The Response of Cell Cultures to Nutrient- and Serum-Induced Changes in the Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Growth
2.2. Composition of the Growth Media
2.3. Test of Cell Permeability
2.4. ATP Determination
2.5. Measurement of the Mitochondrial Membrane Potential
2.6. Autophagy Assay
2.7. Statistical Analysis
3. Results
3.1. Cell Growth
3.2. LDH
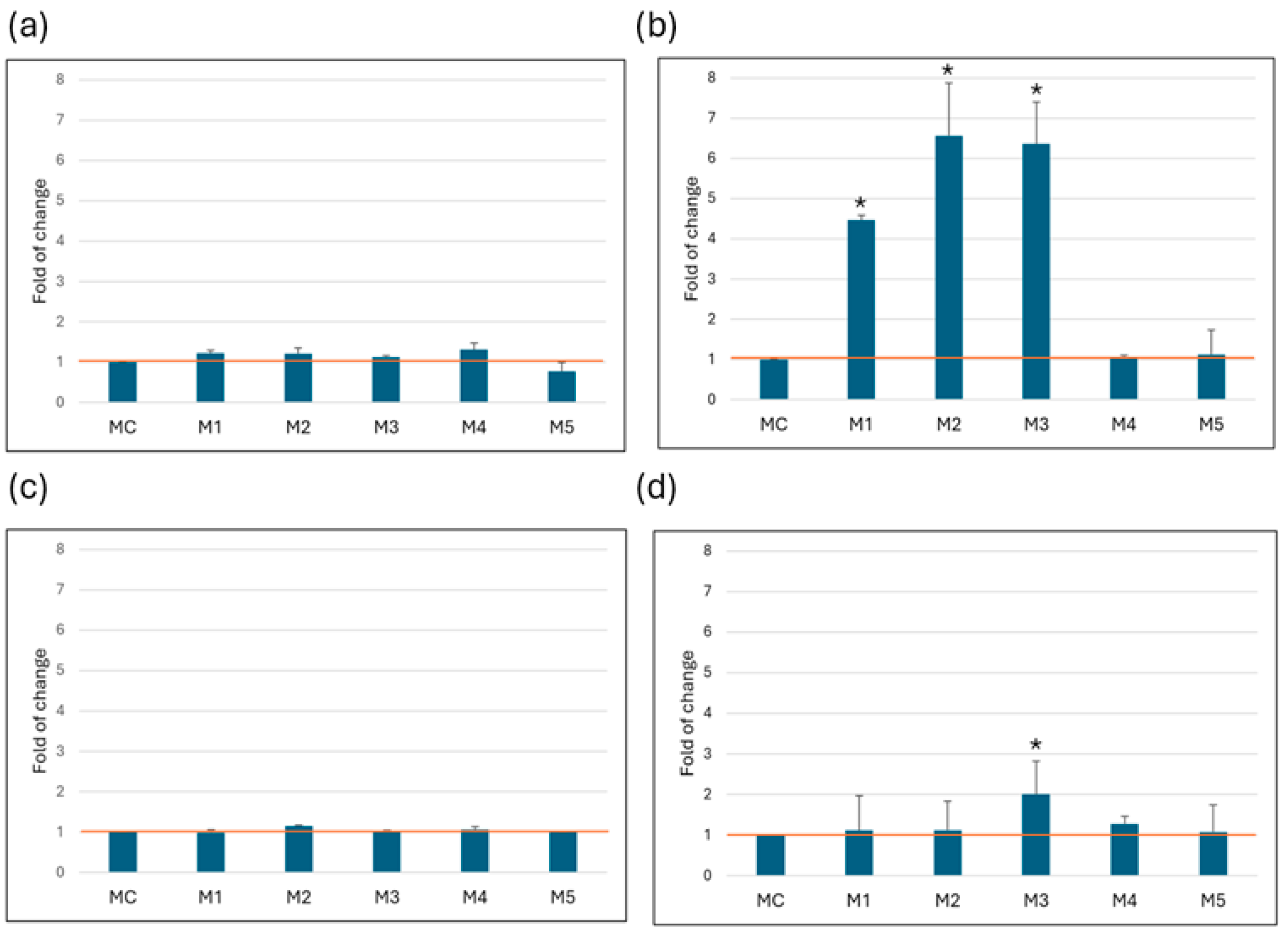
3.3. Autophagy
3.4. Mitochondrial Membrane Potential
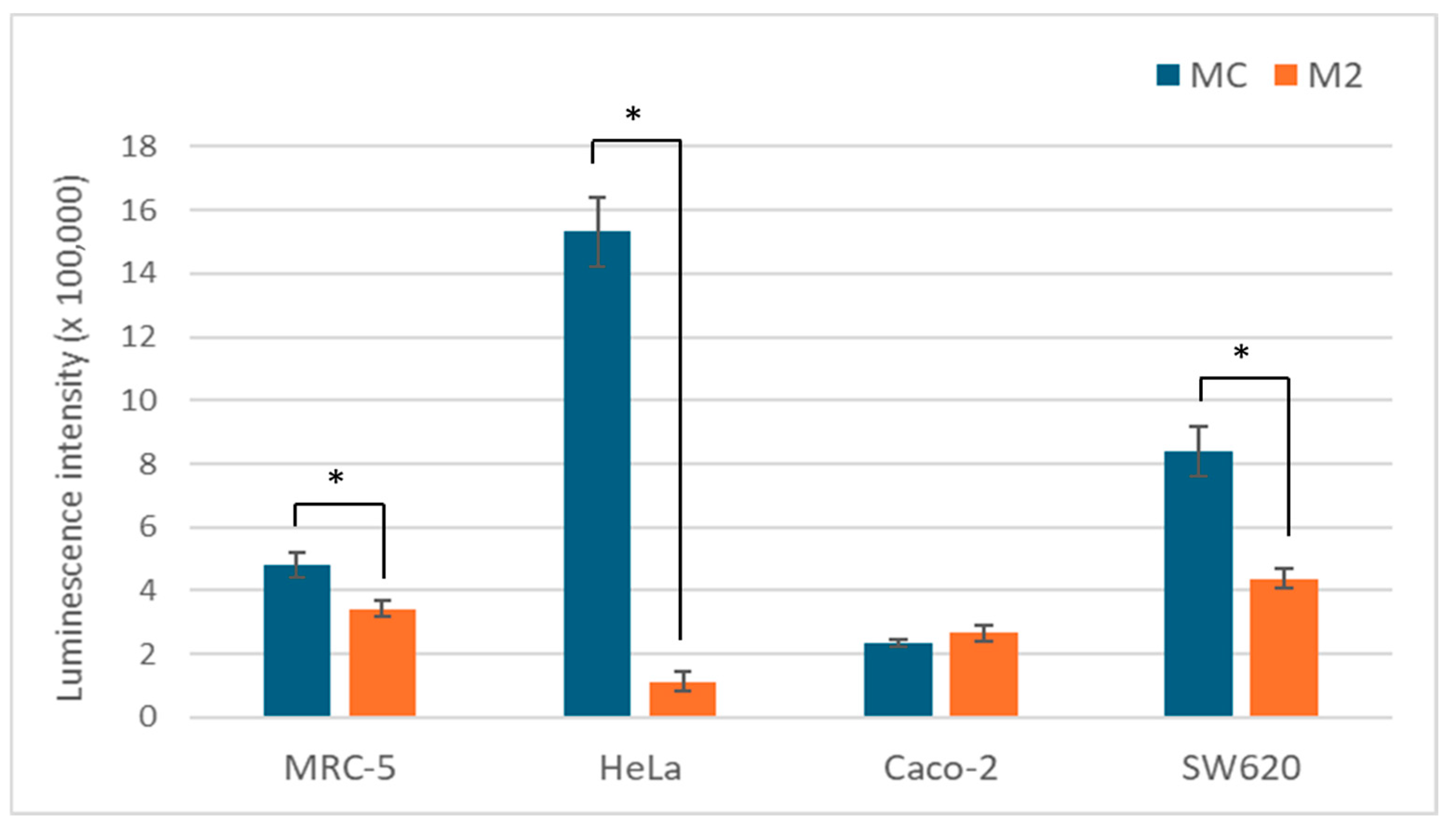
3.5. ATP
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | AMP-Activated Protein Kinase |
| ATP | Adenosine Triphosphate |
| ATPlite | ATP Luminescence Assay Kit |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DRP1 | Dynamin-Related Protein 1 |
| ETC | Electron Transport Chain |
| FBS | Foetal Bovine Serum |
| LDH | Lactate Dehydrogenase |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| NAD+ | Nicotinamide Adenine Dinucleotide (oxidized form) |
| PBS | Phosphate Buffered Saline |
| PINK1 | PTEN-Induced Kinase 1 |
| ROS | Reactive Oxygen Species |
| ULK1 | Unc-51-Like Kinase 1 |
References
- Segeritz, C.-P.; Vallier, L. Cell Culture. In Basic Science Methods for Clinical Researchers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–172. ISBN 978-0-12-803077-6. [Google Scholar]
- Yao, T.; Asayama, Y. Animal-cell Culture Media: History, Characteristics, and Current Issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in Cell Culture Media: Stability and Stabilization Strategies. Biotech. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Subbiahanadar Chelladurai, K.; Selvan Christyraj, J.D.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Chellathurai Vasantha, N.; Selvan Christyraj, J.R.S. Alternative to FBS in Animal Cell Culture-An Overview and Future Perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, S.Y.; Yun, S.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Choi, J.S.; Kim, G.-D.; Joo, S.T.; Choi, I.; et al. Review of the Current Research on Fetal Bovine Serum and the Development of Cultured Meat. Food Sci. Anim. Resour. 2022, 42, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, C.R.; McCahill, K.A.; Rops, J.G.; Dufour, J.M.; Russell, K.A.; Koch, T.G. A Review of Fetal Bovine Serum in the Culture of Mesenchymal Stromal Cells and Potential Alternatives for Veterinary Medicine. Front. Vet. Sci. 2022, 9, 859025. [Google Scholar] [CrossRef]
- Koobotse, M.O.; Schmidt, D.; Holly, J.M.P.; Perks, C.M. Glucose Concentration in Cell Culture Medium Influences the BRCA1-Mediated Regulation of the Lipogenic Action of IGF-I in Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8674. [Google Scholar] [CrossRef]
- Men, X.; Wang, H.; Li, M.; Cai, H.; Xu, S.; Zhang, W.; Xu, Y.; Ye, L.; Yang, W.; Wollheim, C.B.; et al. Dynamin-Related Protein 1 Mediates High Glucose Induced Pancreatic Beta Cell Apoptosis. Int. J. Biochem. Cell Biol. 2009, 41, 879–890. [Google Scholar] [CrossRef]
- Cao, M.; Jiang, J.; Du, Y.; Yan, P. Mitochondria-Targeted Antioxidant Attenuates High Glucose-Induced P38 MAPK Pathway Activation in Human Neuroblastoma Cells. Mol. Med. Rep. 2012, 5, 929–934. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Pan, Z.; Xu, J.; Jiang, T.; Zhang, L.; Zhu, K.; Zhang, D.; Song, J.; Sheng, C.; et al. The Inhibition of PINK1/Drp1-Mediated Mitophagy by Hyperglycemia Leads to Impaired Osteoblastogenesis in Diabetes. iScience 2025, 28, 111519. [Google Scholar] [CrossRef]
- Yen, B.L.; Wang, L.-T.; Wang, H.-H.; Hung, C.-P.; Hsu, P.-J.; Chang, C.-C.; Liao, C.-Y.; Sytwu, H.-K.; Yen, M.-L. Excess Glucose Alone Depress Young Mesenchymal Stromal/Stem Cell Osteogenesis and Mitochondria Activity within Hours/Days via NAD+/SIRT1 Axis. J. Biomed. Sci. 2024, 31, 49. [Google Scholar] [CrossRef]
- Kim, D.-H. Contrasting Views on the Role of AMPK in Autophagy. BioEssays 2024, 46, 2300211. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, D.-H.; Kim, D.-H. Redefining the Role of AMPK in Autophagy and the Energy Stress Response. Nat. Commun. 2023, 14, 2994. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, D.-H. A Paradigm Shift: AMPK Negatively Regulates ULK1 Activity. Autophagy 2024, 20, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Li, F.; Wan, X.; Li, Z.; Zhou, L. High Glucose Inhibits Autophagy and Promotes the Proliferation and Metastasis of Colorectal Cancer through the PI3K/AKT/mTOR Pathway. Cancer Med. 2024, 13, e7382. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Qiu, J.; Zheng, Q.; Meng, X. Hyperglycemia and Chemoresistance in Breast Cancer: From Cellular Mechanisms to Treatment Response. Front. Oncol. 2021, 11, 628359. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.I. Advancing Diabetes Management: Exploring Pancreatic Beta-Cell Restoration’s Potential and Challenges. World J. Gastroenterol. 2024, 30, 4339–4353. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Varano, M.; Mallozzi, C.; Gaddini, L.; Villa, M.; Gabrielli, S.; Formisano, G.; Pricci, F.; Malchiodi-Albedi, F. Primary Retinal Cultures as a Tool for Modeling Diabetic Retinopathy: An Overview. BioMed Res. Int. 2015, 2015, 364924. [Google Scholar] [CrossRef]
- Hattangady, N.; Rajadhyaksha, M. A Brief Review of in Vitro Models of Diabetic Neuropathy. Int. J. Diab Dev. Ctries. 2009, 29, 143. [Google Scholar] [CrossRef]
- Hoang, V.T.; Trinh, Q.-M.; Phuong, D.T.M.; Bui, H.T.H.; Hang, L.M.; Ngan, N.T.H.; Anh, N.T.T.; Nhi, P.Y.; Nhung, T.T.H.; Lien, H.T.; et al. Standardized Xeno- and Serum-Free Culture Platform Enables Large-Scale Expansion of High-Quality Mesenchymal Stem/Stromal Cells from Perinatal and Adult Tissue Sources. Cytotherapy 2021, 23, 88–99. [Google Scholar] [CrossRef]
- Feng, T.; Xu, X.; Wang, X.; Tang, W.; Lu, Y. PGRN Protects against Serum Deprivation-Induced Cell Death by Promoting the ROS Scavenger System in Cervical Cancer. Cell Death Dis. 2024, 15, 889. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 6, pdb-prot095497. [Google Scholar] [CrossRef]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The Usefulness of Lactate Dehydrogenase Measurements in Current Oncological Practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Rashid, M.; Coombs, K.M. Serum-reduced Media Impacts on Cell Viability and Protein Expression in Human Lung Epithelial Cells. J. Cell. Physiol. 2019, 234, 7718–7724. [Google Scholar] [CrossRef] [PubMed]
- Petrović, D.J.; Jagečić, D.; Krasić, J.; Sinčić, N.; Mitrečić, D. Effect of Fetal Bovine Serum or Basic Fibroblast Growth Factor on Cell Survival and the Proliferation of Neural Stem Cells: The Influence of Homocysteine Treatment. Int. J. Mol. Sci. 2023, 24, 14161. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Cardoso, P.E.S.; Diniz, E.A.; Rahhal, J.G.; Sipert, C.R. Different Concentrations of Fetal Bovine Serum Affect Cytokine Modulation in Lipopolysaccharide-Activated Apical Papilla Cells in Vitro. J. Appl. Oral. Sci. 2023, 31, e20230020. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Yang, I.-P.; Tsai, H.-L.; Huang, C.-W.; Lu, C.-Y.; Miao, Z.-F.; Chang, S.-F.; Juo, S.-H.H.; Wang, J.-Y. High Blood Sugar Levels Significantly Impact the Prognosis of Colorectal Cancer Patients through Down-Regulation of microRNA-16 by Targeting Myb and VEGFR2. Oncotarget 2016, 7, 18837–18850. [Google Scholar] [CrossRef]
- Mosaad, H.; Shalaby, S.M.; Mahmoud, N.M.; Ahmed, M.M.; Fayed, A.; Ashour, H.R.; Sarhan, W. LncRNA ANRIL Promotes Glucose Metabolism and Proliferation of Colon Cancer in a High-Glucose Environment and Is Associated with Worse Outcome in Diabetic Colon Cancer Patients. Asian Pac. J. Cancer Prev. 2024, 25, 1371–1381. [Google Scholar] [CrossRef]
- Huschtscha, L.; Rozengurt, E.; Bodmer, W.F. Growth Factor Requirements of Human Colorectal Tumour Cells: Relations to Cellular Differentiation. Eur. J. Cancer Clin. Oncol. 1991, 27, 1680–1684. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, Y.; Zhang, D.; Lian, S.; Liang, H.; Ye, Y.; Xie, R.; Li, S.; Chen, J.; Xue, X.; et al. Metastatic Cancer Cells Compensate for Low Energy Supplies in Hostile Microenvironments with Bioenergetic Adaptation and Metabolic Reprogramming. Int. J. Oncol. 2018, 53, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tollefsbol, T.O. p16INK4a Suppression by Glucose Restriction Contributes to Human Cellular Lifespan Extension through SIRT1-Mediated Epigenetic and Genetic Mechanisms. PLoS ONE 2011, 6, e17421. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Reyes, A.; Romero-Garcia, S.; Prado-Garcia, H. Metabolic Responses of Lung Adenocarcinoma Cells to Survive under Stressful Conditions Associated with Tumor Microenvironment. Metabolites 2024, 14, 103. [Google Scholar] [CrossRef]
- Steenson, S.; Shojaee-Moradie, F.; Lovegrove, J.A.; Umpleby, A.M.; Jackson, K.G.; Fielding, B.A. Dose Dependent Effects of Fructose and Glucose on de Novo Palmitate and Glycerol Synthesis in an Enterocyte Cell Model. Mol. Nutr. Food Res. 2022, 66, 2100456. [Google Scholar] [CrossRef]
- Iurlaro, R.; Püschel, F.; León-Annicchiarico, C.L.; O’Connor, H.; Martin, S.J.; Palou-Gramón, D.; Lucendo, E.; Muñoz-Pinedo, C. Glucose Deprivation Induces ATF4-Mediated Apoptosis through TRAIL Death Receptors. Mol. Cell. Biol. 2017, 37, e00479-16. [Google Scholar] [CrossRef]
- Costa, C.F.; Pinho, S.A.; Pinho, S.L.C.; Miranda-Santos, I.; Bagshaw, O.; Stuart, J.; Oliveira, P.J.; Cunha-Oliveira, T. Mitochondrial and Metabolic Remodeling in Human Skin Fibroblasts in Response to Glucose Availability. bioRxiv 2021. bioRxiv:2021.02.24.432508. [Google Scholar] [CrossRef]
- Visagie, M.H.; Mqoco, T.V.; Liebenberg, L.; Mathews, E.H.; Mathews, G.E.; Joubert, A.M. Influence of Partial and Complete Glutamine-and Glucose Deprivation of Breast-and Cervical Tumorigenic Cell Lines. Cell Biosci. 2015, 5, 37. [Google Scholar] [CrossRef]
- Circu, M.L.; Maloney, R.E.; Aw, T.Y. Low Glucose Stress Decreases Cellular NADH and Mitochondrial ATP in Colonic Epithelial Cancer Cells: Influence of Mitochondrial Substrates. Chem.-Biol. Interact. 2017, 264, 16–24. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Isaac, K.S.; Combe, M.; Hume, S.; Sokolenko, S. Re-Imagining Human Cell Culture Media: Challenges, Innovations, and Future Directions. Biotechnol. Adv. 2025, 81, 108564. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, F.; Hückelhoven-Krauss, A.; Kunz, A.; Jiang, G.; Sauer, T.; Reichman, A.; Neuber, B.; Böpple, K.; Schmitt, A.; Müller-Tidow, C.; et al. Impact of Serum-free Media on the Expansion and Functionality of CD19.CAR T-cells. Int. J. Mol. Med. 2023, 52, 58. [Google Scholar] [CrossRef] [PubMed]


| Medium | Glucose Concentration (g/L) | FBS |
|---|---|---|
| MC * | 4.5 | 10% |
| M1 | 1 | 5% |
| M2 | 1 | 10% |
| M3 | 1 | 15% |
| M4 | 4.5 | 5% |
| M5 | 4.5 | 15% |
| MC | M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|---|
| MRC-5 | 4.24 (±0.84) | 4.53 (±0.76) | 4.36 (±0.87) | 3.97 (±0.99) | 3.98 (±0.65) | 4.47(±0.81) |
| HeLa | 1.68 (±0.55) | 1.62 (±0.38) | 1.72 (±0.50) | 1.90 (±0.79) | 1.75 (±0.29) | 1.82 (±0.82) |
| Caco-2 | 4.44 (±0.59) | 5.29 (±0.51) | 4.96 (±0.58) | 4.85 (±0.57) | 4.47 (±0.64) | 4.46 (±0.82) |
| SW-620 | 2.25 (±0.54) | 3.43 (±0.89) | 2.77 (±0.81) | 2.42 (±0.80) | 2.29 (±0.60) | 2.07 (±0.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leventić, M.; Mišković Špoljarić, K.; Vojvodić, K.; Kovačević, N.; Obradović, M.; Opačak-Bernardi, T. The Response of Cell Cultures to Nutrient- and Serum-Induced Changes in the Medium. Sci 2025, 7, 105. https://doi.org/10.3390/sci7030105
Leventić M, Mišković Špoljarić K, Vojvodić K, Kovačević N, Obradović M, Opačak-Bernardi T. The Response of Cell Cultures to Nutrient- and Serum-Induced Changes in the Medium. Sci. 2025; 7(3):105. https://doi.org/10.3390/sci7030105
Chicago/Turabian StyleLeventić, Marijana, Katarina Mišković Špoljarić, Karla Vojvodić, Nikolina Kovačević, Marko Obradović, and Teuta Opačak-Bernardi. 2025. "The Response of Cell Cultures to Nutrient- and Serum-Induced Changes in the Medium" Sci 7, no. 3: 105. https://doi.org/10.3390/sci7030105
APA StyleLeventić, M., Mišković Špoljarić, K., Vojvodić, K., Kovačević, N., Obradović, M., & Opačak-Bernardi, T. (2025). The Response of Cell Cultures to Nutrient- and Serum-Induced Changes in the Medium. Sci, 7(3), 105. https://doi.org/10.3390/sci7030105






