Hydrogen Sulfide Has a Minor Impact on Human Gut Microbiota Across Age Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ex Vivo Testing Setup
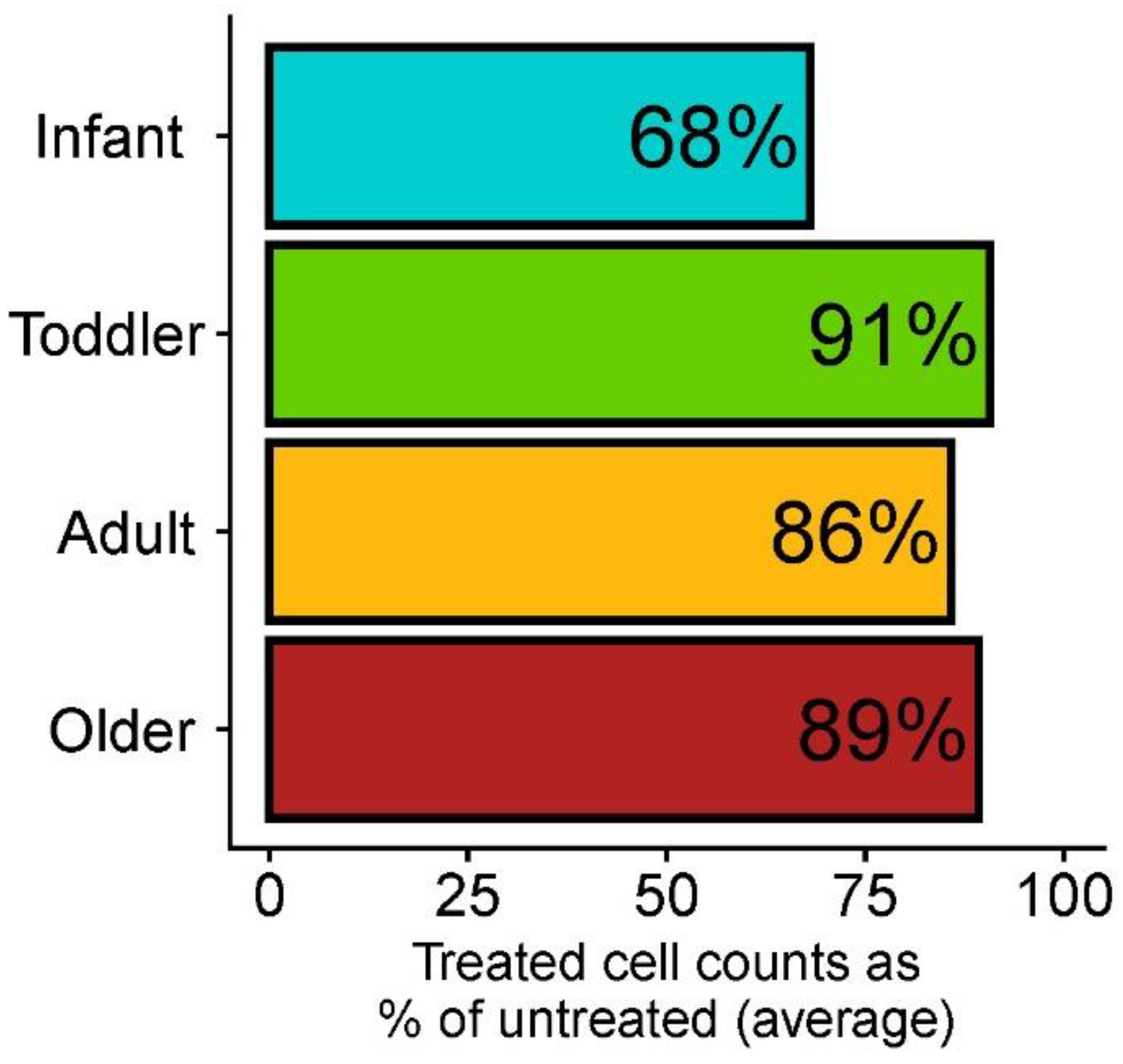
2.3. Bacterial Cell Counts
2.4. SCFA, Lactate, and Gas Measurement
2.5. Transepithelial Electrical Resistance (TEER) Measurement
2.6. DNA Extraction and Sequencing
2.7. Bioinformatics Analysis
2.8. Statistical Analyses
2.9. Data Availability
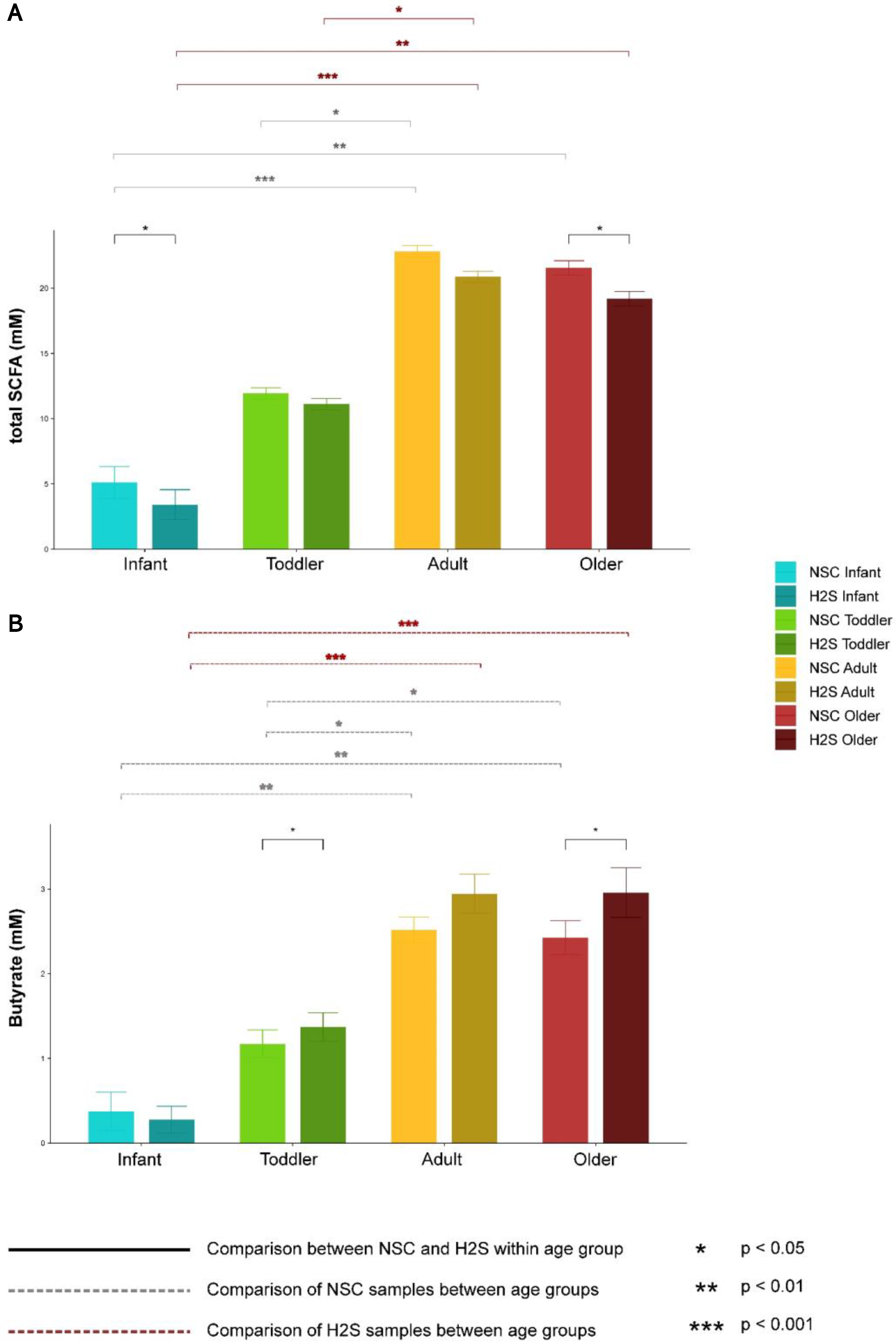
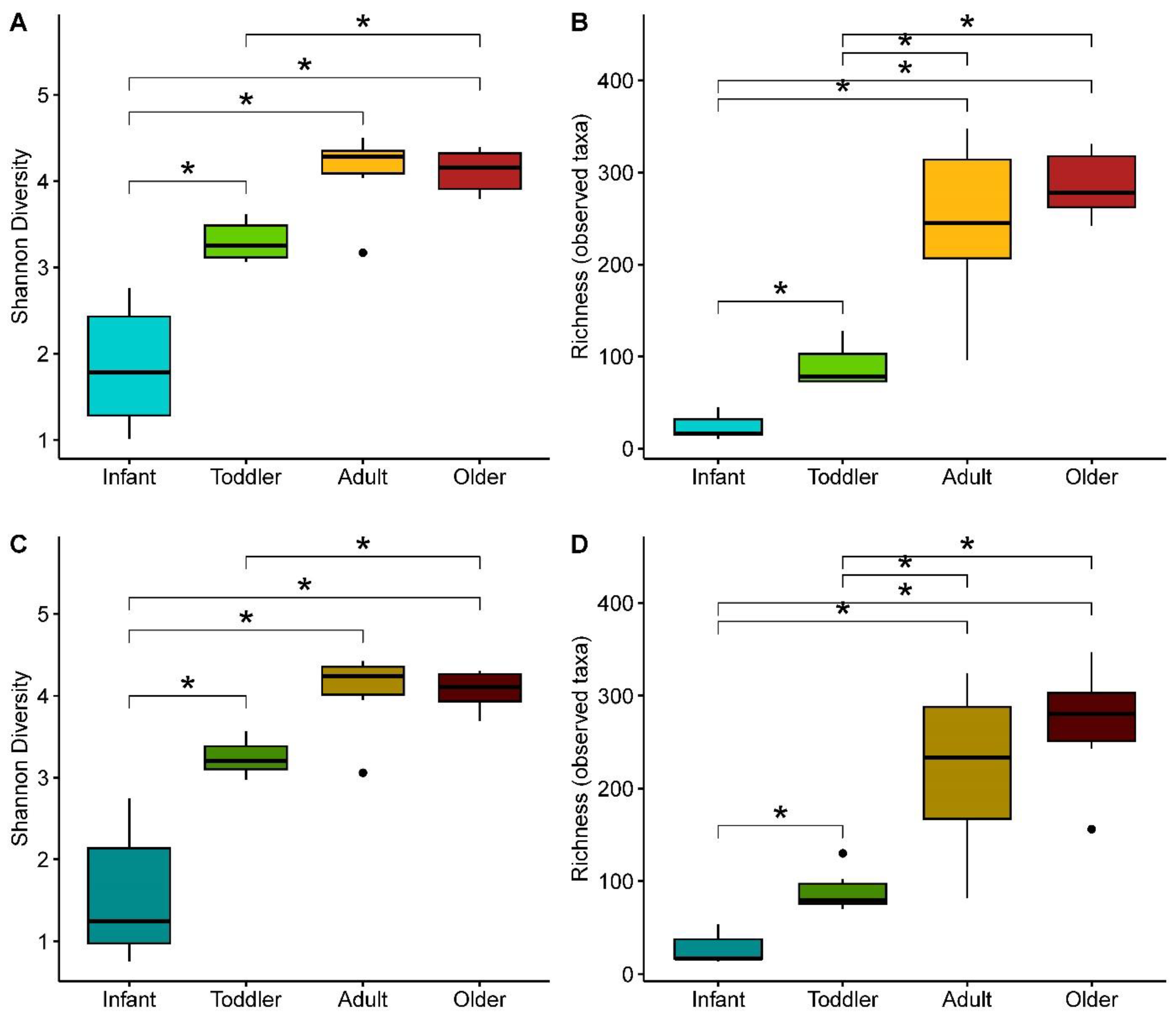
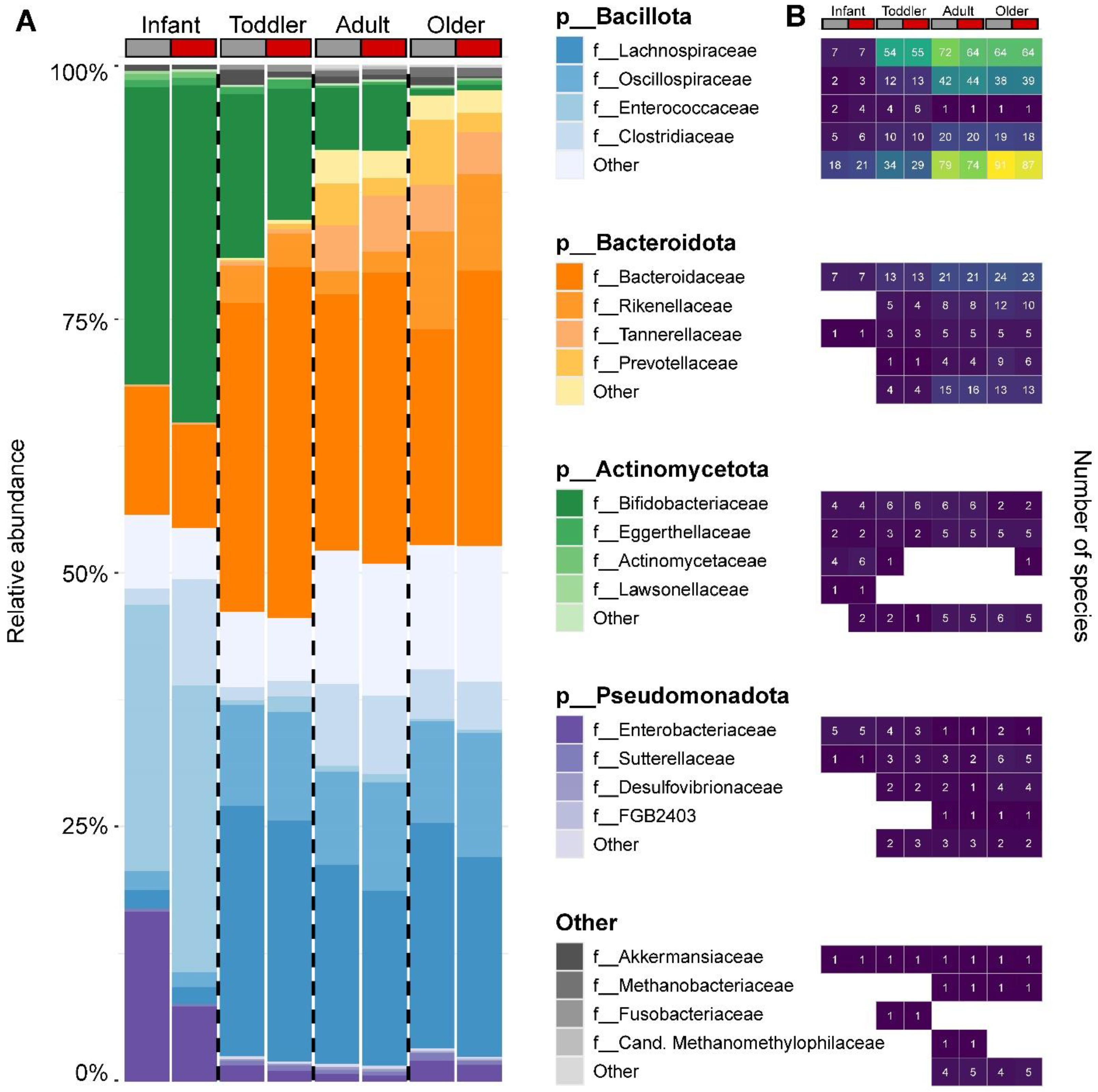
3. Results
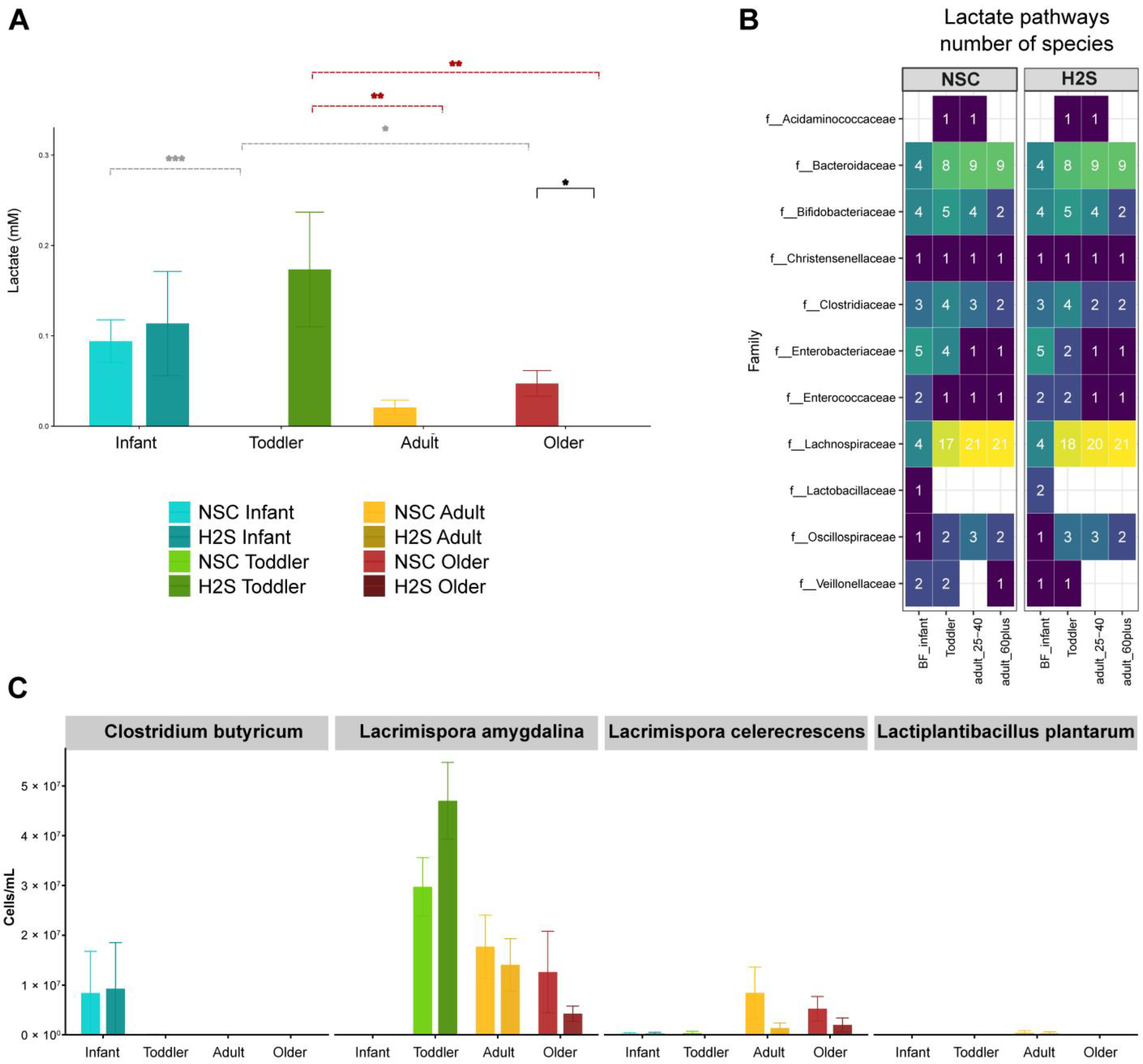
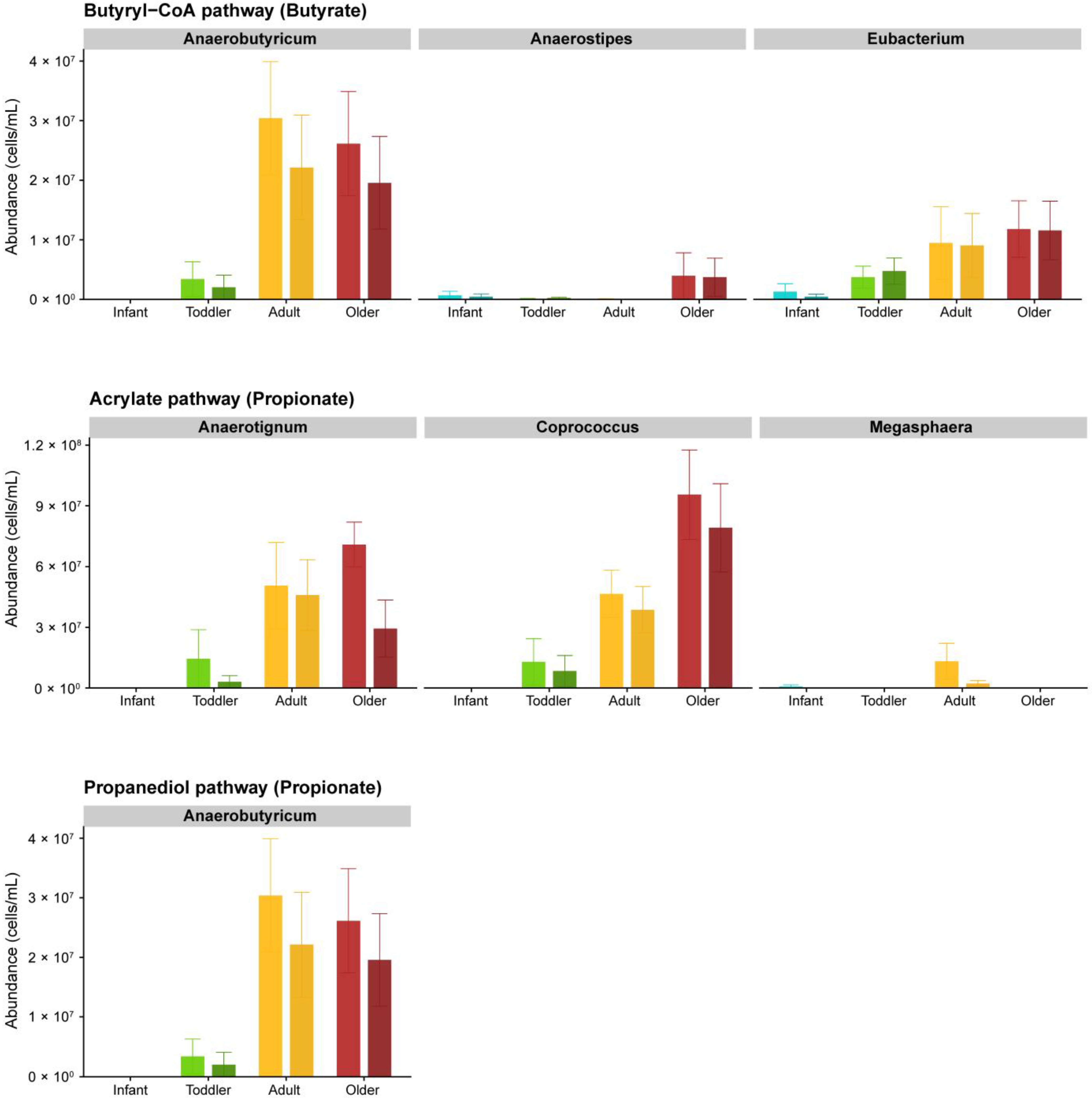
3.1. Effect of SSP on the Metabolism of the Gut Microbial Communities
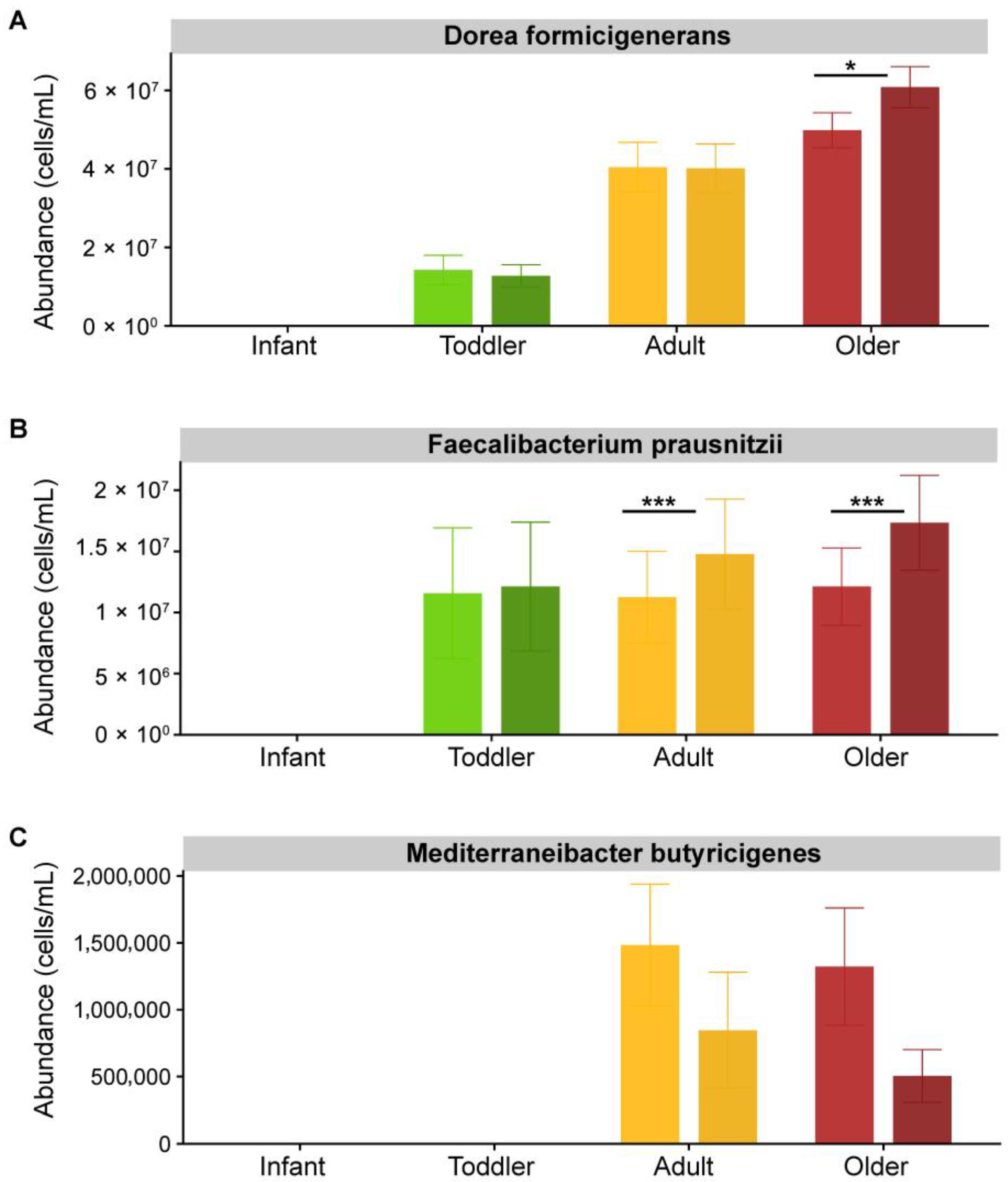
3.2. Effect of SSP Treatment on the Composition and Structure of Gut Microbial Communities
3.3. Lactate Production and Conservation
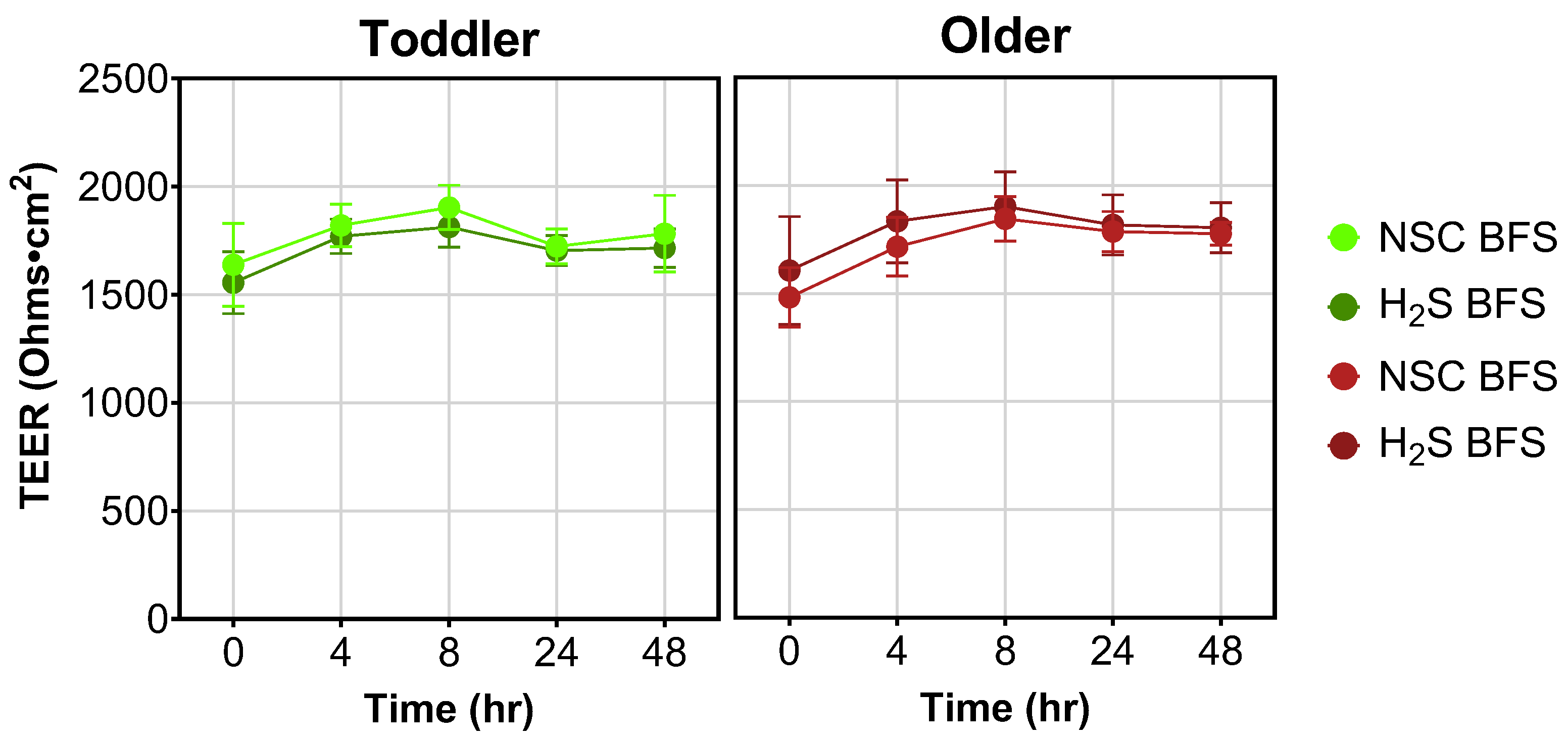
3.4. TEER Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSP | Sodium sulfide preparation |
| GIT | Gastrointestinal tract |
| SCFA | Short-chain fatty acid |
| NSC | No-substrate control |
References
- Fakhraee, M.; Crockford, P.W.; Bauer, K.W.; Pasquier, V.; Sugiyama, I.; Katsev, S.; Raven, M.R.; Gomes, M.; Philippot, P.; Crowe, S.A.; et al. The history of Earth’s sulfur cycle. Nat. Rev. Earth Environ. 2025, 6, 106–125. [Google Scholar] [CrossRef]
- Gupta, S.; Plugge, C.M.; Muyzer, G.; Sánchez-Andrea, I. Harnessing the potential of the microbial sulfur cycle for environmental biotechnology. Curr. Opin. Biotechnol. 2024, 88, 103164. [Google Scholar] [CrossRef]
- Buret, A.G.; Allain, T.; Motta, J.-P.; Wallace, J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid Redox Signal 2022, 36, 211–219. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Kye, B.-H.; Ko, S.-H.; Yoo, R.N. Sulfur Metabolism of the Gut Microbiome and Colorectal Cancer: The Threat to the Younger Generation. Nutrients 2023, 15, 1966. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef]
- Piragine, E.; Malanima, M.A.; Lucenteforte, E.; Martelli, A.; Calderone, V. Circulating Levels of Hydrogen Sulfide (H2S) in Patients with Age-Related Diseases: A Systematic Review and Meta-Analysis. Biomolecules 2023, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Public Health Statement: Toxicological Profile for Hydrogen Sulfide. Agency for Toxic Substances and Disease Registry. December 2016. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp114-c1-b.pdf (accessed on 15 April 2025).
- Chou, C.-H.S.J. Hydrogen Sulfide: Human Health Aspects; Agency for Toxic Substances and Disease Registry; World Health Organization & International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 2003. Available online: https://digitallibrary.un.org/record/505398?v=pdf (accessed on 15 April 2025).
- Tomasova, L.; Konopelski, P.; Ufnal, M. Gut Bacteria and Hydrogen Sulfide: The New Old Players in Circulatory System Homeostasis. Molecules 2016, 21, 1558. [Google Scholar] [CrossRef]
- Tomasova, L.; Grman, M.; Ondrias, K.; Ufnal, M. The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health. Nutr. Metab. 2021, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotech. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Quist, A.J.L.; Johnston, J.E. Malodors as environmental injustice: Health symptoms in the aftermath of a hydrogen sulfide emergency in Carson, California, USA. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 935–940. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Poștaru, M.; Turnea, M.; Rotariu, M.; Galaction, A.I. Hydrogen Sulfide and Gut Microbiota: Their Synergistic Role in Modulating Sirtuin Activity and Potential Therapeutic Implications for Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1480. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Rotariu, M.; Poștaru, M.; Turnea, M.; Galaction, A.I. Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut–Brain Axis: Implications for Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines 2024, 12, 2670. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Teigen, L.M.; Kaiser-Powers, T.; Matson, M.; Elkin, B.; Kabage, A.J.; Hamilton, M.; Vaughn, B.P.; Sadowsky, M.J.; Staley, C.; Khoruts, A. Contribution of Common Sulfur-Containing Substrates to Hydrogen Sulfide Production by Human Gut Microbiota Using an In Vitro Model Standardized for Bacterial Counts. Gut Microbes Rep. 2024, 1, 2361246. [Google Scholar] [CrossRef]
- Hayes, J.A.; Lunger, A.W.; Sharma, A.S.; Fernez, M.T.; Carrier, R.L.; Koppes, A.N.; Koppes, R.; Woolston, B.M. Engineered bacteria titrate hydrogen sulfide and induce concentration-dependent effects on the host in a gut microphysiological system. Cell Rep. 2023, 42, 113481. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Gao, Z.W.; Liu, N.; Zhang, W.H.; Ling, H. Effects of Exogenous Hydrogen Sulfide on Diabetic Metabolic Disorders in db/db Mice are Associated with Gut Bacterial and Fungal Microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 801331. [Google Scholar] [CrossRef]
- Lemons, J.M.S.; Narrowe, A.B.; Firrman, J.; Mahalak, K.K.; Liu, L.S.; Higgins, S.; Moustafa, A.M.; Baudot, A.; Deyaert, S.; Van den Abbeele, P. The food additive butylated hydroxyanisole minimally affects the human gut microbiome ex vivo. Food Chem. 2025, 473, 143037. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítežová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Deyaert, S.; Thabuis, C.; Perreau, C.; Bajic, D.; Wintergerst, E.; Joossens, M.; Firrman, J.; Walsh, D.; Baudot, A. Bridging preclinical and clinical gut microbiota research using the ex vivo SIFR((R)) technology. Front. Microbiol. 2023, 14, 1131662. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Beghini, F.; Cumbo, F.; McIver, L.J.; Thompson, K.N.; Zolfo, M.; Manghi, P.; Dubois, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 2023, 41, 1633–1644. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, e65088. [Google Scholar] [CrossRef] [PubMed]
- Martinez Arbizu, P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis, R package version 0.4. 2020.
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package, R package version 2.8-0. 2025.
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Barker-Tejeda, T.C.; Zubeldia-Varela, E.; Macías-Camero, A.; Alonso, L.; Martín-Antoniano, I.A.; Rey-Stolle, M.F.; Mera-Berriatua, L.; Bazire, R.; Cabrera-Freitag, P.; Shanmuganathan, M.; et al. Comparative characterization of the infant gut microbiome and their maternal lineage by a multi-omics approach. Nat. Commun. 2024, 15, 3004. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs. Res. 2016, 65, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Louis, P.; Duncan, S.H.; Sheridan, P.O.; Walker, A.W.; Flint, H.J. Microbial lactate utilisation and the stability of the gut microbiome. Gut Microbiome 2022, 3, e3. [Google Scholar] [CrossRef]
- Elferink, S.; Krooneman, J.; Gottschal, J.C.; Spoestra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Prabhu, R.; Altman, E.; Eiteman, M.A. Lactate and Acrylate Metabolism by Megasphaera elsdenii under Batch and Steady-State Conditions. Appl. Environ. Microbiol. 2012, 78, 8564–8570. [Google Scholar] [CrossRef]
- Detman, A.; Mielecki, D.; Chojnacka, A.; Salamon, A.; Blaszczyk, M.K.; Sikora, A. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors. Microb. Cell Fact. 2019, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Sjögren, Y.M.; Tomicic, S.; Lundberg, A.; Fagerås-Böttcher, M.; Björkstén, B.; Sverremark-Ekström, E.; Jenmalm, M. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy 2009, 39, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Teigen, L.M.; Geng, Z.; Sadowsky, M.J.; Vaughn, B.P.; Hamilton, M.J.; Khoruts, A. Dietary Factors in Sulfur Metabolism and Pathogenesis of Ulcerative Colitis. Nutrients 2019, 11, 931. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Tannock, G.W.; Liu, Y. Guided dietary fibre intake as a means of directing short-chain fatty acid production by the gut microbiota. J. R. Soc. N. Z. 2020, 50, 434–455. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef]
- Zhanbo, Q.; Jing, Z.; Shugao, H.; Yinhang, W.; Jian, C.; Xiang, Y.; Feimin, Z.; Jian, L.; Xinyue, W.; Wei, W.; et al. Age and aging process alter the gut microbes. Aging 2024, 16, 8. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Mutuyemungu, E.; Singh, M.; Liu, S.; Rose, D.J. Intestinal gas production by the gut microbiota: A review. J. Funct. Foods 2023, 100, 105367. [Google Scholar] [CrossRef]
- Pi, X.; Yu, Z.; Yang, X.; Du, Z.; Liu, W. Effects of Zymosan on Short-Chain Fatty Acid and Gas Production in in vitro Fermentation Models of the Human Intestinal Microbiota. Front. Nutr. 2022, 9, 921137. [Google Scholar] [CrossRef]
- Hodgkinson, K.; Abbar, F.A.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; Preter, V.D.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Immerseel, F.V.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, K.C.; Suh, M.K.; Han, K.I.; Eom, M.K.; Lee, J.H.; Park, S.H.; Kang, S.W.; Park, J.E.; Oh, B.S.; et al. Mediterraneibacter butyricigenes sp. nov., a butyrate-producing bacterium isolated from human faeces. J. Microbiol. 2019, 57, 38–44. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; Angelis, M.D. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef]
- Lee, T.Y. Lactate: A multifunctional signaling molecule. J. Yeungnam Med. Sci. 2021, 38, 183–193. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhao, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal 2023, 21, 212. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. Msystems 2017, 2, e00051-17. [Google Scholar] [CrossRef]
- Weghoff, M.C.; Bertsch, J.; Müller, V. A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol. 2015, 17, 670–677. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberan, A.; Chen, Y.; Yang, F.; Blaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Teklebrhan, T.; Tan, Z. Diet Supplementation with Sulfur Amino Acids Modulated Fermentation Metabolome and Gut Microbiome in Goats. Front. Microbiol. 2022, 13, 870385. [Google Scholar] [CrossRef]
- Tejedor-Sanz, S.; Stevens, E.T.; Li, S.; Finnegan, P.; Nelson, J.; Knoesen, A.; Light, S.H.; Ajo-Franklin, C.M.; Marco, M.L. Extracellular electron transfer increases fermentation in lactic acid bacteria via a hybrid metabolism. eLife 2022, 11, e70684. [Google Scholar] [CrossRef]
- Habicht, K.S.; Salling, L.; Thamdrup, B.; Canfield, D.E. Effect of low sulfate concentrations on lactate oxidation and isotope fractionation during sulfate reduction by Archaeoglobus fulgidus strain Z. Appl. Environ. Microbiol. 2005, 71, 3770–3777. [Google Scholar] [CrossRef]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.M.; Stams, A.J.M. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef]
- Macy, J.M.; Santini, J.M.; Pauling, B.V.; O’Neill, A.H.; Sly, L.I. Two new arsenate/sulfate-reducing bacteria: Mechanisms of arsenate reduction. Arch. Microbiol. 2000, 173, 49–57. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kotrsová, V.; Dordević, D.; Buňková, L.; Vítězová, M.; Amedei, A. Hydrogen Sulfide Effects on the Survival of Lactobacilli with Emphasis on the Development of Inflammatory Bowel Diseases. Biomolecules 2019, 9, 752. [Google Scholar] [CrossRef]
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef]
- Rios-Arce, N.D.; Collins, F.L.; Schepper, J.D.; Steury, M.D.; Raehtz, S.; Mallin, H.; Schoenherr, D.T.; Parameswaran, N.; McCabe, L.R. Epithelial Barrier Function in Gut-Bone Signaling. Adv. Exp. Med. Biol. 2017, 1033, 151–183. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Huang, Z.; Xue, B.; Zhu, J.; You, M.; Liu, X.; Wang, X.; Liu, Y.; Chen, S.; et al. Hydrogen sulfide preserves intestinal barrier repair function through sulfhydration of RPS20 in experimental colitis. Res. Sq. 2024. preprint. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, J.; Chen, G. Higher dietary butyrate intake is associated with better cognitive function in older adults: Evidence from a cross-sectional study. Front. Aging Neurosci. 2025, 17, 1522498. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Qaisar, R.; Iqbal, M.S.; Ahmad, F.; Karim, A. Oral butyrate improves postural balance by repairing leaky gut in geriatric adults. Gait Posture 2025, 121, 370–376. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Lemons, J.M.S.; Firrman, J.; Mahalak, K.K.; Chetty, V.J.; Narrowe, A.B.; Higgins, S.; Moustafa, A.M.; Baudot, A.; Deyaert, S.; et al. Hydrogen Sulfide Has a Minor Impact on Human Gut Microbiota Across Age Groups. Sci 2025, 7, 102. https://doi.org/10.3390/sci7030102
Liu L, Lemons JMS, Firrman J, Mahalak KK, Chetty VJ, Narrowe AB, Higgins S, Moustafa AM, Baudot A, Deyaert S, et al. Hydrogen Sulfide Has a Minor Impact on Human Gut Microbiota Across Age Groups. Sci. 2025; 7(3):102. https://doi.org/10.3390/sci7030102
Chicago/Turabian StyleLiu, Linshu, Johanna M. S. Lemons, Jenni Firrman, Karley K. Mahalak, Venkateswari J. Chetty, Adrienne B. Narrowe, Stephanie Higgins, Ahmed M. Moustafa, Aurélien Baudot, Stef Deyaert, and et al. 2025. "Hydrogen Sulfide Has a Minor Impact on Human Gut Microbiota Across Age Groups" Sci 7, no. 3: 102. https://doi.org/10.3390/sci7030102
APA StyleLiu, L., Lemons, J. M. S., Firrman, J., Mahalak, K. K., Chetty, V. J., Narrowe, A. B., Higgins, S., Moustafa, A. M., Baudot, A., Deyaert, S., & Van den Abbeele, P. (2025). Hydrogen Sulfide Has a Minor Impact on Human Gut Microbiota Across Age Groups. Sci, 7(3), 102. https://doi.org/10.3390/sci7030102







