153–156. ISBN 978-3-031-44970-3. [Google Scholar]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Varma, R.S. A Review of Chitosan Nanoparticles: Nature’s Gift for Transforming Agriculture through Smart and Effective Delivery Mechanisms. Int. J. Biol. Macromol. 2024, 260, 129522. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, Q.; Xiao, J.; Qin, X.; Zhang, Y.; Li, X.; Zheng, W.; Zhang, H. Nanoparticle LDH Enhances RNAi Efficiency of dsRNA in Piercing-Sucking Pests by Promoting dsRNA Stability and Transport in Plants. J. Nanobiotechnol. 2024, 22, 544. [Google Scholar] [CrossRef]
- Poznanski, P.; Shalmani, A.; Bryla, M.; Orczyk, W. Salicylic Acid Mediates Chitosan-Induced Immune Responses and Growth Enhancement in Barley. Int. J. Mol. Sci. 2024, 25, 13244. [Google Scholar] [CrossRef]
- Mukarram, M.; Ali, J.; Dadkhah-Aghdash, H.; Kurjak, D.; Kačík, F.; Ďurkovič, J. Chitosan-Induced Biotic Stress Tolerance and Crosstalk with Phytohormones, Antioxidants, and Other Signalling Molecules. Front. Plant Sci. 2023, 14, 1217822. [Google Scholar] [CrossRef]
- Usman, S.; Shah, A.A.; Kaleem, M.; Noreen, Z.; Xu, W.; Mahmoud, E.A.; Elansary, H.O. Chitosan-Copper Nanocomposites Exterminate Cd Toxicity in Capsicum annuum L. through Improving Photosynthetic Attributes, Antioxidant Defense, and Reduced Cd Uptake. ACS Omega 2025, 10, 32879–32894. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, N.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Ritenour, M.A.; Lin, Y. Effects of Chitosan Treatment on the Storability and Quality Properties of Longan Fruit during Storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Y.; Liu, Y.; Li, X.; Wu, S. Application of Chitosan in Fruit Preservation: A Review. Food Chem. X 2024, 23, 101589. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, G.; Lin, H.; Lin, M.; Wang, H.; Lin, Y. Chitosan Postharvest Treatment Suppresses the Pulp Breakdown Development of Longan Fruit through Regulating ROS Metabolism. Int. J. Biol. Macromol. 2020, 165, 601–608. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Do, T.V.T.; Reungsang, A.; Chaiwong, N.; Rachtanapun, P. Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers 2021, 13, 3430. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Xue, S.; Gong, D.; Wang, B.; Zheng, X.; Xie, P.; Bi, Y.; Prusky, D. Preharvest Multiple Sprays with Chitosan Promotes the Synthesis and Deposition of Lignin at Wounds of Harvested Muskmelons. Int. J. Biol. Macromol. 2022, 206, 167–174. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, F.; Cai, W.; Peng, B.; Ning, M.; Shan, C.; Yang, X. Chitosan Treatment Reduces Softening and Chilling Injury in Cold-Stored Hami Melon by Regulating Starch and Sucrose Metabolism. Front. Plant Sci. 2022, 13, 1096017. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Gu, J.; Chen, C.; Liu, J.; Zhang, Z.; Hua, B.; Miao, M. The Sink-Source Relationship in Cucumber (Cucumis sativus L.) Is Modulated by DNA Methylation. Plants 2023, 13, 103. [Google Scholar] [CrossRef]
- Valantin, M.; Gary, C.; Vaissière, B.E.; Frossard, J.S. Effect of Fruit Load on Partitioning of Dry Matter and Energy in Cantaloupe (Cucumis melo L.). Ann. Bot. 1999, 84, 173–181. [Google Scholar] [CrossRef]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-Red Radiation Stimulates Dry Mass Partitioning to Fruits by Increasing Fruit Sink Strength in Tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef]
- Brown, A.; Al-Azawi, T.N.I.; Methela, N.J.; Rolly, N.K.; Khan, M.; Faluku, M.; Huy, V.N.; Lee, D.-S.; Mun, B.-G.; Hussian, A.; et al. Chitosan-Fulvic Acid Nanoparticles Enhance Drought Tolerance in Maize via Antioxidant Defense and Transcriptional Reprogramming. Physiol. Plant. 2024, 176, e14455. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Prajapati, D.; Pal, A.; Dimkpa, C.; Harish; Singh, U.; Devi, K.A.; Choudhary, J.L.; Saharan, V. Chitosan Nanomaterials: A Prelim of next-Generation Fertilizers; Existing and Future Prospects. Carbohydr. Polym. 2022, 288, 119356. [Google Scholar] [CrossRef]
- Bécquer Granados, C.J.; González Cañizares, P.J.; Ávila Cordoví, U.; Nápoles Gómez, J.Á.; Galdo Rodríguez, Y.; Muir Rodríguez, I.; Hernández Obregón, M.; Quintana Sanz, M.; Medinilla Nápoles, F. Efecto de La Inoculación de Microorganismos Benéficos y Quitomax® En Cenchrus ciliaris L., En Condiciones de Sequía Agrícola. Pastos y Forrajes 2019, 42, 39–47. [Google Scholar]
- Dawa, K.K.; Metwaly, E.E.; Swelam, W.M.E.; Morgan, A.F.F.M. Response of Some Cucumber Cultivars Grown under High Plastic Tunnels to Grafting and Some Foliar Application Treatments. J. Plant Prod. 2022, 13, 861–867. [Google Scholar] [CrossRef]
- Cice, D.; Ferrara, E.; Pecoraro, M.T.; Capriolo, G.; Petriccione, M. An Innovative Layer-by-Layer Edible Coating to Regulate Oxidative Stress and Ascorbate–Glutathione Cycle in Fresh-Cut Melon. Horticulturae 2024, 10, 465. [Google Scholar] [CrossRef]
- Robles-Ozuna, L.E.; Goycoolea, F.M.; Silveira, M.I. Uso Del Quitosano Durante El Escaldado Del Nopal (Opuntia ficus indica) y Efecto Sobre Su Calidad. Rev. Mex. De Ing. Química 2007, 6, 193–201. [Google Scholar]
- Dai, L.; Wang, X.; Zhang, J.; Li, C. Application of Chitosan and Its Derivatives in Postharvest Coating Preservation of Fruits. Foods 2025, 14, 1318. [Google Scholar] [CrossRef]
- Dwivedi, S.; Anand, G.; Yadav, S.; Yadav, D. An Insight into Production Strategies for Microbial Pectinases: An Overview. In Microbial Enzymes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2025; pp. 87–118. ISBN 978-3-527-84434-0. [Google Scholar]
- Sun, H.; Hao, D.; Tian, Y.; Huang, Y.; Wang, Y.; Qin, G.; Pei, J.; Abd El-Aty, A.M. Effect of Chitosan/Thyme Oil Coating and UV-C on the Softening and Ripening of Postharvest Blueberry Fruits. Foods 2022, 11, 2795. [Google Scholar] [CrossRef]
- Kassem, H.S.; Tarabih, M.E.; Ismail, H.; Eleryan, E.E. Influence of Nano-Silica/Chitosan Film Coating on the Quality of ‘Tommy Atkins’ Mango. Processes 2022, 10, 279. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Vieira, J.M.; Rocha, C.M.R.; Lagarón, J.M.; Cerqueira, M.A.; Jasso de Rodríguez, D.; Vicente, A.A. Postharvest Quality Improvement of Tomato (Solanum lycopersicum L.) Fruit Using a Nanomultilayer Coating Containing Aloe vera. Foods 2024, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan Coating with Trans-Cinnamaldehyde Improves Structural Integrity and Antioxidant Metabolism of Fresh-Cut Melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Sree Rayanoothala, P.; Dweh, T.J.; Mahapatra, S.; Kayastha, S. Unveiling the Protective Role of Chitosan in Plant Defense: A Comprehensive Review with Emphasis on Abiotic Stress Management. Crop Des. 2024, 3, 100076. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-Harvest Treatment of Chitosan Oligosaccharides Improved Strawberry Fruit Quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Ackah, S.; Xue, S.; Osei, R.; Kweku-Amagloh, F.; Zong, Y.; Prusky, D.; Bi, Y. Chitosan Treatment Promotes Wound Healing of Apple by Eliciting Phenylpropanoid Pathway and Enzymatic Browning of Wounds. Front. Microbiol. 2022, 13, 828914. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving Abiotic Stress Tolerance in Plants through Antioxidative Defense Mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Characterization, Phenolic Compounds and Functional Properties of Cucumis melo L. Peels. Food Chem. 2017, 221, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Martínez González, L.; Reyes Guerrero, Y.; Falcón Rodríguez, A.; Núñez Vázquez, M. Efecto Del Tratamiento a Las Semillas Con Quitosana En El Crecimiento de Plántulas de Arroz (Oryza sativa L.) Cultivar INCA LP-5 En Medio Salino. Cultiv. Trop. 2015, 36, 143–150. [Google Scholar]
- Hajihashemi, S.; Kazemi, S. The Potential of Foliar Application of Nano-Chitosan-Encapsulated Nano-Silicon Donor in Amelioration the Adverse Effect of Salinity in the Wheat Plant. BMC Plant Biol. 2022, 22, 148. [Google Scholar] [CrossRef]
- Qiao, J.; Li, D.; Guo, L.; Hong, X.; He, S.; Huo, J.; Sui, X.; Zhang, Y. Enhancing Postharvest Quality and Antioxidant Capacity of Blue Honeysuckle Cv. ‘Lanjingling’ with Chitosan and Aloe vera Gel Edible Coatings during Storage. Foods 2024, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Ackah, S.; Bi, Y.; Xue, S.; Yakubu, S.; Han, Y.; Zong, Y.; Atuna, R.A.; Prusky, D. Post-Harvest Chitosan Treatment Suppresses Oxidative Stress by Regulating Reactive Oxygen Species Metabolism in Wounded Apples. Front. Plant Sci. 2022, 13, 959762. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- de Goes, G.B.; Dias, T.J.; Neri, D.K.P.; Filho, P.L.; da Silva Leal, M.P.; Henschel, J.M.; Batista, D.S.; da Silva Ribeiro, J.E.; da Silva, T.I.; de Mello Oliveira, M.D.; et al. Bioactivator, Phosphorus and Potassium Fertilization and Their Effects on Soil, Physiology, Production and Quality of Melon. Acta Physiol. Plant 2023, 45, 56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preciado-Rangel, P.; Marín-Gómez, E.R.; Ortega-Ortiz, H.; Hernández-Montiel, L.G.; Núñez-Ramírez, F.; Reyes-Pérez, J.J.; Torres-Rodriguez, J.A. Chitosan Nanoparticles Enhance Yield and Bioactive Compounds in Melon Fruits. Sci 2025, 7, 166. https://doi.org/10.3390/sci7040166
Preciado-Rangel P, Marín-Gómez ER, Ortega-Ortiz H, Hernández-Montiel LG, Núñez-Ramírez F, Reyes-Pérez JJ, Torres-Rodriguez JA. Chitosan Nanoparticles Enhance Yield and Bioactive Compounds in Melon Fruits. Sci. 2025; 7(4):166. https://doi.org/10.3390/sci7040166
Chicago/Turabian StylePreciado-Rangel, Pablo, Edgar R. Marín-Gómez, Hortensia Ortega-Ortiz, Luis Guillermo Hernández-Montiel, Fidel Núñez-Ramírez, Juan José Reyes-Pérez, and Juan Antonio Torres-Rodriguez. 2025. "Chitosan Nanoparticles Enhance Yield and Bioactive Compounds in Melon Fruits" Sci 7, no. 4: 166. https://doi.org/10.3390/sci7040166
APA StylePreciado-Rangel, P., Marín-Gómez, E. R., Ortega-Ortiz, H., Hernández-Montiel, L. G., Núñez-Ramírez, F., Reyes-Pérez, J. J., & Torres-Rodriguez, J. A. (2025). Chitosan Nanoparticles Enhance Yield and Bioactive Compounds in Melon Fruits. Sci, 7(4), 166. https://doi.org/10.3390/sci7040166










