The Detection of Different Cancer Types Using an Optimized MoS2-Based Surface Plasmon Resonance Multilayer System
Abstract
1. Introduction
2. Methodology
2.1. Numerical Framework
- The sensitivity enhancement regarding the baseline sensors after/before analyte adsorption:
- The sensitivity to the refractive index after analyte adsorption:where is the angle shift variation and is the refractive index variation (dimensionless).
- The detection accuracy (DA) is expressed in terms of and FWHM (in degrees) as:
- The quality factor (QF) is denoted in terms of and FWHM:
- The figure of merit (FoM) is expressed as:where shows the lowest normalized reflection value from the SPR curve.
- The limit of detection (LoD) is calculated as:
- The comprehensive sensitivity factor (CSF) ratio is computed based on Ref. [33] (and reference inside):represents the maximum reflectance before deep resonance. The investigation is conducted with a data sampling of points. Notably, all simulations assume TM-polarized light, as required for surface plasmon excitation at the metal–dielectric interface.
2.2. Systems and Initial Parameters
3. Results and Discussion
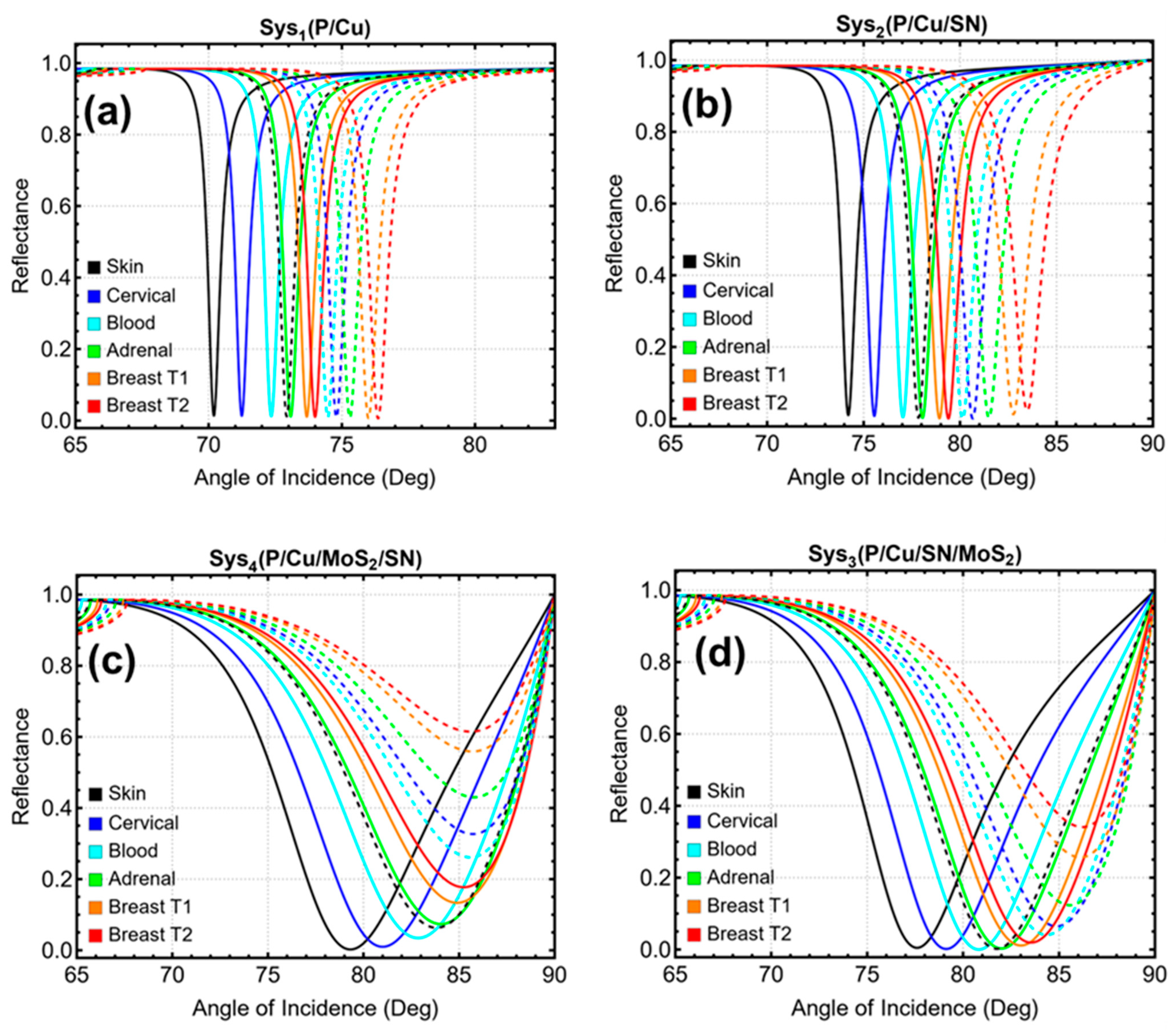
3.1. Systems Under Consideration
3.2. Cooper Optimization
3.3. Silicon Nitride Optimization
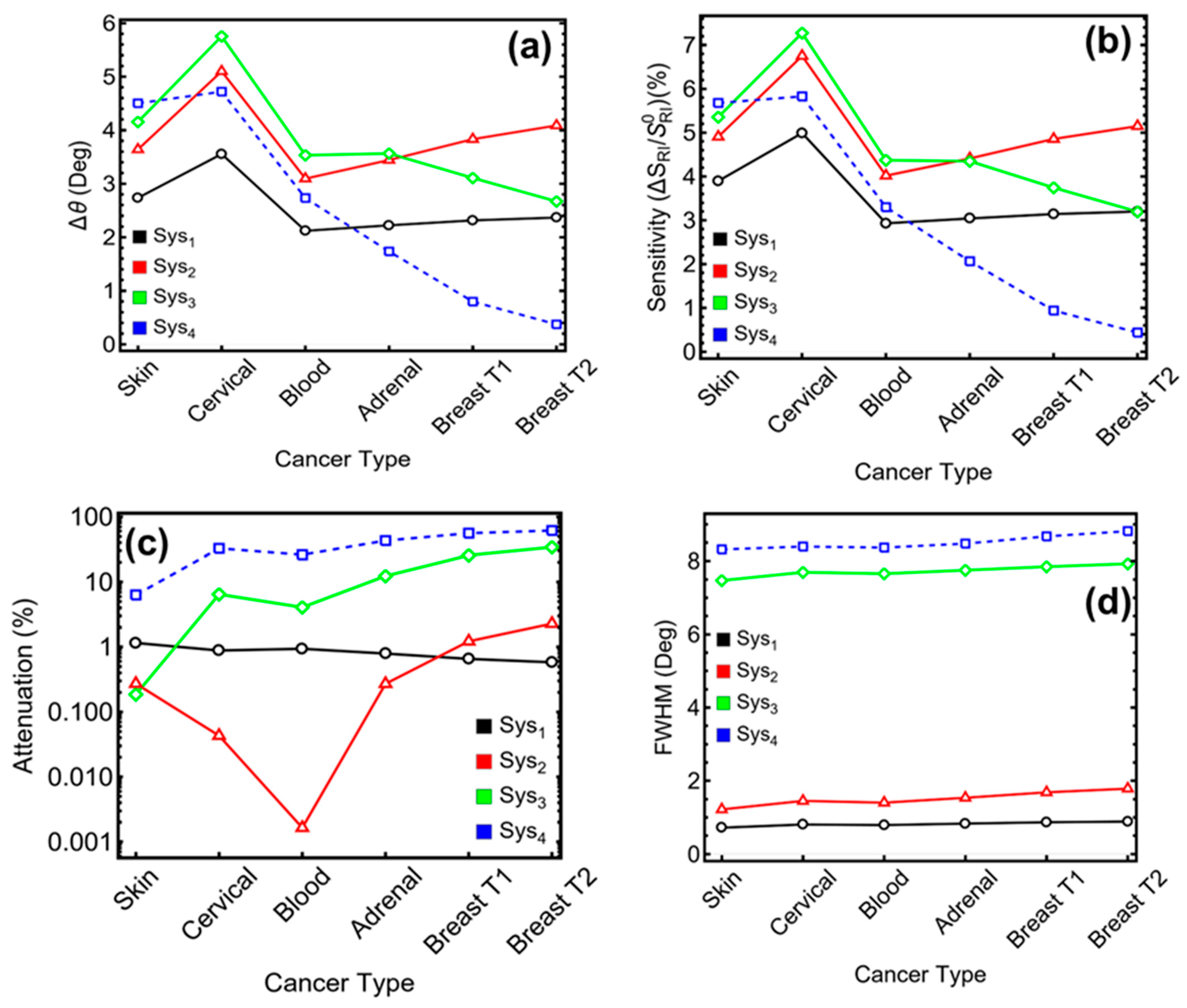
- Sys2 achieves Δθ = 3.7°, sensitivity = 5.4%, attenuation = 1.1%, and FWHM = 0.96°.
- Sys3 records Δθ = 4.0°, sensitivity = 5.6%, attenuation = 12.9%, and FWHM = 5.36°.
- Sys4 offers Δθ = 3.9°, sensitivity = 5.5%, attenuation = 19.8%, and FWHM = 5.06°.
3.4. Molybdenum Disulfide Optimization
3.5. Optimized Parameters and Cancer Samples Tested
3.6. Cancer Detection
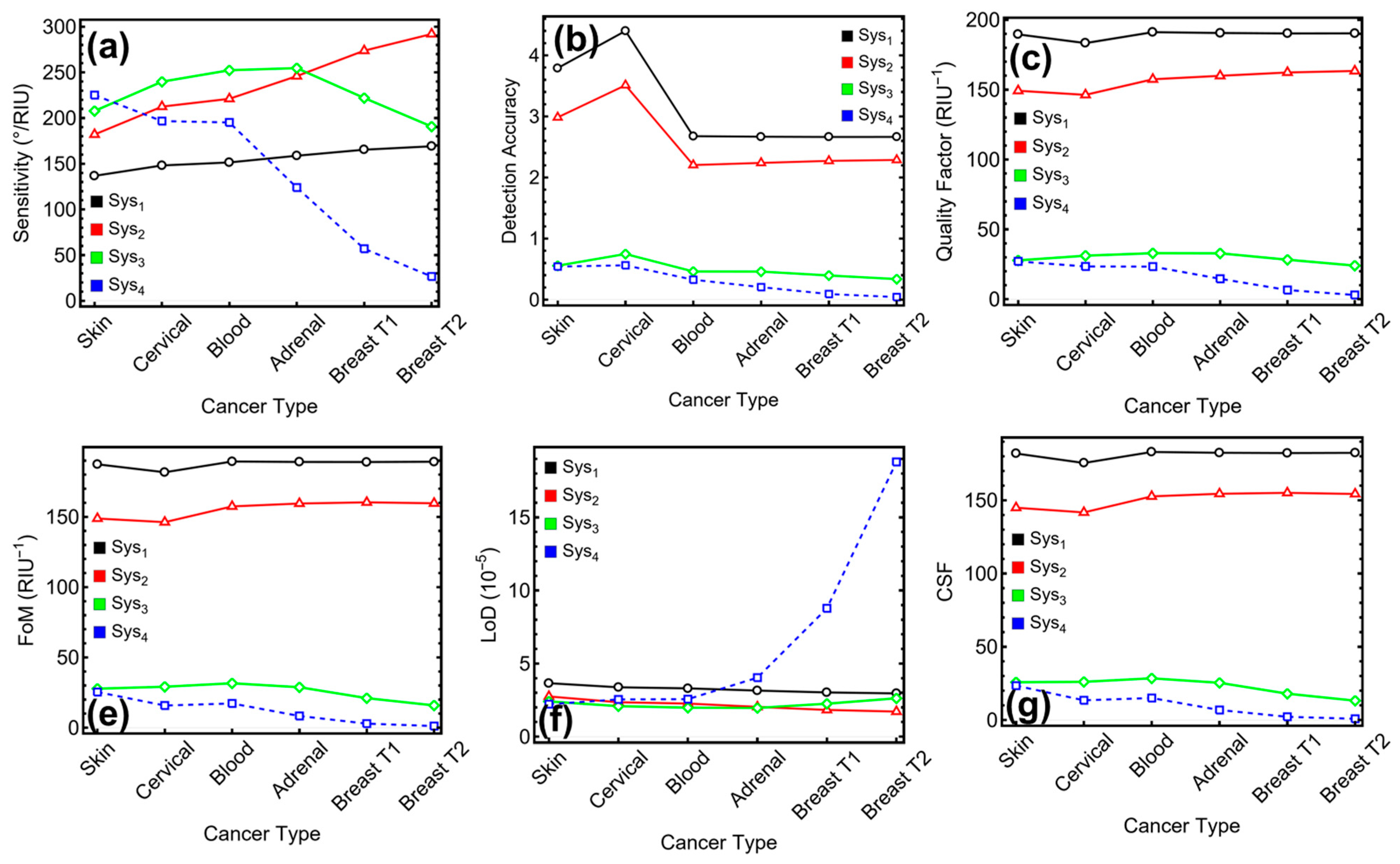
3.7. Performance Metrics of the Biosensor
3.8. Literature Comparison
3.9. Potential Fabrication of the Proposed Biosensors
- BK7 glass substrates can be cleaned using piranha solution, rinsed with deionized water, and dried under nitrogen.
- A Cu film (45–55 nm) can be deposited via thermal evaporation or sputtering under high vacuum. Immediate processing is recommended to minimize oxidation.
- A 7 nm Si3N4 film can be deposited by low-temperature PECVD or ALD, depending on the configuration.
- Few-layer MoS2 can be transferred using a PMMA-assisted wet transfer method from CVD-grown wafers, followed by PMMA removal and gentle annealing.
- Optional thermal annealing (~150 °C, inert atmosphere) may be applied to improve interfacial quality and reduce transfer residues.
- AFM, ellipsometry, and Raman spectroscopy may be used to validate layer thickness and material integrity prior to SPR interrogation.
3.10. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, L.-Y.; Zhao, J.-Q.; Zou, T.-T.; Xu, Z.-H.; Lv, Y. Cervical Cancer Burden and Attributable Risk Factors across Different Age and Regions from 1990 to 2021 and Future Burden Prediction: Results from the Global Burden of Disease Study 2021. Front. Oncol. 2025, 15, 1541452. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, H.; Lian, M.; He, Q.; Lv, M.; Zhai, L.; Zhou, J.; Wu, K.; Yi, M. Global Status and Attributable Risk Factors of Breast, Cervical, Ovarian, and Uterine Cancers from 1990 to 2021. J. Hematol. Oncol. 2025, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhong, Y.; Han, L.; Xie, Y.; Wan, M. Global, Regional, and National Trends in the Burden of Melanoma and Non-Melanoma Skin Cancer: Insights from the Global Burden of Disease Study 1990–2021. Sci. Rep. 2025, 15, 5996. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Kumar, V.; Kumar, D. Molecular Mechanism of Genetic, Epigenetic, and Metabolic Alteration in Lung Cancer. Med. Oncol. 2025, 42, 61. [Google Scholar] [CrossRef]
- Esmaeili, A.; Awasthi, P.; Tabaee, S. Beyond Immortality: Epstein–Barr Virus and the Intricate Dance of Programmed Cell Death in Cancer Development. Cancer Treat. Res. Commun. 2025, 43, 100880. [Google Scholar] [CrossRef]
- Piscone, A.; Gorini, F.; Ambrosio, S.; Noviello, A.; Scala, G.; Majello, B.; Amente, S. Targeting the 8-oxodG Base Excision Repair Pathway for Cancer Therapy. Cells 2025, 14, 112. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast Cancer: Pathogenesis and Treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Wu, P.; Huang, X.; Ma, D. Advances in Cervical Cancer: Current Insights and Future Directions. Cancer Commun. 2025, 45, 77–109. [Google Scholar] [CrossRef]
- Hossain, R.A.; Murshedul, I.M.; Fuad, A.A.M.; Sagline, M.M.; Zihad, M.M.; Nurul, A.M.; Ashiq, M.M.; Peng, L. Overview of Skin Cancer Types and Prevalence Rates across Continents. Cancer Pathog. Ther. 2025, 3, 89–100. [Google Scholar] [CrossRef]
- Singh, A.; Hammer, M.M.; Byrne, S.C. Incidentally Detected Adrenal Nodules on Lung Cancer Screening CT. J. Am. Coll. Radiol. 2025, 22, 291–296. [Google Scholar] [CrossRef]
- Lue, J.C.; Radisky, D.C. From Embryogenesis to Senescence: The Role of Mammary Gland Physiology in Breast Cancer Risk. Cancers 2025, 17, 787. [Google Scholar] [CrossRef] [PubMed]
- Rosko, A.E.; Huang, Y.; Wall, S.A.; Mims, A.; Woyach, J.; Presley, C.; Williams, N.O.; Stevens, E.; Han, C.J.; Von Ah, D.; et al. Predictive Ability of the Cancer and Aging Research Group Chemotherapy Toxicity Calculator in Hematologic Malignancy. J. Geriatr. Oncol. 2025, 16, 102144. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L. Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration. Biomolecules 2025, 15, 444. [Google Scholar] [CrossRef] [PubMed]
- Karthiga, R.; Narasimhan, K.; Raju, N.; Amirtharajan, R. Automatic Approach for Breast Cancer Detection Based on Deep Belief Network Using Histopathology Images. Multimed. Tools Appl. 2025, 84, 4733–4750. [Google Scholar] [CrossRef]
- Fadel, E.F.; Tolba, M.E.M.; Ahmed, A.M.; El-Hady, H.A. Serological and Molecular Detection of Toxoplasma gondii among Cancer Patients in Sohag, Upper Egypt: A Case–Control Study. Sci. Rep. 2025, 15, 5236. [Google Scholar] [CrossRef]
- Sadr, S.; Rahdar, A.; Pandey, S.; Hajjafari, A.; Soroushianfar, M.; Sepahvand, H.; Sasani, B.; Salimpour Kavasebi, S.; Borji, H. Revolutionizing Cancer Detection: Harnessing Quantum Dots and Graphene-Based Nanobiosensors for Lung and Breast Cancer Diagnosis. BioNanoScience 2024, 15, 111. [Google Scholar] [CrossRef]
- Butt, M.A. Surface Plasmon Resonance-Based Biodetection Systems: Principles, Progress and Applications—A Comprehensive Review. Biosensors 2025, 15, 35. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Fan, H.; Liu, Z.; Donati, P.; Amin, Y.; Yap, W.F.; Liang, J.; Pompa, P.P.; He, S. Progress in Surface Plasmon and Other Resonance Biosensors for Biomedical Applications. Adv. Mater. Technol. 2025, 2500536. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Bhat, A.A.; Singh, M.; Li, K. Recent Advances of Guided Mode Resonant Sensors Applied to Cancer Biomarker Detection. Photonics 2025, 12, 424. [Google Scholar] [CrossRef]
- Abul Rub, F.; Moursy, N.; Alhedeithy, N.; Mohamed, J.; Ifthikar, Z.; Elahi, M.A.; Mir, T.A.; Rehman, M.U.; Tariq, S.; Alabudahash, M.; et al. Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer. Biosensors 2025, 15, 203. [Google Scholar] [CrossRef]
- Sangeetha, P.; Ayyanar, N.; Prabhakar, G.; Rajaram, S. Study Review of Optical Biosensors Based on 2D Materials. Plasmonics 2025. [CrossRef]
- Ashrafi, T.M.S.; Mohanty, G. Surface Plasmon Resonance Sensors: A Critical Review of Recent Advances, Market Analysis, and Future Directions. Plasmonics 2025. [CrossRef]
- Ebnonnasir, A.; Narayanan, B.; Kodambaka, S.; Ciobanu, C.V. Tunable MoS₂ Bandgap in MoS₂–Graphene Heterostructures. Appl. Phys. Lett. 2014, 105, 031603. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.; Naylor, C.H.; Ee, H.-S.; Park, J.; Johnson, A.T.C.; Agarwal, R. Strong Exciton–Plasmon Coupling in MoS₂ Coupled with Plasmonic Lattice. Nano Lett. 2016, 16, 1262–1269. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Naylor, C.H.; Lee, B.; Zheng, B.; Liu, G.; Johnson, A.T.C.; Pan, A.; Agarwal, R. Understanding the Different Exciton–Plasmon Coupling Regimes in Two-Dimensional Semiconductors Coupled with Plasmonic Lattices: A Combined Experimental and Unified Equation of Motion Approach. ACS Photonics 2018, 5, 192–200. [Google Scholar] [CrossRef]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-Dimensional Layered MoS₂ Biosensors Enable Highly Sensitive Detection of Biomolecules. Sci. Rep. 2014, 4, 7352. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Cutting-Edge MoS₂-Based Biosensing Platforms for Detecting Contaminants in Food Samples. TrAC Trends Anal. Chem. 2025, 188, 118239. [Google Scholar] [CrossRef]
- Ngan, D.T.T.; Thuy, V.T.; Van Tuan, D.; Dien, N.D.; Tam, P.D. MoS₂/Ag Composite-Based Biosensor with Improved Sensitivity and Selectivity for Glucose Detection. J. Electron. Mater. 2025, 54, 3981–3993. [Google Scholar] [CrossRef]
- Tene, T.; Bellucci, S.; Vacacela Gomez, C. SPR Biosensor Based on Bilayer MoS₂ for SARS-CoV-2 Sensing. Biosensors 2025, 15, 21. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Guang, Z.; Hu, J.; Zhao, F.; Liu, Y.; Shao, L. MoS₂ Surface Plasmon Resonance Based High-Resolution THz Biosensor Using a Dual D-Shaped Channel Micro-Structured Fiber. Opt. Laser Technol. 2025, 180, 111387. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly Sensitive Graphene Biosensors Based on Surface Plasmon Resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Svozilík, J.; Colcha, D.; Cevallos, Y.; Vinueza-Naranjo, P.G.; Vacacela Gomez, C.; Bellucci, S. The Tunable Parameters of Graphene-Based Biosensors. Sensors 2024, 24, 5049. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Coello-Fiallos, D.; Palacios Robalino, M.L.; Londo, F.; Vacacela Gomez, C. The Effect of MoS₂ and Si₃N₄ in Surface Plasmon Resonance Biosensors for HIV DNA Hybridization Detection: A Numerical Study. Micromachines 2025, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mishra, M.; Sahoo, N.; Tripathy, S.K. Highly Sensitive Nitride-Based SPR Biosensor for Efficient Adrenal Gland/Blood/Breast/Cervical/Skin Cancer Detection. Sens. Bio-Sens. Res. 2024, 45, 100684. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Srivastava, S.K. Silicon Nitride-BP-Based Surface Plasmon Resonance Highly Sensitive Biosensor for Virus SARS-CoV-2 Detection. Plasmonics 2022, 17, 1065–1077. [Google Scholar] [CrossRef]
- Lu, X.; Yu, X.; Zhou, J.; Chang, M.; Lu, D. An Ultra-Wide Range D-Shaped Fiber SPR Sensor with a Nanostructure of Gold–MoS2 and Sodium for the Simultaneous Measurement of Refractive Index and Temperature. Sensors 2025, 25, 377. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Romero, P.; Guapi, A.; Gahramanli, L.; Vacacela Gomez, C. SARS-CoV-2 Detection by Surface Plasmon Resonance Biosensors Based on Graphene-Multilayer Structures. Front. Phys. 2024, 12, 1503400. [Google Scholar] [CrossRef]
- Akib, T.B.A.; Rana, M.M.; Mehedi, I.M. Multi-Layer SPR Biosensor for In-Situ Amplified Monitoring of the SARS-CoV-2 Omicron (B.1.1.529) Variant. Biosens. Bioelectron. 2024, 16, 100434. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.-F. Surface Plasmon Resonance Biosensor Performance Analysis on 2D Material Based on Graphene and Transition Metal Dichalcogenides. ECS J. Solid State Sci. Technol. 2020, 9, 115023. [Google Scholar] [CrossRef]
- Panda, A.; Pukhrambam, P.D. Modeling of High-Performance SPR Refractive Index Sensor Employing Novel 2D Materials for Detection of Malaria Pathogens. IEEE Trans. NanoBiosci. 2022, 21, 312–319. [Google Scholar] [CrossRef]
- Park, Y.-L.; Chen, B.-R.; Wood, R.J. Design and Fabrication of Soft Artificial Skin Using Embedded Microchannels and Liquid Conductors. IEEE Sens. J. 2012, 12, 2711–2718. [Google Scholar] [CrossRef]
- Tene, T.; Tubon-Usca, G.; Tixi Gallegos, K.; Mendoza Salazar, M.J.; Vacacela Gomez, C. MoS₂-Based Biosensor for SARS-CoV-2 Detection: A Numerical Approach. Front. Nanotechnol. 2024, 6, 1505751. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chen, Y.-H.; Darius, E.; Shi, H.-F.; Yeh, C.-H.; Hsu, J.-Y.; Liu, K.-K. Two-Dimensional MoS₂ Field-Effect Biosensor for Highly Sensitive Detection of Cardiac Troponin I. ACS Appl. Mater. Interfaces 2025, 17, 30740–30746. [Google Scholar] [CrossRef] [PubMed]
- Crowson, A.N. Basal Cell Carcinoma: Biology, Morphology and Clinical Implications. Mod. Pathol. 2006, 19 (Suppl. 2), S127–S147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-Dimensional Printing of HeLa Cells for Cervical Tumor Model In Vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, P.R.C.; Wang, X.-B.; Huang, Y.; Becker, F.F. Dielectrophoretic Separation of Cancer Cells from Blood. IEEE Trans. Ind. Appl. 1997, 33, 670–678. [Google Scholar] [CrossRef]
- Jockenhövel, F.; Hoermann, R.; Mann, K. Tumors of the Adrenals. Onkologie 1995, 18, 317–323. [Google Scholar] [CrossRef]
- Russo, J.; Hu, Y.-F.; Yang, X.; Russo, I.H. Chapter 1: Developmental, Cellular, and Molecular Basis of Human Breast Cancer. J. Natl. Cancer Inst. Monogr. 2000, 2000, 17–37. [Google Scholar] [CrossRef]
- Gee, M.S.; Upadhyay, R.; Bergquist, H.; Alencar, H.; Reynolds, F.; Maricevich, M.; Weissleder, R.; Josephson, L.; Mahmood, U. Human Breast Cancer Tumor Models: Molecular Imaging of Drug Susceptibility and Dosing during HER2/neu-Targeted Therapy. Radiology 2008, 248, 925–935. [Google Scholar] [CrossRef]
- Karki, B.; Sarkar, P.; Dhiman, G.; Srivastava, G.; Kumar, M. Platinum Diselenide and Graphene-Based Refractive Index Sensor for Cancer Detection. Plasmonics 2024, 19, 953–962. [Google Scholar] [CrossRef]
- Tene, T.; Arias, F.A.; Guamán-Lozada, D.F.; Guadalupe Alcoser, M.A.; Gahramanli, L.; Vacacela Gomez, C.; Bellucci, S. Advanced SPR-Based Biosensors for Potential Use in Cancer Detection: A Theoretical Approach. Sensors 2025, 25, 2685. [Google Scholar] [CrossRef]
- Schranghamer, T.F.; Sharma, M.; Singh, R.; Das, S. Review and comparison of layer transfer methods for two-dimensional materials for emerging applications. Chem. Soc. Rev. 2021, 50, 11032–11054. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Meenakshi, G.A.; Vinothini, S.; Yu, C.L.; Chen, C.L.; Chiu, T.W.; Vittayakorn, N. A Review of Thin-Film Growth, Properties, Applications, and Future Prospects. Processes 2025, 13, 587. [Google Scholar] [CrossRef]
- Sindona, A.; Pisarra, M.; Bellucci, S.; Tene, T.; Guevara, M.; Vacacela Gomez, C. Plasmon oscillations in two-dimensional arrays of ultranarrow graphene nanoribbons. Phys. Rev. B 2019, 100, 235422. [Google Scholar] [CrossRef]
- Ryou, J.; Kim, Y.S.; Kc, S.; Cho, K. Monolayer MoS2 bandgap modulation by dielectric environments and tunable bandgap transistors. Sci. Rep. 2016, 6, 29184. [Google Scholar] [CrossRef] [PubMed]
- Khakbaz, P.; Waldhoer, D.; Bahrami, M.; Knobloch, T.; Pourfath, M.; Davoudi, M.R.; Zhang, Y.; Gao, X.; Peng, H.; Waltl, M. Two-dimensional Bi2SeO2 and Its Native Insulators for Next-Generation Nanoelectronics. ACS Nano 2025, 19, 9788–9800. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Enhanced adsorption sites in monolayer MoS2 pyramid structures for highly sensitive and fast hydrogen sensor. Int. J. Hydrogen Energy 2020, 45, 9268–9277. [Google Scholar] [CrossRef]

| Sys No. | Code | Full Name | Nick Name |
|---|---|---|---|
| 0 | Sys0 | Prism/Copper/PBS | P/Cu/PBS |
| 1 | Sys1 | Prism/Copper/Cancer Sample | P/Cu/MCancer |
| 2 | Sys2 | Prism/Copper/Si3N4 | P/Cu/SN/MCancer |
| 3 | Sys3 | Prism/Copper/Si3N4/Molybdenum disulfide/Cancer Sample | P/Cu/SN/MoS2/MCancer |
| 4 | Sys4 | Prism/Copper/Molybdenum disulfide/Si3N4/Cancer Sample | P/Cu/MoS2/SN/MCancer |
| Material | Refractive Index | Thickness (nm) | Refs. |
|---|---|---|---|
| BK-7 (P) | 1.5151 | --- | [37] |
| Copper (Cu) | 0.0369 + 4.5393i | 45.0 | [38] |
| Si3N4 (SN) | 2.0394 | 5.00 | [35] |
| Molybdenum disulfide (MoS2) | 5.0805 + 1.1723i | 0.65 | [39,40,41] |
| PBS (M) | 1.335 | --- | [34] |
| Cancer Sample () | 1.349 | --- | [34] |
| Material | Refractive Index (RI) | Thickness (nm) |
|---|---|---|
| Sys1 | ||
| BK7 (P) | 1.5151 | --- |
| Cu | 0.0369 + 4.5393 | 55.0 |
| Sys2 | ||
| BK7 (P) | 1.5151 | --- |
| Cu | 0.0369 + 4.5393 | 55.0 |
| Si3N4 (SN) | 2.0394 | 7.0 |
| Sys3 | ||
| BK7 (P) | 1.5151 | --- |
| Cu | 0.056253 + 4.2760 | 45.0 |
| Si3N4 (SN) | 2.0394 | 7.0 |
| Molybdenum disulfide (MoS2) | 5.0805 + 1.1723 i | 0.65 * L (L = 2) |
| Sys4 | ||
| BK7 (P) | 1.5151 | --- |
| Cu | 0.0369 + 4.5393 | 45.0 |
| Molybdenum disulfide (MoS2) | 5.0805 + 1.1723 i | 0.65 * L (L = 3) |
| Si3N4 (SN) | 2.0394 | 7.0 |
| Cancer Type | Cell Line | Refractive Index (Normal) | Refractive Index (Cancerous) | Reported Concentration/Ratio | Specificity/Detection Method Description |
|---|---|---|---|---|---|
| Breast Cancer (Type 1) | MDA-MB-231 | 1.368 | 1.397 | 80% cancer cells | SPR detection based on refractive index modulation induced by MDA-MB-231 morphology; label-free physical interaction |
| Breast Cancer (Type 2) | MCF-7 | 1.368 | 1.401 | 80% cancer cells | SPR response enhanced by multilayer design; detects RI shift induced by MCF-7 cell optical profile |
| Cervical Cancer | HeLa | 1.368 | 1.392 | 80% cancer cells | SPR configuration using Si3N4; monitors HeLa cell-induced RI variations without surface binding agents |
| Skin Cancer | Basal cells | 1.368 | 1.382 | 80% cancer cells | SPR reflectance profile adjusted to identify basal-cell-induced RI changes in a non-functionalized setting |
| Adrenal Cancer | PC-12 | 1.368 | 1.385 | 80% cancer cells | SPR simulation of RI shift due to PC-12 cell morphology; detection through physical adsorption effects only |
| Blood Cancer | Jurkat/JM | 1.368 | 1.389 | 80% cancer cells | Detection via RI perturbation from Jurkat/JM cells; no biochemical tags or functionalization involved |
| Cancer Type | ) | DA | QF (RIU−1) | FoM (RIU−1) | LoD (10−5) | CSF |
|---|---|---|---|---|---|---|
| Sys1 | ||||||
| Skin | 136.750 | 3.791 | 189.560 | 187.376 | 3.656 | 181.93 |
| Cervical | 148.125 | 4.399 | 183.328 | 181.712 | 3.375 | 175.48 |
| Blood | 151.429 | 2.676 | 191.165 | 189.373 | 3.301 | 182.98 |
| Adrenal | 158.750 | 2.667 | 190.569 | 189.062 | 3.149 | 182.44 |
| Breast T1 | 165.357 | 2.663 | 190.250 | 189.004 | 3.023 | 182.25 |
| Breast T2 | 169.107 | 2.664 | 190.316 | 189.211 | 2.956 | 182.39 |
| Sys2 | ||||||
| Skin | 182.000 | 2.984 | 149.216 | 148.810 | 2.747 | 144.92 |
| Cervical | 212.500 | 3.510 | 146.277 | 146.214 | 2.352 | 141.72 |
| Blood | 221.071 | 2.203 | 157.428 | 157.425 | 2.261 | 152.68 |
| Adrenal | 245.893 | 2.238 | 159.916 | 159.483 | 2.033 | 154.46 |
| Breast T1 | 273.750 | 2.272 | 162.305 | 160.324 | 1.826 | 155.09 |
| Breast T2 | 291.964 | 2.287 | 163.384 | 159.653 | 1.712 | 154.33 |
| Sys3 | ||||||
| Skin | 207.750 | 0.556 | 27.808 | 27.757 | 2.406 | 25.77 |
| Cervical | 239.792 | 0.747 | 31.163 | 29.149 | 2.085 | 26.06 |
| Blood | 252.321 | 0.461 | 32.954 | 31.611 | 1.981 | 28.45 |
| Adrenal | 254.643 | 0.459 | 32.851 | 28.799 | 1.963 | 25.39 |
| Breast T1 | 221.964 | 0.395 | 28.283 | 21.035 | 2.252 | 17.96 |
| Breast T2 | 190.714 | 0.336 | 24.060 | 15.862 | 2.621 | 13.19 |
| Sys4 | ||||||
| Skin | 225.125 | 0.541 | 27.082 | 25.371 | 2.220 | 23.44 |
| Cervical | 196.667 | 0.562 | 23.439 | 15.782 | 2.542 | 13.45 |
| Blood | 195.179 | 0.326 | 23.340 | 17.244 | 2.561 | 15.00 |
| Adrenal | 123.929 | 0.204 | 14.630 | 8.338 | 4.034 | 6.81 |
| Breast T1 | 56.964 | 0.092 | 6.571 | 2.898 | 8.777 | 2.18 |
| Breast T2 | 26.607 | 0.042 | 3.019 | 1.163 | 18.791 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Vique López, D.F.; Valverde Aguirre, P.E.; Monge Moreno, A.M.; Vacacela Gomez, C. The Detection of Different Cancer Types Using an Optimized MoS2-Based Surface Plasmon Resonance Multilayer System. Sci 2025, 7, 76. https://doi.org/10.3390/sci7020076
Tene T, Vique López DF, Valverde Aguirre PE, Monge Moreno AM, Vacacela Gomez C. The Detection of Different Cancer Types Using an Optimized MoS2-Based Surface Plasmon Resonance Multilayer System. Sci. 2025; 7(2):76. https://doi.org/10.3390/sci7020076
Chicago/Turabian StyleTene, Talia, Diego Fabián Vique López, Paulina Elizabeth Valverde Aguirre, Adriana Monserrath Monge Moreno, and Cristian Vacacela Gomez. 2025. "The Detection of Different Cancer Types Using an Optimized MoS2-Based Surface Plasmon Resonance Multilayer System" Sci 7, no. 2: 76. https://doi.org/10.3390/sci7020076
APA StyleTene, T., Vique López, D. F., Valverde Aguirre, P. E., Monge Moreno, A. M., & Vacacela Gomez, C. (2025). The Detection of Different Cancer Types Using an Optimized MoS2-Based Surface Plasmon Resonance Multilayer System. Sci, 7(2), 76. https://doi.org/10.3390/sci7020076








