Antibacterial Properties of Submerged Cultivated Fomitopsis pinicola, Targeting Gram-Negative Pathogens, Including Borrelia burgdorferi
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cell Lines
2.2. Mushroom Inoculum Preparation and Biomass Growth Parameters
2.3. Preparation of Mushroom Extracts
2.4. Scavenging Activity on 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radicals
2.5. Qualitative Analysis of Bioactive Constituents in Fomitopsis Pinicola Extracts
2.6. Cell Culture Assays
2.6.1. Cell Treatment Procedure
2.6.2. Cell Viability by WST-1 Assay
2.7. Bacterial Culture Assays
2.8. Minimal Bactericidal Concentration (MBC)
2.9. SYBR Green I/PI Assay
2.10. Evaluation of Antibacterial Effect of Fomitopsis pinicola Extracts Against B. burgdorferi
2.11. Crystal Violet Biofilm Assay
2.12. Statistical Analysis
3. Results
3.1. Yield and Abbreviation of Extracts Obtained from Fomitopsis pinicola Mycelial Biomass
3.2. DPPH Free Radical Scavenging Activity of Fomitopsis pinicola Extracts
3.3. Qualitative Analysis of Bioactive Constituents in Fomitopsis pinicola Extracts
3.4. Antibacterial Efficiency of Extracts from Fomitopsis pinicola Mycelial Biomass
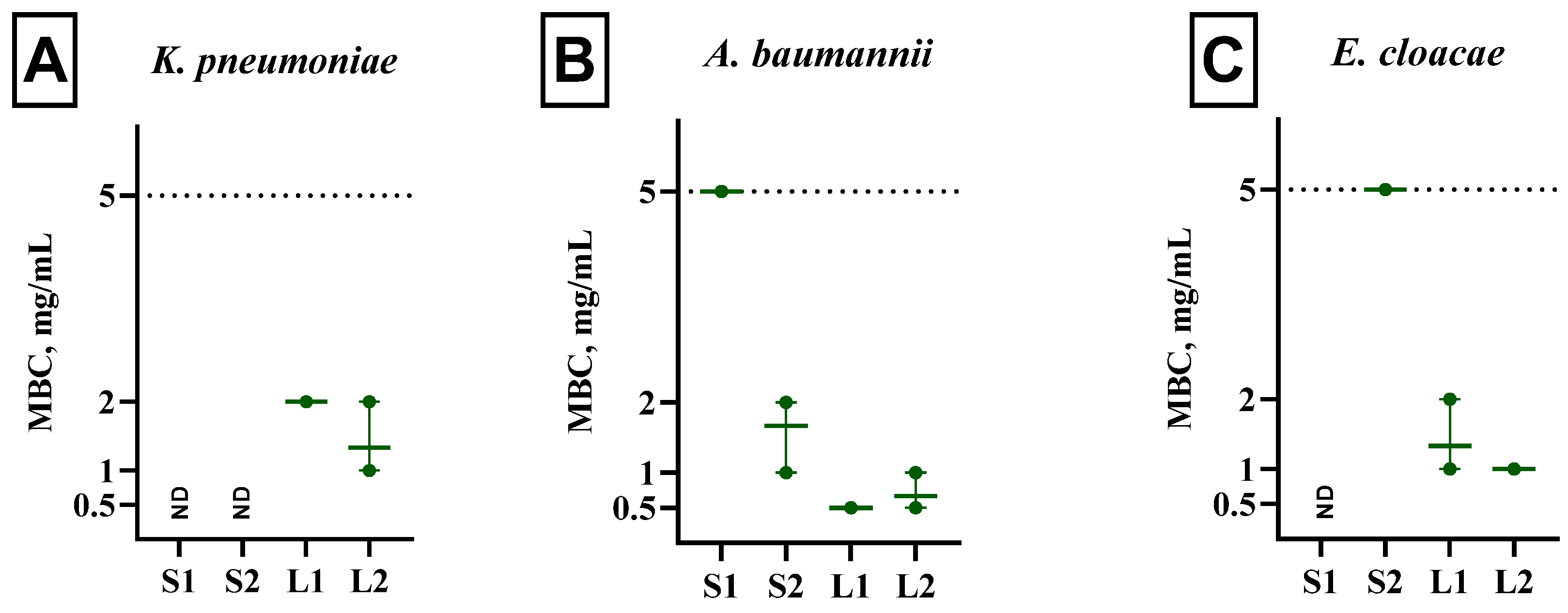
3.5. Bactericidal Activity of Extracts from Fomitopsis pinicola Mycelial Biomass
3.6. Anti-B. burgdorferi Activity of Extracts from Fomitopsis pinicola Mycelial Biomass
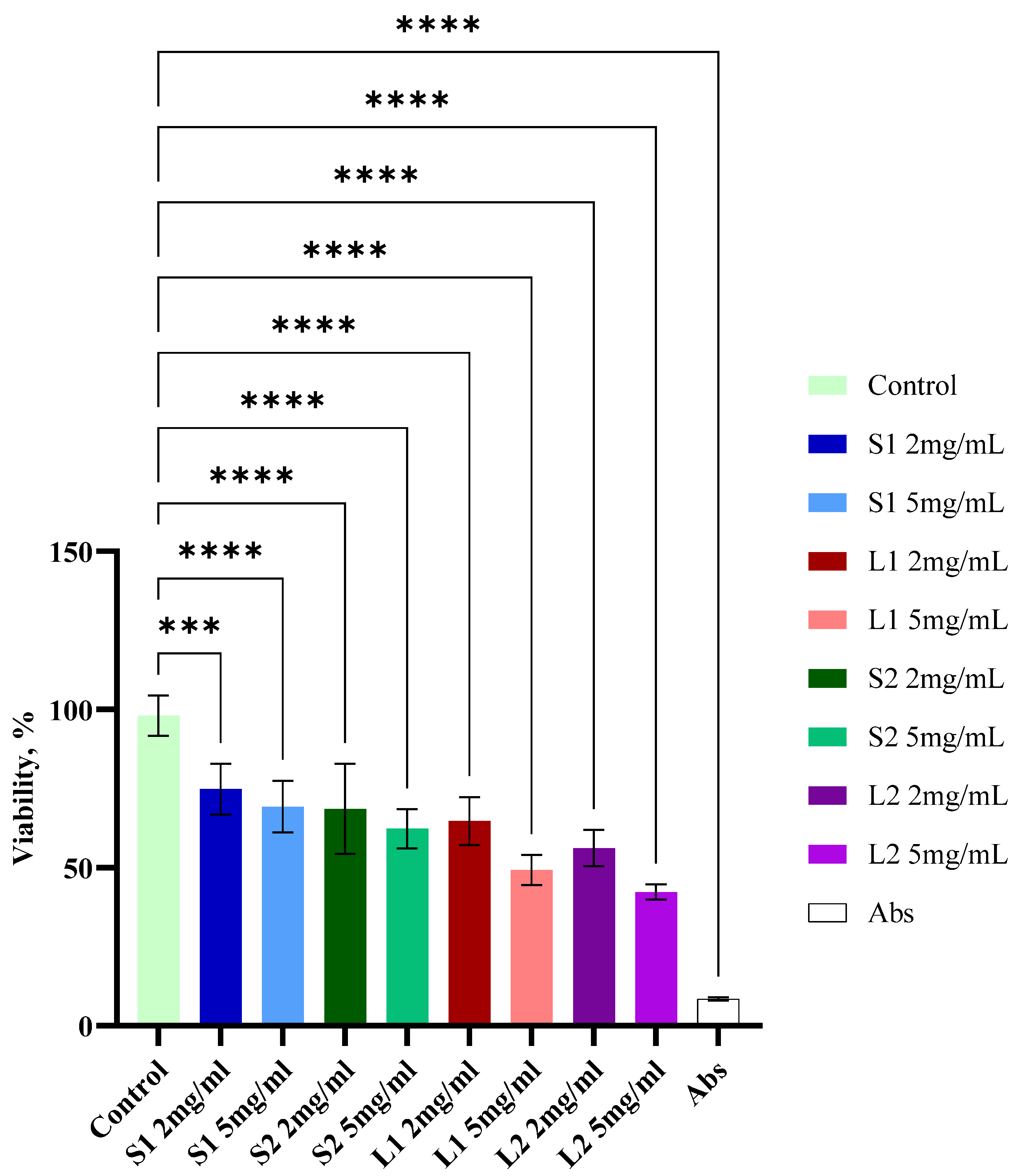
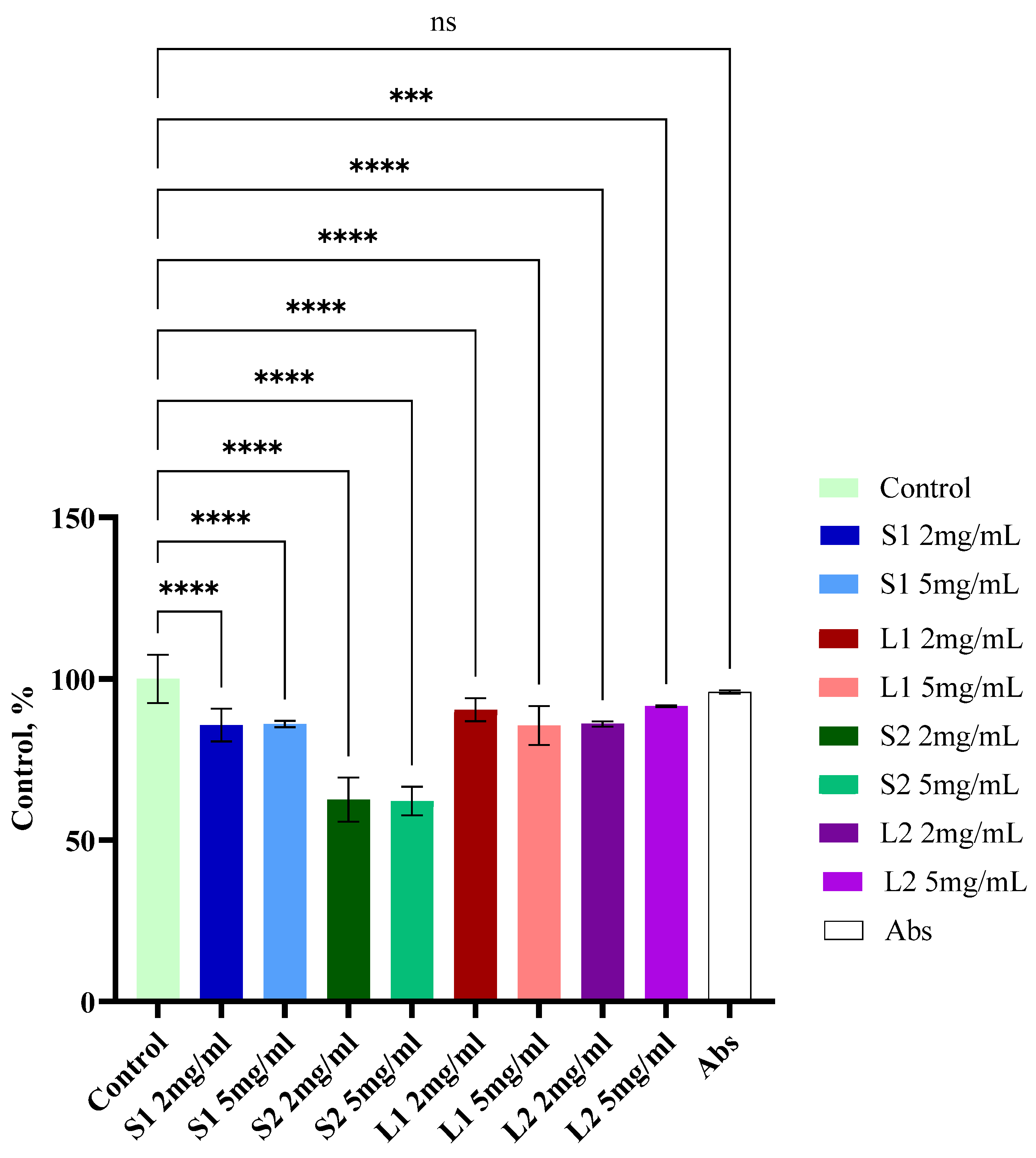
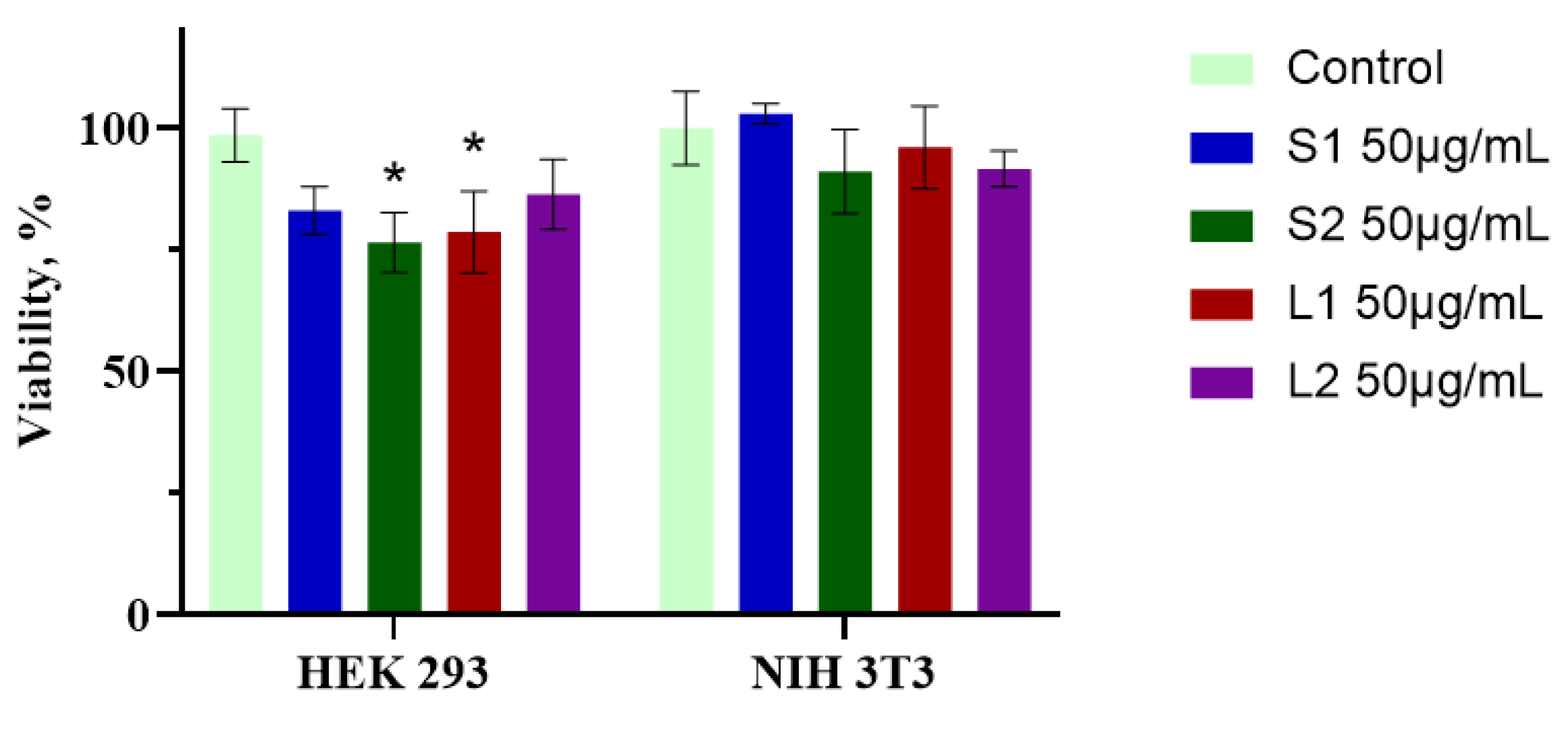
3.7. Cytotoxic Activity of Extracts from Submerged Fomitopsis pinicola Mycelial Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug-resistant bacteria |
| MBC | Minimal bactericidal concentration |
| MP | Mandarin pomace (juice production waste) |
| DW | Dry weight |
References
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Lavigne, J.P.; Pages, J.M. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Intra, J.; Carcione, D.; Sala, R.M.; Siracusa, C.; Brambilla, P.; Leoni, V. Antimicrobial Resistance Patterns of Enterobacter cloacae and Klebsiella aerogenes Strains Isolated from Clinical Specimens: A Twenty-Year Surveillance Study. Antibiotics 2023, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- de Man, T.J.B.; Lutgring, J.D.; Lonsway, D.R.; Anderson, K.F.; Kiehlbauch, J.A.; Chen, L.; Walters, M.S.; Sjolund-Karlsson, M.; Rasheed, J.K.; Kallen, A.; et al. Genomic Analysis of a Pan-Resistant Isolate of Klebsiella pneumoniae, United States 2016. MBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Chaddad, A.; Hanna, J.; Matta, J.; Daoud, Z. Understanding the Epidemiology of Multi-Drug Resistant Gram-Negative Bacilli in the Middle East Using a One Health Approach. Front. Microbiol. 2019, 10, 1941. [Google Scholar] [CrossRef]
- O’Connell, S.; Wolfs, T.F. Lyme borreliosis. Pediatr. Infect Dis. J. 2014, 33, 407–409. [Google Scholar] [CrossRef][Green Version]
- Zajkowska, J.; Lewczuk, P.; Strle, F.; Stanek, G. Lyme borreliosis: From pathogenesis to diagnosis and treatment. Clin. Dev. Immunol. 2012, 2012, 231657. [Google Scholar] [CrossRef][Green Version]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Brorson, O.; Brorson, S.H. Grapefruit seed extract is a powerful in vitro agent against motile and cystic forms of Borrelia burgdorferi sensu lato. Infection 2007, 35, 206–208. [Google Scholar] [CrossRef]
- Feng, J.; Leone, J.; Schweig, S.; Zhang, Y. Evaluation of Natural and Botanical Medicines for Activity Against Growing and Non-growing Forms of B. burgdorferi. Front. Med. 2020, 7, 6. [Google Scholar] [CrossRef]
- Heggers, J.P.; Cottingham, J.; Gusman, J.; Reagor, L.; McCoy, L.; Carino, E.; Cox, R.; Zhao, J.G. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: II. Mechanism of action and in vitro toxicity. J. Altern. Complement Med. 2002, 8, 333–340. [Google Scholar] [CrossRef]
- Liebold, T.; Straubinger, R.K.; Rauwald, H.W. Growth inhibiting activity of lipophilic extracts from Dipsacus sylvestris Huds. roots against Borrelia burgdorferi ss in vitro. Pharmazie 2011, 66, 628–630. [Google Scholar]
- Rauwald, H.W.; Liebold, T.; Grotzinger, K.; Lehmann, J.; Kuchta, K. Labdanum and Labdanes of Cistus creticus and C. ladanifer: Anti-Borrelia activity and its phytochemical profiling. Phytomedicine 2019, 60, 152977. [Google Scholar] [CrossRef]
- Laanet, P.R.; Bragina, O.; Joul, P.; Vaher, M. Plantago major and Plantago lanceolata Exhibit Antioxidant and Borrelia burgdorferi Inhibiting Activities. Int. J. Mol. Sci. 2024, 25, 7112. [Google Scholar] [CrossRef] [PubMed]
- Saar-Reismaa, P.; Bragina, O.; Kuhtinskaja, M.; Reile, I.; Laanet, P.R.; Kulp, M.; Vaher, M. Extraction and Fractionation of Bioactives from Dipsacus fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity. Pharmaceuticals 2022, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.S.; Ramya, H.; Ajith, T.A.; Shah, M.A.; Janardhanan, K.K. Bioactive extract of Fomitopsis pinicola rich in 11-α-acetoxykhivorin mediates anticancer activity by cytotoxicity, induction of apoptosis, inhibition of tumor growth, angiogenesis and cell cycle progression. J. Funct. Foods 2021, 78, 104372. [Google Scholar] [CrossRef]
- Grienke, U.; Zoll, M.; Peintner, U.; Rollinger, J.M. European medicinal polypores—A modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Q.; Xu, T.C.; Wang, Y.; Xu, W.F.; Li, M.; Zhang, X.; Zhao, C.; Zhang, D.L.; Jin, P.F.; et al. Bioactive terpenoids and sterols from the fruiting bodies of Fomitopsis pinicola. Phytochemistry 2025, 236, 114510. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.S. Characterisation of Extracts and Anti-Cancer Activities of Fomitopsis pinicola. Nutrients 2020, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, M.L.; Xu, T.M.; Wang, Y.; Wu, D.M.; Cui, B.K. Taxonomy and Phylogeny of the Fomitopsis pinicola Complex With Descriptions of Six New Species From East Asia. Front. Microbiol. 2021, 12, 644979. [Google Scholar] [CrossRef] [PubMed]
- Krupodorova, T.; Barshteyn, V.; Dzhagan, V.; Pluzhnyk, A.; Zaichenko, T.; Blume, Y. Enhancement of antioxidant activity and total phenolic content of Fomitopsis pinicola mycelium extract. Fungal. Biol. Biotechnol. 2024, 11, 18. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Spirovic-Trifunovic, B.; Miletic, S.; Lazic, V.; Zizak, Z.; Vunduk, J. Bioprospecting of Selected Species of Polypore Fungi from the Western Balkans. Molecules 2024, 29, 314. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Wang, P.; Wang, L.; Fan, J.; Wang, X.; Liu, Q. Investigating migration inhibition and apoptotic effects of Fomitopsis pinicola chloroform extract on human colorectal cancer SW-480 cells. PLoS ONE 2014, 9, e101303. [Google Scholar] [CrossRef]
- Peng, X.R.; Su, H.G.; Liu, J.H.; Huang, Y.J.; Yang, X.Z.; Li, Z.R.; Zhou, L.; Qiu, M.H. C30 and C31 Triterpenoids and Triterpene Sugar Esters with Cytotoxic Activities from Edible Mushroom Fomitopsis pinicola (Sw. Ex Fr.) Krast. J. Agric. Food Chem. 2019, 67, 10330–10341. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Li, M.; Yuan, T. Lanostane triterpenoids from the fruiting bodies of Fomitopsis pinicola and their anti-inflammatory activities. Phytochemistry 2022, 193, 112985. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Shnyreva, A.V.; Barkhudaryan, A. Antimicrobial Activity of Different Collections of Medicinal Polypore Fungus Fomitopsis pinicola (Agaricomycetes). Int. J. Med. Mushrooms 2024, 26, 33–48. [Google Scholar] [CrossRef]
- Lomascolo, A.; Cayol, J.L.; Roche, M.; Guo, L.; Robert, J.L.; Record, E.; Lesage-Meessen, L.; Ollivier, B.; Sigoillot, J.C.; Asther, M. Molecular clustering of Pycnoporus strains from various geographic origins and isolation of monokaryotic strains for laccase hyperproduction. Mycol. Res. 2002, 106, 1193–1203. [Google Scholar] [CrossRef]
- Asatiani, M.D.; Sharvit, L.; Barseghyan, G.S.; Chan, J.S.L.; Elisashvili, V.; Wasser, S.P. Cytotoxic Activity of Medicinal Mushroom Extracts on Human Cancer Cells. SF J. Biotechnol. Biomed. Eng. 2018, 1, 1–7. [Google Scholar]
- Huang, C.W.; Hung, Y.C.; Chen, L.Y.; Asatiani, M.; Elisashvili, V.I.; Klarsfeld, G.; Melamed, D.; Fares, B.; Wasser, S.P.; Mau, J.L. Chemical Composition and Antioxidant Properties of Different Combinations of Submerged Cultured Mycelia of Medicinal Mushrooms. Int. J. Med. Mushrooms 2021, 23, 1–24. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Shaikh, J.R.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Rao, A.; Kumari, S.; Laura, J.S.; Dhania, G. Qualitative Phytochemical Screening of Medicinal Plants Using Different Solvent Extracts. Orient. J. Chem. 2023, 39, 621–626. [Google Scholar] [CrossRef]
- Mohan, M.K.; Kaur, H.; Rosenberg, M.; Duvanova, E.; Lukk, T.; Ivask, A.; Karpichev, Y. Synthesis and Antibacterial Properties of Novel Quaternary Ammonium Lignins. ACS Omega 2024, 9, 39134–39145. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive Phytochemical Constituents of Wild Edible Mushrooms from Southeast Asia. Molecules 2020, 25, 1972. [Google Scholar] [CrossRef]
- Vyshnavi, D.V.; Swaroop, S.S.; Sudheer, A.; Kanala, S.R.; Naik, R.M.; Varalakshmi, O. Plant-Derived Selected Bioactive Saponins and Tannins: An Overview of their Multi-Target Mechanisms and Diverse Biological Activities. Pharmacogn. Res. 2023, 15, 623–635. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res. Int. 2022, 5445291. [Google Scholar] [CrossRef]
- Yu, W.B.; Zhang, Y.F.; Lu, Y.; Ouyang, Z.W.; Peng, J.H.; Tu, Y.Y.; He, B. Recent research on the bioactivity of polyphenols derived from edible fungi and their potential in chronic disease prevention. J. Funct. Foods 2025, 124, 106627. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Evidente, A. Structures and Biological Activities of Alkaloids Produced by Mushrooms, a Fungal Subgroup. Biomolecules 2022, 12, 1025. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.S.; Akowuah, G.A.; Chye, F.Y.; Sia, C.M.; Okechukwu, P.N.; Ho, C.W. Identification of Apigenin-7-Glucoside and Luteolin-7-Glucoside in Pleurotus porrigens and Schizophyllum commune Mushrooms by Liquid Chromatography—Ion Trap Tandem Mass Spectrometry. Curr. Bioact. Compd. 2015, 11, 202–208. [Google Scholar] [CrossRef]
- Wu, T.; He, M.Y.; Zang, X.X.; Zhou, Y.; Qiu, T.F.; Pan, S.Y.; Xu, X.Y. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tan, S.N.; Cui, J.; Guo, N.; Wang, W.; Zu, Y.G.; Jin, S.; Xu, X.X.; Liu, Q.; Fu, Y.J. PA-1, a novel synthesized pyrrolizidine alkaloid, inhibits the growth of Escherichia coli and Staphylococcus aureus by damaging the cell membrane. J. Antibiot. 2014, 67, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Larghi, E.L.; Bracca, A.B.; Arroyo Aguilar, A.A.; Heredia, D.A.; Pergomet, J.L.; Simonetti, S.O.; Kaufman, T.S. Neocryptolepine: A Promising Indoloisoquinoline Alkaloid with Interesting Biological Activity. Evaluation of the Drug and its Most Relevant Analogs. Curr. Top. Med. Chem. 2015, 15, 1683–1707. [Google Scholar] [CrossRef]
- Kelley, C.; Lu, S.F.; Parhi, A.; Kaul, M.; Pilch, D.S.; LaVoie, E.J. Antimicrobial activity of various 4-and 5-substituted 1-phenylnaphthalenes. Eur. J. Med. Chem. 2013, 60, 395–409. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Dresch, P.; MN, D.A.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Erdogan Eliuz, E.A. Antibacterial activity and antibacterial mechanism of ethanol extracts of Lentinula edodes (Shiitake) and Agaricus bisporus (button mushroom). Int. J. Environ. Health Res. 2022, 32, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Michalak, K.; Winiarczyk, S.; Adaszek, L.; Kosikowska, U.; Andrzejczuk, S.; Garbacz, K.; Dobrut, A.; Jarosz, L.; Czupryna, W.; Pietras-Ozga, D. Antioxidant and antimicrobial properties of an extract rich in proteins obtained from Trametes versicolor. J. Vet. Res. 2023, 67, 209–218. [Google Scholar] [CrossRef] [PubMed]


| Extracts from Biomass, Cultivated in a Glycerol-Containing Synthetic Medium | ||
|---|---|---|
| Extracts | Abbreviations | Yield of Extracts (g/g DW) |
| Water | S1 | 0.386 |
| Ethanol | S2 | 0.342 |
| Extracts from biomass, cultivated in a lignocellulose (MP)-based medium | ||
| Water | L1 | 0.391 |
| Ethanol | L2 | 0.369 |
| Class of Compound | Test | Extract-S1 | Extract-S2 | Extract-L1 | Extract-L2 |
|---|---|---|---|---|---|
| Peptide bond | Biuret | + | − | − | − |
| Saponins | Foam | + | * | + | * |
| Phenols | Iodine | + | + | + | + |
| Alkaloids | Mayer’s | − | + | − | + |
| Tannins | Braymer’s | + | + | + | + |
| Reducing sugars | Fehling | + | + | + | + |
| Glycosides | Salkowski’s | + | + | + | + |
| Flavonoids | Lead acetate | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragina, O.; Kuhtinskaja, M.; Elisashvili, V.; Asatiani, M.; Kulp, M. Antibacterial Properties of Submerged Cultivated Fomitopsis pinicola, Targeting Gram-Negative Pathogens, Including Borrelia burgdorferi. Sci 2025, 7, 104. https://doi.org/10.3390/sci7030104
Bragina O, Kuhtinskaja M, Elisashvili V, Asatiani M, Kulp M. Antibacterial Properties of Submerged Cultivated Fomitopsis pinicola, Targeting Gram-Negative Pathogens, Including Borrelia burgdorferi. Sci. 2025; 7(3):104. https://doi.org/10.3390/sci7030104
Chicago/Turabian StyleBragina, Olga, Maria Kuhtinskaja, Vladimir Elisashvili, Mikheil Asatiani, and Maria Kulp. 2025. "Antibacterial Properties of Submerged Cultivated Fomitopsis pinicola, Targeting Gram-Negative Pathogens, Including Borrelia burgdorferi" Sci 7, no. 3: 104. https://doi.org/10.3390/sci7030104
APA StyleBragina, O., Kuhtinskaja, M., Elisashvili, V., Asatiani, M., & Kulp, M. (2025). Antibacterial Properties of Submerged Cultivated Fomitopsis pinicola, Targeting Gram-Negative Pathogens, Including Borrelia burgdorferi. Sci, 7(3), 104. https://doi.org/10.3390/sci7030104







