Solubility Modeling of Sabah Green Robusta Coffee (Coffea canephora) Bean Oil Extracted Using Supercritical Carbon Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Robusta Coffee Beans Preparation
2.2. Supercritical Carbon Dioxide (SC-CO2) Extraction
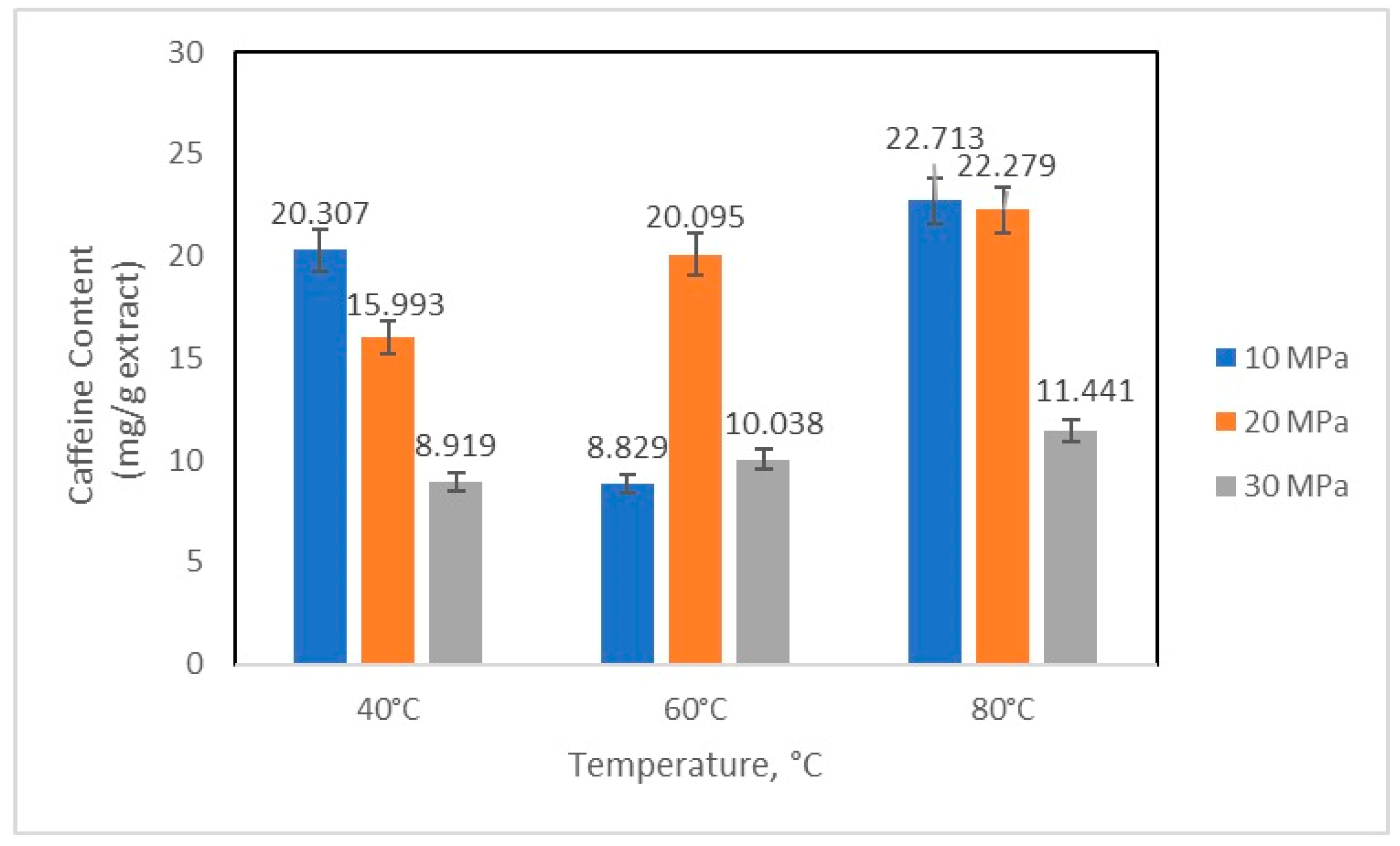
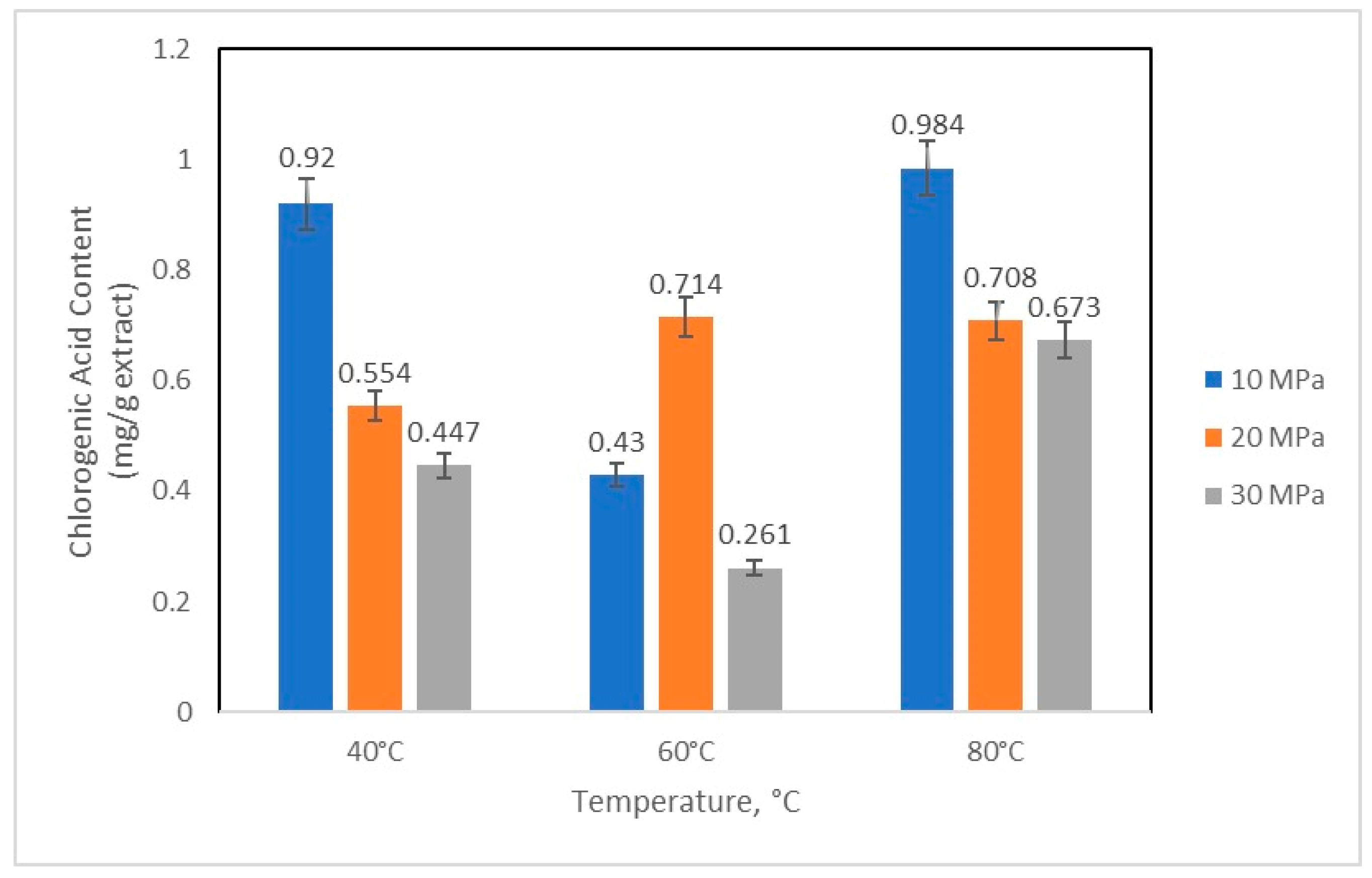
2.3. Quantification of Caffeine and Chlorogenic Acid
2.4. Solubility Determination and Correlation Model
2.5. Data Validation
3. Results and Discussion
3.1. Extraction of Sabah Green Coffee Bean Oil
3.2. Solubility of Sabah Green Coffee Bean Oil
3.3. Correlation Data of Sabah Green Coffee Bean Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SC-CO2 | Supercritical Carbon Dioxide Extraction |
| CO2 | Carbon Dioxide |
| HPLC | High-Performance Liquid Chromatography |
| CER | Constant Extraction Rate |
| AARD% | Average Absolute Relative Deviation |
References
- Ribeiro, R.C.; Mota, M.F.S.; Silva, R.M.; Silva, D.C.; Novaes, F.J.; da Veiga, V.F., Jr.; Bizzo, H.R.; Teixeira, R.S.S.; Rezende, C.M. Coffee Oil Extraction Methods: A Review. Foods 2024, 13, 2601. [Google Scholar] [CrossRef]
- Omar, N.R.N.; Ahmad, A.A.; Nor, N.A.A.L.M.; Abidin, A.Z.Z.; Sulaiman, N.H.; Ahmad, B. Coffee industry in Malaysia: An overview and potential. Econ. Technol. Manag. Rev. 2022, 19, 103–114. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Esquivel, P.; Jimenez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Makiso, M.U.; Tola, Y.B.; Ogah, O.; Endale, F.L. Bioactive compounds in coffee and their role in lowering the risk of major public health consequences: A review. Food Sci. Nutr. 2024, 12, 734–764. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose–response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids—Occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; WenHua, L.; XiaoHui, Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Mohd Idrus, N.F.; Putra, N.R.; Awang, M.A.; Idham, Z.; Mamat, H.; Che Yunus, M.A. Solubility of rosmarinic acid in supercritical carbon dioxide extraction from Orthosiphon stamineus leaves. ChemEngineering 2022, 6, 59. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Nian Yian, L.; Mohd Rasidek, N.A.; Che Yunus, M.A. Parametric and kinetic study of supercritical carbon dioxide extraction on sinensetin from Orthosiphon stamineus Benth. leaves. Sep. Sci. Technol. 2022, 57, 444–453. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Yian, L.N.; Ramli, W.D.; Yunus, M.A.C. Optimization of supercritical carbon dioxide and co-solvent ethanol extraction of wasted peanut skin using response surface methodology. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 02005. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Mamat, H.; Jusoh, W.M.S.W.; Idham, Z.; Che Yunus, M.A.; Irianto, I. Influence of particle size in supercritical carbon dioxide extraction of roselle (Hibiscus sabdariffa) on bioactive compound recovery, extraction rate, diffusivity, and solubility. Sci. Rep. 2023, 13, 10871. [Google Scholar] [CrossRef]
- Arsad, N.H.; Putra, N.R.; Idham, Z.; Norodin, N.S.M.; Yunus, M.A.C.; Aziz, A.H.A. Solubilization of eugenol from Piper betle leaves to supercritical carbon dioxide: Experimental and modelling. Results Eng. 2023, 17, 100914. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Idham, Z.; Qomariyah, L.; Yunus, M.A.C. Extraction rate of valuable compounds from peanut skin waste by ethanol-assisted supercritical carbon dioxide: Modelling and optimization. Malays. J. Fundam. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1988, 27, 1551–1553. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Machmudah, S.; Jumakir, J.; Waluyo, W.; Che Yunus, M.A. Procyanidin and proanthocyanidin extraction from Arachis hypogaea skins by using supercritical carbon dioxide: Optimization and modeling. J. Food Process. Preserv. 2021, 45, e15689. [Google Scholar] [CrossRef]
- Fajara, B.E.P.; Susanti, H. HPLC determination of caffeine in coffee beverage. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 259, p. 012011. [Google Scholar]
- De Azevedo, A.B.A.; Mazzafera, P.; Mohamed, R.S.; Melo, S.A.B.; Kieckbusch, T.G. Extraction of caffeine, chlorogenic acids and lipids from green coffee beans using supercritical carbon dioxide and co-solvents. Braz. J. Chem. Eng. 2008, 25, 543–552. [Google Scholar] [CrossRef]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Moon, J.K.; Yoo, H.S.; Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef]
- Azevedo, Á.B.A.D.; Kieckbusch, T.G.; Tashima, A.K.; Mohamed, R.S.; Mazzafera, P.; Melo, S.A.B.V.D. Supercritical CO2 recovery of caffeine from green coffee oil: New experimental solubility data and modeling. Química Nova 2008, 31, 1319–1323. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer Science & Business Media: Berlin, Germany, 2013; Volume 4. [Google Scholar]
- Özkal, S.G.; Yener, M.E.; Bayındırlı, L. The solubility of apricot kernel oil in supercritical carbon dioxide. Int. J. Food Sci. Technol. 2006, 41, 399–404. [Google Scholar] [CrossRef]
- Maheshwari, P.; Nikolov, Z.L.; White, T.M.; Hartel, R. Solubility of fatty acids in supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1992, 69, 1069–1076. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Özkal, S.G.; Salgın, U.; Yener, M.E. Supercritical carbon dioxide extraction of hazelnut oil. J. Food Eng. 2005, 69, 217–223. [Google Scholar] [CrossRef]
- Uquiche, E.L.; Toro, M.T.; Quevedo, R.A. Supercritical extraction with carbon dioxide and co-solvent from Leptocarpha rivularis. J. Appl. Res. Med. Aromat. Plants 2019, 14, 100210. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Zuknik, M.H.; Norulaini, N.N.; Dalila, W.W.N.; Ali, N.R.; Omar, A.M. Solubility of virgin coconut oil in supercritical carbon dioxide. J. Food Eng. 2016, 168, 240–244. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Solubility of grape seed oil in supercritical CO2: Experiments and modeling. J. Chem. Thermodyn. 2016, 100, 44–52. [Google Scholar] [CrossRef]
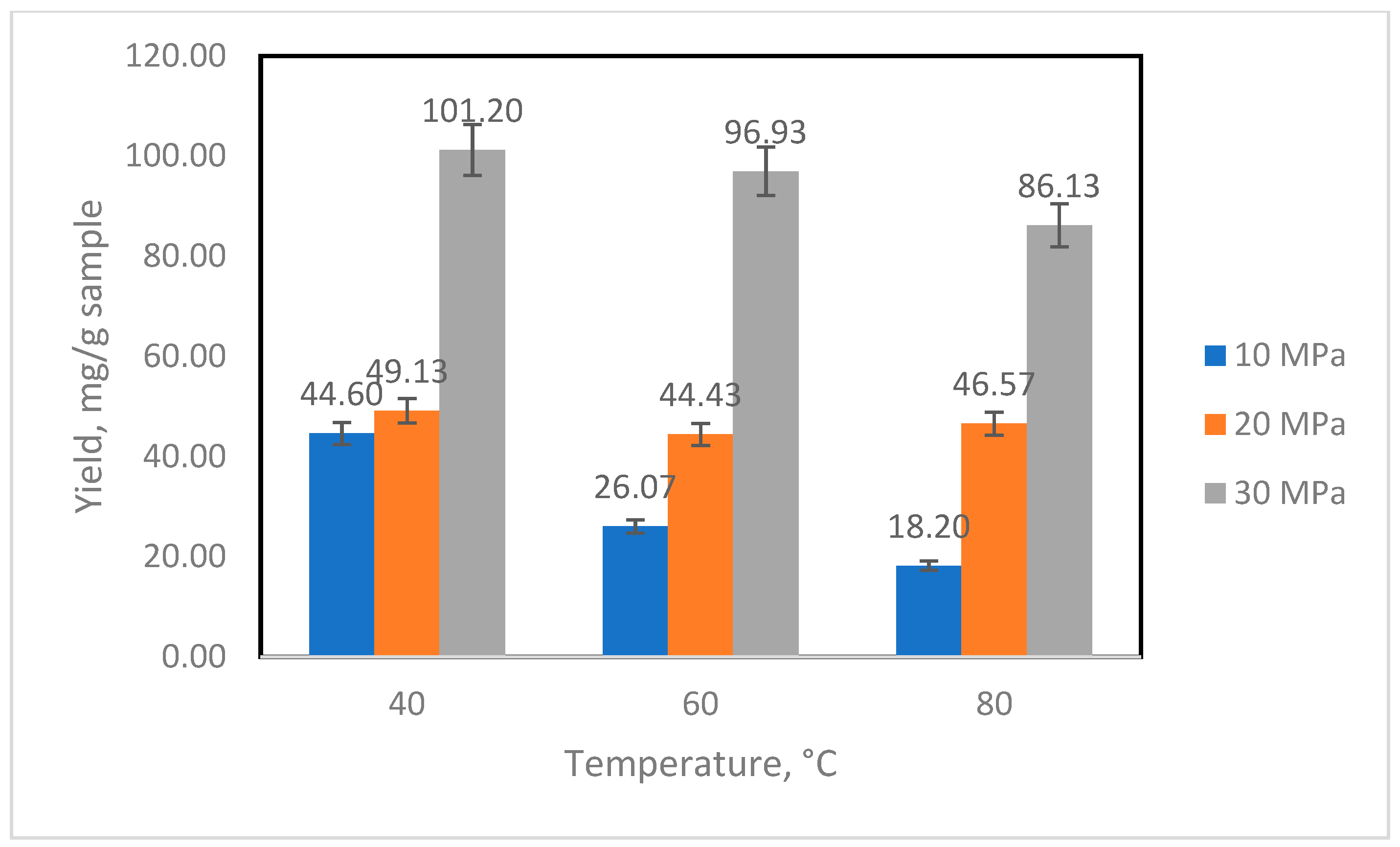

| Temperature (°C) | Pressure (MPa) | Solvent Density (g/L) | Yield (mg/g Sample) | Experimental Solubility (mg Extract/g Solvent) | Calculated Solubility-Chrastil (mg Extract/g Solvent) | Calculated Solubility-dVa (mg Extract/g Solvent) |
|---|---|---|---|---|---|---|
| 80 | 10 | 221.6 | 18.20 | 0.542 | 0.543 | 0.542 |
| 60 | 10 | 290.0 | 26.07 | 0.559 | 0.573 | 0.512 |
| 40 | 10 | 628.7 | 44.60 | 0.948 | 0.948 | 0.661 |
| 80 | 20 | 594.1 | 46.57 | 0.440 | 0.439 | 0.665 |
| 60 | 20 | 723.8 | 44.43 | 0.444 | 0.536 | 0.661 |
| 40 | 20 | 839.9 | 49.13 | 0.656 | 0.656 | 0.656 |
| 80 | 30 | 746.0 | 86.13 | 1.727 | 1.727 | 1.926 |
| 60 | 30 | 830.0 | 96.93 | 2.167 | 2.131 | 2.167 |
| 40 | 30 | 910.0 | 101.20 | 2.681 | 2.682 | 2.488 |
| Models | Model Constants | Model Coefficient |
|---|---|---|
| Chrastil | k | 0.510 |
| a | 228.977 | |
| b | −3.912 | |
| AARD% | 3.37 | |
| Del Valle-Aguilera | k | 0.169 |
| a | −214.975 | |
| b | −3.912 | |
| c | −0.357 | |
| AARD% | 14.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurun Hizar, S.A.; Mamat, H.; Pindi, W.; Julmohammad, N.; Mohd Amin, S.F.; Awang, M.A.; Roslan, J.; Ahmad Zaini, M.A.; Putra, N.R.; Jaziri, A.A.; et al. Solubility Modeling of Sabah Green Robusta Coffee (Coffea canephora) Bean Oil Extracted Using Supercritical Carbon Dioxide. Sci 2025, 7, 139. https://doi.org/10.3390/sci7040139
Khurun Hizar SA, Mamat H, Pindi W, Julmohammad N, Mohd Amin SF, Awang MA, Roslan J, Ahmad Zaini MA, Putra NR, Jaziri AA, et al. Solubility Modeling of Sabah Green Robusta Coffee (Coffea canephora) Bean Oil Extracted Using Supercritical Carbon Dioxide. Sci. 2025; 7(4):139. https://doi.org/10.3390/sci7040139
Chicago/Turabian StyleKhurun Hizar, Sarah Aisyah, Hasmadi Mamat, Wolyna Pindi, Norliza Julmohammad, Siti Faridah Mohd Amin, Mohd Azrie Awang, Jumardi Roslan, Muhammad Abbas Ahmad Zaini, Nicky Rahmana Putra, Abdul Aziz Jaziri, and et al. 2025. "Solubility Modeling of Sabah Green Robusta Coffee (Coffea canephora) Bean Oil Extracted Using Supercritical Carbon Dioxide" Sci 7, no. 4: 139. https://doi.org/10.3390/sci7040139
APA StyleKhurun Hizar, S. A., Mamat, H., Pindi, W., Julmohammad, N., Mohd Amin, S. F., Awang, M. A., Roslan, J., Ahmad Zaini, M. A., Putra, N. R., Jaziri, A. A., Ishak, N., & Abdul Aziz, A. H. (2025). Solubility Modeling of Sabah Green Robusta Coffee (Coffea canephora) Bean Oil Extracted Using Supercritical Carbon Dioxide. Sci, 7(4), 139. https://doi.org/10.3390/sci7040139








