Abstract
Aging is a complex biological and physiological change that leads to a loss of function in all living organisms. Although the mechanism behind the aging process is still largely unknown, scientific studies have shown that oxidative stress and age-related low autophagy, which are associated with various chronic diseases such as cancer, diabetes, cardiovascular diseases, and neurodegenerative diseases, promote aging. Interestingly, many medicinal plants and their biologically active compounds have the ability to extend lifespan as they can inhibit oxidative stress and promote autophagy. This review evaluates and provides up-to-date information on the anti-aging potential of bioactive compounds in edible medicinal plants. In this study, seventeen (17) biologically active compounds from edible medicinal plants with anti-aging effects were reviewed. In vivo and in vitro studies showed that these biologically active compounds exhibit anti-aging effects via various mechanisms such as the activation of autophagy, increases in antioxidant enzymes, reductions in reactive oxygen species, the inhibition of inflammatory markers, and the downregulation of senescence genes. This study suggests that edible medicinal plants containing these bioactive compounds may promote health and extend lifespan. However, the exact mechanisms, effective doses, clinical trials, and chronic and genotoxic effects of bioactive compounds as anti-aging agents should be further investigated.
1. Introduction
Aging is an irreversible and unavoidable biological phenomenon that is characterized by the impairment of physiological functions, ultimately leading to the development of chronic diseases and the death of living organisms [1,2]. This intricate process involves various biochemical and genetic signaling pathways, including the mammalian target of rapamycin, insulin-like growth factor-1, factor forkhead box O-3, nuclear factor kappa B, nuclear factor erythroid 2-related factor 2, and sirtuins, all of which play important roles in determining lifespan and the onset of age-related diseases [3,4]. Globally, healthy aging is a glorious adventure. According to estimates by World Population Prospects [5], approximately 994 million (12%) and 1.6 billion (16%) people worldwide will be aged 65 years and older by 2030 and 2050, respectively. However, aging is associated with a multitude of chronic diseases, such as cardiovascular diseases, dementia, musculoskeletal disorders, cancer, diabetes, glaucoma, and neurodegenerative diseases [6,7,8,9]. These diseases pose a significant threat to life and can have profound psychological and economic impacts on both the affected individuals and their families [7]. Although the exact molecular mechanisms underlying the aging process remain largely unknown, scientific research has indicated that lifestyle, genetic factors, dietary habits, oxidative stress, genomic instability, decreased age-related autophagy, and cellular senescence play a crucial role in its regulation [9,10,11]. In particular, oxidative stress has been identified as one of the main factors in the development of many chronic diseases associated with aging. Consequently, strategies aimed at delaying or attenuating these factors, particularly oxidative stress, have great promise for promoting healthy aging.
Research studies have provided evidence that edible medicinal plants contain a variety of bioactive compounds that have the potential to reduce the risk of chronic diseases, delay the aging process, and extend lifespans [2,12]. These plants serve as a rich and renewable source of bioactive phytochemicals, i.e., non-nutritive components that confer protective properties to plants. Edible medicinal plants, including fruits and vegetables, are particularly rich in these bioactive compounds. Consequently, these plants represent an invaluable reservoir of innovative pharmacological substances that can be utilized in the development of novel drugs and bioactive foods.
Notably, conventional drugs used in the treatment of diseases, such as artemisinin and quinine for combating malaria, vinblastine for cancer therapy, morphine and codeine for use as opioid analgesics, and aspirin for use as a non-steroidal anti-inflammatory drug, are derived from various medicinal plants [13,14,15]. These phytochemicals can be classified into different groups, including polyphenols (flavonoids, phenolic acids, lignans, stilbenes, and tannins) [2,16], carotenoids (carotenes and xanthophylls) [17], phytosterols (sterols and stanols) [18], and terpenoids (monoterpenoids, diterpenoids, hemiterpenoids, triterpenoids, tetraterpenoids, sesquiterpenoids, polyterpenoids, and sesterterpenoids) [2,19]. Through various mechanisms such as oxidative stress suppression, the regulation of age-related genes, immunomodulation, apoptosis regulation, autophagy regulation, cellular senescence suppression, and the regulation of mitochondrial function and biogenesis [2,3,7,12], these bioactive compounds exhibit anti-aging effects and contribute to lifespan extension.
Scientific investigations have provided evidence that bioactive compounds possess remarkable properties, counteracting the aging process in different model organisms [20,21,22,23]. For instance, Kim et al. [20] demonstrated that quercetin effectively mitigates the senescence of vascular smooth muscle cells induced by H2O2 through the regulation of apoptosis via the AMPK signaling pathway. Similarly, Liu et al. [21] discovered that lycopene inhibits oxidative stress and apoptosis in the ovaries of aging hens by activating the Nrf2/HO-1 signaling pathway in hens with D-galactose-induced aging. The therapeutic advantages of the various common compounds derived from natural sources have been extensively examined in the treatment of chronic ailments. These compounds include epigallocatechin gallate from green tea, resveratrol from grapes, chlorogenic acid from carrots and apples, curcumin from turmeric, and quercetin from sage and onions [22,23]. In light of this context, this article presents a comprehensive review of seventeen biologically active compounds found in edible medicinal plants. These compounds, namely, chlorogenic acid, caffeic acid, quercetin, rutin, anthocyanins, resveratrol, β-carotene, lycopene, lutein, diosgenin, β-sitosterol, epigallocatechin gallate, curcumin, ellagic acid, luteolin, fisetin, and kaempferol, have been extensively investigated for their potential to promote healthy aging and prolong lifespan (Figure 1). This review covers the edible sources of these medicinal plants, the chemical constituents of the bioactive compounds, their mechanisms of action, and in vitro and in vitro anti-aging activities in various experimental models. The significance of this research lies in its emphasis on identifying the bioactive compounds found in medicinal plants and their anti-aging potentials.
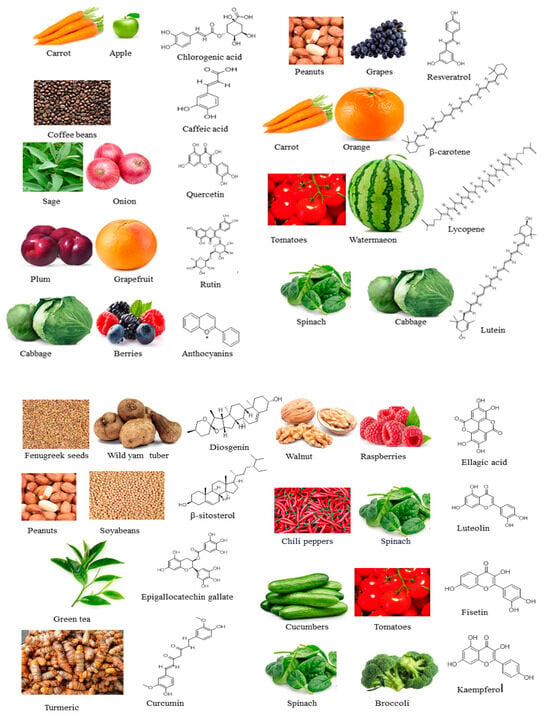
Figure 1.
Edible medicinal plants: bioactive compounds with anti-aging potentials found in them and their chemical structures.
2. Materials Used for the Study
The literature materials utilized in this current investigation were sourced from various databases, namely, Springer, Sciencedirect, Frontiers, Wiley, PubMed, and MDPI. The articles utilized in this study spanned from January 2002 to March 2024. The search terms used include “anti-aging” and “bioactive compounds”, with the addition of specific compounds such as chlorogenic acid, caffeic acid, quercetin, rutin, anthocyanins, resveratrol, β-carotene, lycopene, lutein, diosgenin, β-sitosterol, epigallocatechin gallate, curcumin, ellagic acid, luteolin, fisetin, and kaempferol. Only papers published in the English language were considered for inclusion in this study. The mechanisms of action for the selected bioactive compounds against anti-aging were detailed in Table 1. The chemical structures of the selected bioactive compounds were obtained from the PubChem and NIST Chemistry databases, and subsequently they were redrawn using ChemDraw software (version 12.0.2), as shown in Figure 1.
3. Proposed Mechanism of Action of the Bioactive Compounds
Various signaling pathways have been identified as regulators of aging and age-related diseases. These pathways include insulin-like growth factor-1 (IGF-1), the mammalian target of rapamycin (mTOR), the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), factor forkhead box O-3 (FOXO3), nuclear factor erythroid 2-related factor 2 (Nfr2), and sirtuins (SIRT) [3,4]. Recent research suggests that reducing insulin/IGF-1 signaling can extend life expectancy and improve health by increasing stress resistance (Figure 2). In situations where there is a deficiency of nutrients, insulin production and IGF-1 signaling are reduced, leading to the activation of transcription factor proteins called FOXO. These proteins then stimulate the production of various protective proteins, such as superoxide dismutase, catalase, glutathione S-transferase, and chaperones [24,25,26]. These proteins play a critical role in protecting cells and delaying the aging process by neutralizing reactive oxygen species (ROS), repairing DNA damage, maintaining protein structure, and detoxifying heavy metals. Interestingly, a specific variant of the foxo3 gene has been linked to a longer lifespan in humans, suggesting that the activation of FOXO transcription factors also has anti-aging effects in humans [27,28].
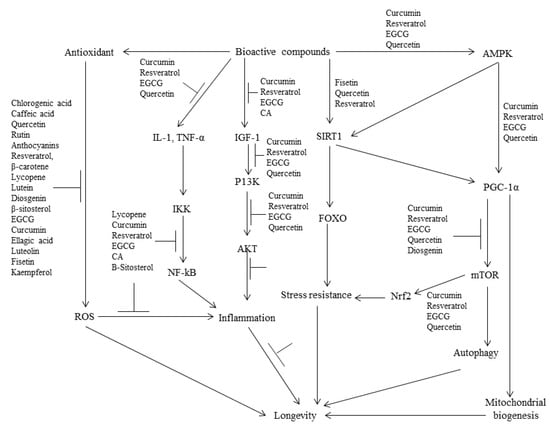
Figure 2.
Proposed mechanisms of the anti-aging effects of the bioactive compounds isolated from edible medicinal plants.
Recent studies have revealed that quercetin has a positive impact on the lifespan of worms. Specifically, it has been observed that quercetin can increase both the average and median lifespan of worms by 18% and 21%, respectively. However, it does not seem to affect the maximum lifespan of these organisms [29]. Interestingly, the life-extending effect of quercetin is not observed in worms that lack the daf-2 gene, which encodes a receptor similar to the human IGF-1 receptor. On the other hand, the mutation of the daf-16 gene does not impede the life-prolonging effects of quercetin, suggesting that other pathways may be involved in mediating these effects on lifespan [29]. In a separate study conducted by Baur et al. [30], the potential of resveratrol, a phenolic compound found in grapes, blueberries, and red wine, to extend the lifespan of mice fed on a high-calorie diet was demonstrated. The researchers observed that resveratrol improved insulin sensitivity and reduced IGF-1 levels in the blood, indicating a decrease in the insulin/IGF-1 signaling pathway. Additionally, the substance exerted positive effects on motor and balance functions as the performance of treated mice in athletic challenges improved over time. Resveratrol was also found to mitigate the age-related degeneration of cognitive function, blood vessels, and bone. However, it is important to note that resveratrol did not extend the lifespan of mice that were not fed a high-calorie or high-fat diet, as evidenced by studies conducted by Pearson et al. [31] and Miller et al. [32].
A recent study conducted by Mao et al. [33] demonstrated that the lifespan of aged female mice was extended by 9% through the targeting of the IGF-1 receptor using a monoclonal antibody. This intervention not only reduced inflammation but also inhibited tumor formation. However, the effectiveness of interventions targeting IGF-1 to promote longevity in humans has yet to be determined. mTOR, a kinase responsible for regulating cell growth, metabolism, and nutrient recognition, is suppressed in conditions of amino acid or glucose deficiency, such as during fasting, calorie restriction, and prolonged exercise. This suppression of mTOR halts cell growth in order to maintain existing nutrient and energy levels [34]. On the other hand, autophagy plays a vital role in sustaining nutrient and energy levels by eliminating damaged molecules and organelles, thereby rejuvenating cells and tissues [35,36]. The involvement of mTOR and autophagy in the aging process is supported by various observations. The inhibition of mTOR is mediated by AMPK, as depicted in Figure 2. The activation of AMPK not only enhances ATP production by promoting lipid oxidation but also inhibits ATP-consuming pathways involved in liver gluconeogenesis, such as the biosynthesis of new molecules. Furthermore, AMPK activation stimulates the expression of genes associated with lipid oxidation and mitochondrial biogenesis through the activation of PGC-1α [37]. In a study conducted on mice fed on a high-calorie diet, resveratrol was found to extend lifespan by increasing AMPK activity. This increase in AMPK activity subsequently led to an elevation in PGC-1α activity and the generation of new mitochondria [30]. Other natural compounds, such as epigallocatechin gallate (EGCG) found in green tea and curcumin extracted from turmeric, have also been shown to activate AMPK [38,39].
There are natural compounds, including fisetin and quercetin that have demonstrated the ability to inhibit mTOR and potentially induce autophagy [40,41,42]. The inhibition of mTOR is widely recognized as an effective mechanism for extending lifespan, making these natural compounds and their derivatives promising candidates for the development of anti-aging nutraceuticals.
Sirtuins, on the other hand, play a critical role in regulating various cellular metabolic pathways, thereby influencing lifespan and aging. One notable sirtuin is sirtuin-1, which functions by deacetylating and activating PGC-1α. This activation leads to an increase in fatty acid oxidation and mitochondrial biogenesis, both of which are crucial for cellular function [43]. Additionally, sirtuin-1 also deacetylates FOXO proteins, resulting in their activation (Figure 2).
This activation, in turn, stimulates the transcription of proteins that enhance stress resistance and ultimately contribute to a longer lifespan [44]. Furthermore, specific polyphenolic compounds found in edible medicinal plants, such as fisetin, quercetin, and resveratrol, have been shown to activate sirtuin-1 (Figure 2). These compounds have the potential to modulate sirtuin activity and potentially influence lifespan and aging [45].
Moreover, it has been suggested that the process of aging is primarily attributed to a persistent imbalance between reactive oxygen species (ROS) and antioxidants, commonly referred to as oxidative stress. This imbalance ultimately leads to cellular senescence, functional alterations, and the onset of various diseases, such as cardiovascular disease, diabetes, and cancer [16,46]. Polyphenols possess the ability to act as exogenous antioxidants, effectively mitigating the harmful impacts of reactive oxygen species (ROS) through various mechanisms. One such mechanism involves the direct elimination of ROS owing to the presence of phenolic hydroxyl groups on the polyphenol molecules. In addition, polyphenols can augment cellular antioxidant activity by modulating the NF-kB- and Nrf2-mediated pathways (Figure 2). Several polyphenols, including EGCG, luteolin, and curcumin, have been identified as potent inhibitors of ROS [16].
4. Bioactive Compounds
4.1. Chlorogenic Acid
Chlorogenic acid (CGA) is a polyphenol found in vegetables, coffee, fruits, and tea and has been associated with some of the beneficial effects found in the consumption of these substances [47,48,49]. In most cases, these beneficial properties are associated with aging, such as the improvement in cognitive function and protection from neurodegenerative conditions such as Parkinson and Alzheimer’s disease [50,51]. According to the research carried out by Zheng et al. [52], investigating the direct impact of CGA on the aging process, the potential of the bioactive compounds to extend the lifespan of the organism Caenorhabditis elegans was examined. The nematode was chosen as an excellent model for this observation due to its amenability to genetic manipulation and suitability to testing for the ability of a compound to prolong lifespan. By treating the wild-type worms with different concentrations of CGA (0–2000 µM) through a lifespan assay, a concentration of 50 µM was observed as extending the adult mean lifespan of the worms by 20.1%. In addition, aging has been established as a contributing factor to the decrease in body movement observed in nematodes [53]. However, treating the worms with CGA caused a significant delay in the decline of body movement [52]. Further investigation into the molecular mechanism responsible for the role of CGA in lifespan extension and delaying age-dependent decline in body movement revealed that CGA modulates the insulin/insulin-like signaling pathway through the DAF-16 transcription factor.
In a similar experiment, Siswanto et al. [54] demonstrated that the treatment of Hep3B and HeLa cell lines with a concentration of 20 and 40 µM of CGA led to a significant increase in the lifespan of Caenorhabditis elegans. However, lower doses of about 4–10 µM did not achieve the same result. In their research, the increase in lifespan was associated with the induction of SKN-1. In previous studies, SKN-1 and DAF-16 have been found to protect the organism from stress and also promote longevity [55]. In yeast, CGA obtained from almond skin extract was found to improve the lifespan of the organism [56]. Using a CGA concentration of 25 µM, the chronological lifespan of the yeast cells was improved by 0.08 days.
Through the administration of 20 or 40 mg/kg of CGA to mice that were 17 months old for a period of two weeks, Hada et al. [57] examined the effect of the bioactive compound in terms of regulating vascular changes in the cell. By using saline and angiotensin as negative and positive controls, respectively, the research examined the in vivo effect of CGA on vascular senescence. Using the SA-β-gal assay, the aorta of the mice was examined for the phenotype of senescence in the presence and absence of CGA. The result of the experiment showed that angiotensin- and H2O2-induced senescence was suppressed in mice treated with CGA in a dose-dependent manner [57]. The suitable concentration was observed to be between 0.5 µM and 1.0 µM because, at a concentration of about 5.0 µM, CGA was found to be toxic, leading to cell damage and reduced cell proliferation. In addition, through an in vitro analysis, increased expression levels of SIRT1 and eNOS, which are known to be senescence-related markers, were found in Human Umbilical Vein Endothelial Cells (HUVECs) incubated with CGA [57].
Based on the relationship between aging and certain health conditions such as dementia, Kato et al. [58] examined the capacity of CGA to improve cognitive function. In their experiment, 28 elderly individuals who lived in communities were placed on a 6-month intake of beverage which contained 330 mg of CGA dissolved in 100 mL of water. Through a cognitive assessment and an examination of central nervous system vital signs after the period of nutritional intervention, the result showed a significant improvement in cognitive function. Specific factors such as verbal memory, attention, cognitive flexibility, motor speed, and executive function were shown to have improved significantly. To ascertain these effects in the short term, Suzukamo et al. [59] examined the cognitive response of individuals who were placed on an active beverage containing 270 mg of CGA for two weeks. A comparison of baseline to end-point values showed that CNS vital signs, such as psychomotor speed and motor speed, improved drastically in an individual that had the CGA beverage. Similarly, Saitou et al. [60] affirmed the potential of CGA to delay cognitive decline and improve cognitive functions in individuals who complained of subjective memory loss.
In UV-induced fibroblast cells, the anti-aging properties of CGA were observed by treating the cells with CGA for four days [61]. Previous studies have shown a direct relationship between exposure to UV rays and an increase in ROS level in cells resulting in apoptosis [62]. Therefore, the detection assay test kit was used to measure ROS levels in the cells before and after treatment with CGA. In addition, the expression of the COL-3 gene, which is known to be abundant in younger individuals was measured, using a real-time quantitative reverse transcription polymerase chain reaction. In the result obtained, ROS levels in the cells decreased and COL-3 gene expression increased when the models were compared with untreated cells [63]. In a similar experiment using human fibroblasts and keratinocytes exposed to ultraviolet rays, [64] examined the anti-aging effect of CGA. In their experiment, CGA was obtained from a Cecropia obtuse extract and was identified through LC-MS as the major compound in the plant extract. Therefore, using a highest concentration of 20 µg/mL and a lowest concentration of 5 µg/mL, CGA prevented an increase in protein carbonyl content in the cell, which can lead to oxidative damage [65]. Also, treatment of the cells with CGA led to an increase in HA and collagen content and a decrease in MMP-1.
CGA was also recognized as a major compound in the plant Aster koraiensis and its effect on retinal pathogenic neovascularization was examined [66]. In individuals above the age of 65, retinal pathogenic neovascularization has been associated with irreversible vision loss [67]. Therefore, using a mouse model with oxygen-induced retinopathy, Kim et al. [66] observed a significant reduction in retinal vascular changes upon treatment with 25 and 50 mg/kg/day of CGA.
4.2. Quercetin
Quercetin, which is chemically known as 3,5,7,3′,4′-pentahydroxyflavone, is a dietary flavonoid and can be found in certain foods such as red wine, apples, and onions [68,69]. The pharmaceutical properties of quercetin have been attributed to its ability to scavenge free radicals [70]. Quercetin exhibits various biological activities, such as anti-inflammatory, anti-obesity, antioxidant, neuroprotection, antimicrobial, anticancer, and antiviral activity [71]. In addition, the neuroprotective effect of the compound has been observed due to its capacity to protect neurons from the effect of toxic agents and injury, reduce oxidative stress, and modulate certain mechanisms associated with cell death [70,72]. Yang et al. [73] examined the anti-aging effect of quercetin using the organism Simocephalus vetulus. This organism is known for its small size, short lifespan, and the presence of genes, which could be relevant to determining aging in humans. Through proteomic analysis, the research investigated the lifespan and proteins expressed in the organism upon treatment with different concentrations of quercetin. At concentrations of 1 and 2.5 mg/L, a significant increase in the average lifespan of S. vetuculus was recorded. Maximally, a lifespan increase of about 22% was recorded in the group treated with 1 mg/L of quercetin. In addition, the research established the ability of quercetin to increase lifespans, without affecting other growth parameters such as reproduction and intrinsic growth rate. Using human erythrocytes with induced aging, Remigante et al. [74] examined the protective role quercetin could play in minimizing oxidative damage in cells. Having treated the cells with 100 mM of D-Gal for 24 h, which is expected to accelerate aging, the cells were then treated with 10 µM of quercetin for 1 h. Results from the experiment showed the capacity of quercetin to protect the membrane lipids from oxidative damage, which could lead to aging. Previous studies have shown that when erythrocytes age, they are highly susceptible to oxidative damage due to their inability to neutralize reactive oxygen species [75]. Therefore, inducing the red blood cells to have D-galactose negatively impacted lipid peroxidation and increased glycated hemoglobin levels. However, when the cells were pretreated with quercetin, the examination of different markers associated with oxidation and lipid peroxidation showed that the cells were protected from oxidative damage and that lipid peroxidation was ameliorated. In the fruit Phyllanthus emblica, quercetin was found to be an abundant flavonoid and this was attributed to the capacity of the fruit extract to protect against aging in Caenorhabditis elegans [76]. The result of the experiment showed that the lifespan of the worm was extended by about 18.53%. This has been associated with signaling pathways such as SIRT1, which is known to regulate aging-related diseases [77]. By combining quercetin with resveratrol, [78] reported a reduction in aging biomarkers in human kidney cell cultures in a dose-dependent manner, thereby affirming the anti-aging potential of these compounds. Quercetin has also been identified as a senolytic compound due to its ability to reduce the burden of senescent cells and also reduce the expression of pro-inflammatory cytokines [79,80]. Using hydrogen peroxide to induce senescence in pre-adipocytes and adipocyte cells, Zoico et al. [81] reported that the treatment of these cells with quercetin led to a reduction in beta galactosidase activity through the downregulation of miR-155-5p expression and upregulation of the SIRT-1 pathway. While investigating the effect of quercetin on UV-mediated aging, Shin et al. [82] treated human skin tissues with quercetin and irradiated the skin tissues with ultraviolet rays for 10 days. The results of the experiment showed that quercetin suppressed expression of MMP-1, which is associated with wrinkle formation in the skin. Also, inflammatory responses in the skin tissues were attenuated due to a reduction in COX-2 expression, thereby showing that quercetin possesses anti-wrinkle and anti-inflammatory properties.
4.3. Rutin
Rutin is also known as 3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside and it is found in fruits such as apples and in certain plants such as buckwheat, passion flower, and tea. Rutin is a modified version of quercetin that is mostly abundant in fruits and vegetables [83]. Li et al. [84] examined the effect of rutin on age-related metabolic disorders using aged rats. By maintaining the rats on a standard diet and comparing them with those who had 2% rutin incorporated into their diet, Li et al. [84] discovered that rutin significantly inhibited increases in fasting blood glucose, insulin levels, and blood pressure. Also, the IPGTT and IPITT tests that were carried out showed that rutin improved glucose and insulin tolerance in the aged rats. Although the antioxidative and anti-inflammatory properties of rutin were established in previous research [85,86,87], Choi et al. [88] discovered that rutin could reduce skin aging. Using human dermal fibroblasts (HDFs), the experiment showed that rutin was non-toxic to cells at concentrations of less than 100 µM, inhibited cell damage, and protected the cells from hydrogen peroxide-induced oxidative damage and senescence. In addition, through the regulation of enzymes in the extracellular matrix, the treatment of HDF with rutin resulted in an increase in dermal density and elasticity, which helped to reduce skin aging.
Due to cellular senescence being the central hallmark of aging, other studies have also confirmed the ability of rutin to target senescent cells, thereby having the potential to be a senotherapeutic agent. Through the screening of a library of natural medicinal agents derived from plants, Liu et al. [89] observed that rutin could reduce the expression of senescence-associated secretory phenotypes. In another study, Chattopadhay et al. [90] observed that the ability of rutin to extend the lifespan of Drosophila melanogaster was due to its capacity to mediate hormesis. By treating the fruitfly with different concentrations of rutin of about 100–800 µM, the median lifespan of the fruitfly increased at specific concentrations of 200 and 400 µM. Therefore, just like other hormetins, rutin could extend the lifespan of the organism at lower concentrations without resulting in adverse effects. By supplementing the drinking water administered to 8-month-old mice with 0.2 mg/mL of sodium rutin, a 10% lifespan extension was observed in the models [91]. When compared to caffeic acid, rutin was found to exhibit greater anti-aging effects due to its ability to scavenge hydrogen peroxide and inhibit the activities of tyrosinase, elastase, and hyaluronidase. The secretion of these enzymes has been greatly associated with the aging process.
4.4. Anthocyanins
Found in abundance in the flesh, skin, and roots of grains and colored fruits and vegetables such as berries and potatoes, anthocyanins are water-soluble plant pigments known for their beneficial effects on health [92,93]. In addition, research has shown that the consumption of foods rich in anthocyanins may lower the risk of certain chronic illnesses [92]. Due to their known antioxidative potential, the capacity of anthocyanins to delay aging or the progression of age-related diseases has therefore been explored [94,95]. For instance, purple sweet potato extracts, known to contain a high quantity of anthocyanins, have been investigated for their life-prolonging potential in Drosophila melanogaster [96]. In their research, NAME examined the effect of purified purple sweet potato extract on female wild-type D. melanogaster. While the treatment groups were fed with different concentrations of the extract (0.5, 2.0. 5.0. 8.0 and 10 mg/mL), the control group was fed with a basal diet. The lifespan assay that was carried out to the determine maximum lifespan showed that the lifespan of the organism significantly increased from being fed the diet in a concentration-dependent manner. Lifespans of about 2.84%, 10.78%, 16.34%, 18.57%, and 20.46% were recorded when compared to the control. In addition, the extract was non-toxic and no lethal effect was observed in the organism. Aside from the prolonged lifespan, other physical activities such as crawling ability, stress tolerance and intestinal activity were improved in the purple sweet potato extract treatment group when compared to the control group. These beneficial physiological functions of the plant extract have been attributed to the antioxidative effect of anthocyanins, expressed through the activation of the autophagy pathway [97]. Similarly, using concentrations of 0.5 and 2.0 mg/mL of anthocyanin extract from purple sweet potato extracts, Han et al. [98] also reported a similar finding for male Drosophila melanogaster.
Wang et al. [99] examined the effect of cranberry anthocyanin extract on age-related genes in fruit flies. By feeding the fruit flies for 81 days with a diet containing either 5 mg/mL or 20 mg/mL of cranberry anthocyanin extract, the maximum lifespan of the flies was examined against a control group. On average, the lifespan of the fruit flies fed with the cranberry anthocyanin extract was found to increase in a concentration-dependent manner with the 20 mg/mL group, resulting in a 10% increase in lifespan when compared to the control. By quantifying the expression of SOD, MTH, InR, TOR, Hep, and PEPCK genes in the treatment and control group, the real-time PCR experiment showed that this could be attributed to the upregulation of the SOD gene and downregulation of the MTH, InR, TOR, Hep, and PEPCK genes. These findings were found to be consistent with other studies where the upregulation of SOD gene was associated with longevity and where the downregulation of MTH, InR, TOR, Hep and PEPCK was associated with lifespan extension, the delayed onset of age-related diseases, and the management of type 2 diabetes [100,101,102]. Yan et al. [103] discovered that the mulberry anthocyanin extract had the potential to prevent oxidative damage and increase the lifespan of Caenorhabditis elegans. The MTT assay carried out using Hep 2 cells, revealing that the extract was non-toxic and exhibited antioxidative properties through the activation of Nrf2 signalling pathway. This also corresponds to the findings of Li et al. [104], where anthocyanins prepared from the Dendrobium officinale flower were found to increase the lifespan of Caenorhabditis elegans. This was attributed to the upregulation of the SOD gene, which resulted in a 56.25% increase in lifespan of the organism, and was also associated with other factors such as improved resistance to stress.
Liu et al. [105] investigated the effect of blueberry anthocyanin on epithelium cells relating to retinal pigment. These are known for their susceptibility to injury as aging occurs. Using in vitro models with light-induced retinal damage, the blueberry anthocyanin extract was found to be non-toxic. At concentrations of 1 or 10 µg/mL, the blueberry anthocyanin extracts were found to decrease the percentage of senescent cells in the retinal pigment, and they also reduced intracellular ROS levels.
4.5. Resveratrol
Resveratrol is a widely recognized polyphenolic compound found in different plant sources like grape, peanut, and berry fruits [106]. It has gained considerable attention due to its various health benefits, including its potential as an anti-obesity agent, cardioprotective and neuroprotective properties, antitumor activity, antidiabetic effects, antioxidant properties, and anti-aging effects, as well as its role in glucose metabolism. Notably, this compound has shown promising therapeutic properties in various diseases, such as cancer, neurodegeneration, and atherosclerosis. These effects are mediated through the regulation of multiple synergistic pathways that modulate oxidative stress, cell death, and inflammation [106].
Using a mice model with age-related Alzheimer’s disease, Porquet et al. [107] examined the effect of resveratrol in terms of promoting longevity. The SAMP8 mice models used were fed with a resveratrol diet and compared to models fed with a normal standard diet for a period of 7 months. The results from the experiment showed that the models placed on the resveratrol diet had an increased life expectancy of more than 33% when compared to the control group. Previous research has also shown that, in mice that are about 9 months old, cognitive impairment may set in, which can lead to age-related decline [108]. However, Porquet et al. [107] observed that when mice models that were about 9 months old were fed the resveratrol supplement, memory was not impaired. The molecular mechanism behind this cognitive protection, as revealed by the Western blot analysis carried out, showed that SIRT1 and AMPK levels were increased in the models, while p53 acetylation decreased. This aligned with previous reports examining the properties of SIRT1 in different animal models and their capacity to protect against cognitive stress [109,110].
In similar research using silkworms, activation of the SIRT7 pathway, which was induced at different stages of the life cycle, was said to be responsible for the potential of resveratrol to extend lifespans [111]. Song et al. [111] examined the effect of resveratrol on the silkworm Bombyx mori. By treating worms with 500 µM of the solution, a lifespan assay was carried out to ascertain survival. In addition, other factors such as body weight, fecundity, stress tolerance, and level of antioxidants were measured. Results from the experiment showed that the mean and maximum lifespan of the worms treated with resveratrol increased by 3.18% and 2.31%, respectively. Additionally, it was observed that treatment with resveratrol had no side effects on the silkworms, offered protection from environmental stress, and improved the antioxidative properties of the organism.
In the model organism Drosophila melanogaster, exposure to 7.5, 15, 30 and 60 mg/kg concentrations of resveratrol for 7 days led to an increase in lifespan of about 18.6%, 20.9%, 39.5%, and 41.9%, respectively [112]. Further investigation to examine if resveratrol can improve oxidative damage caused by 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) showed that it improves behavioral deficits in flies, such as climbing rates; reverses the accumulation of hydrogen peroxide; and reverses lesions formation due to oxidative damage.
In some age-related conditions, such as oculopathy, which can lead to visual impairment and blindness, mitochondrial dysfunction has been recognized as a major contributing factor [113,114]. Wang et al. [115] examined the effect of resveratrol on mitochondrial quality and function in aging zebra fish retina. By treating the 19–23-month-old zebra fish with 20 mg/L of resveratrol for 10 days, the researchers examined mitochondrial quality and function. The research showed that upon the treatment of the aged zebra fish retina with resveratrol, mitochondrial integrity could be likened to that of a 5-day-old retina. Also, the fusion and fission that took place in the mitochondria were found to improve in the aging zebra fish retina due to resveratrol treatment.
Through genetic modification, a type of rice known as the resveratrol rice has been developed by modifying rice with stilbene synthase, which is known as the resveratrol biosynthase gene [116]. Islam et al. [117] examined the anti-aging effect of resveratrol rice by feeding grains to Drosophila melanogaster. By supplementing the standard corn meal with resveratrol, the lifespan assay carried out revealed that flies whose meals were supplemented with resveratrol had a significantly extended median lifespan in both males and females. Also, locomotive deterioration, which we expected to be evident in aged flies, was ameliorated in the flies that fed on the resveratrol supplemented grains. In addition, other features such as a healthy body weight, the inhibition of eye degeneration, and the amelioration of neurodegeneration were observed in D. melanogaster, thereby affirming the potential of resveratrol as an anti-aging compound.
In a similar experiment, Khan et al. [118] examined the effect of a callus culture of resveratrol rice on the lifespan of Drosophila melanogaster. In their research, the callus culture was established as containing 180 times more resveratrol than the rice itself. Therefore, by maintaining the matured flies in the callus media, a lifespan assay was carried out to examine survivability. The results of the experiment showed that all the fly strains maintained in the callus media had an extended lifespan, with a highest increase of 50% when compared to the control group. Also, as the age of the flies progressed, the deterioration of locomotion was ameliorated in the treatment group.
4.6. Epigallocatechin Gallate
Epigallocatechin gallate, also known as tea catechin due to its primary sources, has been identified for its pharmacological activities and beneficial roles in human health [119,120]. This has been mostly associated with its antioxidative functions and its potential to protect cells against stress-related diseases. As a result, several studies have been carried out to determine the effect of epigallocatechin gallate on aging. Cai et al. [121] examined the in vitro effect of epigallocatechin gallate on the formation of lipofuscin, a pigment known for its role in aging. Using human serum albumin, a concentration of 6 mg/kg of epigallocatechin gallate was compared to a control group where only distilled water was administered. Histological studies carried out in the brain region showed that epigallocatechin gallate had neuroprotective potential, preventing the deposition of lipofuscin.
In preadipocytes, the potential of EGCG to inhibit or eliminate senescence has also been examined [122]. By adopting the use of 3T3-L1 preadipocytes cells with hydrogen peroxide-induced senescence, the effect of 50 and 100 µM of EGCG was assessed. The results from the experiment showed that at both concentrations, EGCG inhibited the development of hydrogen peroxide-induced senescent cells and downregulated the expression of cell-cycle inhibitors. Further research on the underlying mechanism responsible for this result revealed that this inhibition was primarily due to the potential of EGCG to modulate the pro- and anti-apoptotic pathways. This result was similar to the findings of Lilja et al. [123], where the potential of EGCG to delay senescence and other senescence-induced inflammatory processes in 3T3-L1 preadipocytes was compared to that of other compounds. The outcome also showed that at concentrations of 50 and 100 µM, EGCG effectively reduced the secretion of proinflammatory cytokines, thereby affirming its antioxidative and anti-inflammatory potential, which is important in the treatment of metabolic disorders.
Using the model organism Drosophila melanogaster, the potential of EGCG to increase the lifespan of the organism was also discovered [124]. By placing the fruit flies on a diet supplemented with EGCG, a lifespan experiment was carried out and the results were compared with those of another group fed a control diet but with all other parameters being equal. The results of the research showed that there was a significant increase in the lifespan and survival rate of flies whose diet contained an EGCG concentration of 10 mg/mL. In addition, the climbing assay revealed a higher fitness level when compared to the control.
In healthy and obese rats, the potential of EGCG to extend lifespan has also been examined [125,126]. In these experiments, the incorporation of EGCG into the diets of the rat delayed death and also improved other parameters associated with aging, such as serum glucose levels, inflammation, and oxidative stress. The ethanolic extracts of the plant Sclerocarya birrea have also been found to contain EGCG, whose anti-aging potential has been discovered at concentration of about 5 µg/mL using an anti-collagenase assay [127]. Therefore, the plant extract has been recommended for its suitability to be incorporated as an anti-aging ingredient. When combined with hyaluronic acid, EGCG exhibited improved anti-aging potential and protection against skin damage due to UV exposure [128].
4.7. Caffeic Acid/Dihydrocaffeic Acid
Caffeic acid is a phenol and is also known as 3,4-hydroxycinnamic acid. Several in silico studies have confirmed its antioxidative potential [129,130,131]. Found in high concentrations in several plants and food derived from plants such as coffee, olive oil, and wine, caffeic acid is confirmed to be a naturally occurring flavonoid [132].
Girsang et al. [133] examined the anti-aging potential of caffeic acid in an in vitro study using several inhibitory assays such as collagenase, elastase, hyalurodinase, and tyrosinase. Results from the assays revealed that the inhibitory potential of caffeic acid was concentration-dependent, as a higher concentration of the compound resulted in greater inhibition of these compounds which have been associated with skin aging. This was attributed to the potential of caffeic acid to attach to proteins associated with aging [134]. In Drosophila, caffeic acid was discovered to protect against intestinal stem cell aging [135]. By examining the adult stem cells of the Drosophila midgut, Sheng et al. [135] observed that, at an optimal concentration of 0.2 mg/mL, supplementation with caffeic acid delayed the onset of intestinal stem cells and progenitor cells. Also, at the same optimal concentration, caffeic acid prevented an age-related decline in intestinal function in aged Drosophila by regulating the acid–base homeostasis, strengthening intestinal barrier integrity, and increasing the lifespan of the organism.
By synthesizing caffeic acid with a bioactive peptide (APPPKK), Lee et al. [136] examined the effect of the conjugate on skin aging. The caffeic acid and peptide conjugate were found to be biocompatible as the cell viability essay revealed the 90% viability of fibroblast cells, even at a concentration of 100 µM. Using a DPPH antioxidant assay, the research revealed that the caffeic acid and peptide conjugate exhibited anti-aging effects by reducing stress in the cells. In addition, different concentrations of the conjugate ranging from 1 to 100 µM did not result in ROS generation when compared to the control and reduced hydrogen peroxide-induced oxidative stress in the cells.
Also, using young and old fibroblast cells, Okada and Okada [137] studied the effect of quercetin, resveratrol, and caffeic acid on the expression of different aging-related genes. By adopting quantitative real-time PCR analysis, the research reported that these three compounds regulated the aging process in the cells through different mechanisms. Using a multifunctional microfluidic device, Zhang et al. [106] examined the anti-aging potential of caffeic acid phenethylester in Caenorhabditis elegans. Different concentrations of caffeic acid phenethylester were used, such as 20, 50, 100, and 120 µM. At a concentration of 100 µM, the mean lifespan of the worms was increased by 15.8% and the resistance of the worms to heat stress was enhanced at concentrations of 20, 50, and 100 µM. In addition, the survival rate of the worms was found to be highest at 54.3% when a concentration of 50 µM was used. In a similar study, Gutierrez-Zetina et al. [138] reported that 200 µM of caffeic acid increased the survival rate of Caenorhabditis elegans undergoing thermal stress. In addition, the maximum lifespan of the worms was also found to increase, and this was attributed to a reduction in intracellular reactive oxygen species and the activation of genes associated with longevity.
4.8. Lycopene
Lycopene is a carotenoid pigment that is found in abundance in red fruits and vegetables such as tomato, pawpaw, grapefruit, and watermelon [139,140]. It is a compound known for its various bioactivities and its potential as an effective antioxidant has been extensively explored [141,142]. According to this research, inflammation and oxidative stress due to aging are associated with the development of neurodegenerative conditions [143,144]. Therefore, the potential of lycopene to offer protection against conditions such as cancer, diabetes, and inflammatory diseases has been explored [145]. Zhao et al. [146] examined the effect of lycopene, a carotenoid pigment, on cognitive impairment in mice and the associated mechanisms. In their research, behavioral tests, biochemical analysis, and quantitative real-time PCR were carried out to determine the effect of lycopene on aged mice whose diet had been supplemented with lycopene for 2 months. The results from their experiment showed that, in the lycopene-supplemented group, aging-induced spatial working memory was attenuated when compared to the control. Also, other factors were improved, such as age-induced neuronal degeneration, MDA, and BDNF expression, which is associated with learning and memory. In addition, lycopene was discovered to reduce the accumulation of the amyloid beta peptide, which is known for its role in the development of major neurodegenerative conditions such as Alzheimer. This aligns with previous studies which have identified that the antioxidative potential of lycopene could find application in the management of neurodegenerative conditions [147,148,149].
In a similar study, 100 ng/mL of lycopene was found to reduce oxidative stress, thereby ameliorating ovarian aging in aged chicken [21]. The research reported that in chickens who aged naturally and those in whom aging was induced with D-gal, the administration of 100 ng/mL of lycopene for 72 h resulted in the attenuation of oxidative stress and this was confirmed via the activation of the Nrf2/HO-1 pathway. This result aligns with the findings of Rakha et al. [150], where the administration of 200 nm of lycopene to oocytes resulted in less degeneration, thereby minimizing postovulatory aging, which is known to be as a result of oxidative stress.
Considering other compounds that contain lycopene, Blakeslea trispora powder has been found to contain about 1.9% of lycopene and has been associated with anti-aging effects in mice [151]. By administering different concentrations of the powder (0, 267, 534, 1068 mg/kg) for 30 days, Hu et al. [152] observed that antioxidant activities were improved in mice and this was confirmed through biochemical assessments, which revealed the major pathways affected. By combining lycopene with nicotinamide mononucleotide, a compound known for its role in activating anti-aging pathways, Liu et al. [153] examined how the synergistic potential of both compounds can be beneficial to the aging process. Using male rats which were between 6 and 8 weeks old, the research investigated the effect of nicotinamide mononucleotide and lycopene, both individually and when combined, on the learning and memory of the aging models. At optimal concentrations of 100 mg/kg of nicotinamide mononucleotide and 50 mg/kg of lycopene, the oxidation-associated markers were greatly reduced in the cells, thereby preventing oxidative damage and improved cognitive functioning in the aged models. In addition, the combination of both compounds regulated senescence-related genes in the aging models.
4.9. Lutein
Based on the assessment of human diet, lutein has been found to be the most prevalent carotenoid and can be found in foods such as green vegetables, fruits, and egg yolk [154,155]. It is a bioactive compound known for its biological properties such as its antioxidative potential and its capacity to prevent age-related macular degeneration, cardiovascular diseases, and lung cancer [156]. With respect to its anti-aging potential, Zhang et al. [157] investigated the potential effects of lutein on the lifespan of Drosophila melanogaster. From their research, 0.1 mg/mL of lutein increased the mean lifespan of the organism by 11.35% (from 49.0 to 54.6 days). Also, the survival time of the flies and the maximum lifespan were significantly increased. An assessment of the underlying mechanism revealed that the flies that were fed the lutein-supplemented diet had a decrease in malonyldialdehyde levels, and the PCR experiment revealed the upregulation of antioxidant enzymes.
In a randomized, double-blind trial, the photoprotective potential of lutein was investigated by assessing the minimal erythema dose [158]. In their research, NAME assessed the effect of the dietary lutein supplementation of 20 mg per day for 12 weeks on thirty women. The results revealed a 22% increase in photoprotective activity, marked by a significant increase in MED in the test group. In ARPE-19 cells exposed to high concentrations of glucose to induce senescence, the administration of 1 µg/mL of lutein was found to significantly reduce the activity of SA-b-gal, which is known as a biomarker of cellular senescence [159]. Similarly, a study using the same type of cells [160] reported that at concentrations of 1 to 20 µM, cells treated with lutein exhibited a decrease in SA-b-gal activity in a concentration-dependent manner. Consequently, other parameters such as a decrease in the lysosome content of the cells, the restoration of cell cycling, and the regulation of anti-senescence-related proteins confirmed the role of lutein in preventing aging.
4.10. β-Sitosterol
β-sitosterol is a compound with a chemical structure similar to cholesterol; it is a phytosterol found in various parts of plants such as the fruit, leaves, and rhizomes [161]. Generally, in rats and mice, β-sitosterol is known to be non-toxic, even at concentrations as high as 1000 mg/kg. The compound has been identified for its antioxidative, antidiabetic, and anti-inflammatory properties [119,162,163]. To determine its role in aging, Hah et al. [164] examined the effect of β-sitosterol in the catabolism pathway that occurs as a result of age-related muscle loss. Using male mice, the researchers investigated the muscle mass and strength of the models, which is expected to decline with age and may eventually result in loss of independence. By examining grip strength and conducting a treadmill analysis and histological evaluation of muscle tissue, the results from the experiment confirmed that β-sitosterol protected the mice from muscle loss, which was induced by dexamethasone. In addition, grip strength was restored in the group treated with β-sitosterol.
The presence of β-sitosterol has been identified in a traditional Chinese medicine known as Thamnolia vermicularis [165]. Haiyuan et al. [166] analyzed the protective effect of both β-sitosterol and vermicularin, which are both by-products of Thamnolia vermicularis, on skin aging. By coculturing these compounds with human skin fibroblasts and keratinocytes, their biological effects on skin drying and wrinkling were analyzed. The findings from the research showed that β-sitosterol increased the expression of hyaluronic acid synthases, which is essential in the production of hyaluronic acid, known for its role in skin moisture retention. Also, it contributed to the expression of functional proteins in the skin, which could contribute positively to skin aging.
4.11. Curcumin
Curcumin, also known as 1,7-bis (4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione, is a bioactive compound extracted from Curcuma longa, which is more commonly known as turmeric. Curcumin has been identified for its various biological and pharmacological properties [167]. The antioxidative, anti-inflammatory, and neuroprotective functions of curcumin have been found to slow the aging process [168,169]. As a result, several studies have examined its specific role in alleviating aging symptoms, extending lifespan, and delaying the onset of age-related diseases. Lee et al. [170] reported that curcumin had the potential to extend the adult lifespan and modulate the expression of age-related genes in Drosophila melanogaster. Using two different strains of the organism, employing 100 µM and 250 µM of curcumin led to 16% and 19% increases in lifespan, respectively. Also, the lifespan extension effect of curcumin was reported to be dependent on sex and genetic background. However, certain age-related genes, such as mth, thor, INR, and INK, were also found to be modulated by curcumin supplementation. Even in the larval stage of organisms, Soh et al. [171] reported that the supplementation of larval feed with curcumin could extend the minimum and maximum lifespan of the adult fly. By acting as a diet restriction nutraceutical, larval feeding with curcumin was found to increase development time, but also significantly delay the onset of senescence.
Liao et al. [172] examined the effect of curcumin on lifespan extension in Caenorhabditis elegans. Using 20 µM of curcumin, the result from the experiment revealed that curcumin supplementation can extend the maximum adult lifespan of the organism by 17 days and delay the process of aging. This was attributed to the antioxidative potential of the compound through its reduction of intracellular reactive oxygen species. Sun et al. [173] examined the effect of curcumin on hydrogen peroxide-induced senescence in Human Umbilical Vein Endothelial Cells. The results of the experiment showed that pretreatment of the cells with 25 µM curcumin significantly reduced hydrogen peroxide-induced apoptosis in the cells and protected the cells against cell growth arrest. However, at low concentrations from about 0.1 to 1 µM, Grabowska et al. [174] reported the inability of curcumin to delay senescence in the cells.
For six months, Shailaja et al. [175] examined the effect of curcumin on the inflammatory indices of aging in albino Wistar rats. Using curcumin concentrations of 100, 200, and 400 mg/kg, the findings from the experiment showed that antioxidant capacity was maintained, thereby resulting in a significant reduction in anti-aging inflammatory markers. Using Saccharomyces cerevisiae as the experimental model, Stepien et al. [176] examined the effect of curcumin on cell aging. Going by the strain used (BY4741), 200 µM of curcumin had a positive effect on minimum and maximum lifespans. Additionally, the reproductive lifespan of the yeast strain was extended.
4.12. Luteolin
Luteolin, known chemically as 3′,4′,5,7-tetrahydroxy flavone, can be found in different vegetables and fruits such as carrots, blackberries, grapes, blueberries, cabbage, peppers, broccoli, etc. [177]. This bioactive compound is known for its neuroprotective, antioxidative, anti-inflammatory, and anticancer activities [177,178]. Xing et al. [179] investigated the effect of Luteolin on embryo development using fresh and aged mouse oocytes. The researchers sought to determine the potential of luteolin to delay postovulatory oocyte aging, which is a major disadvantage in artificially assisted reproduction [180]. Using an optimal concentration of 5µM of luteolin, the result from the experiment showed that luteolin, just like some other bioactive compounds like quercetin and resveratrol, could protect the oocyte from post-ovulatory aging. This was attributed to the activation of the SIRT1 gene, which is known for its role in lifespan extension and anti-aging [181]. Xie et al. [182] investigated the protective role of luteolin in preventing the degeneration of the intervertebral disc. This condition is known to result in lower back pain and is primarily caused by cellular senescence or apoptosis. By adopting the use of immortalized nucleus pulposus cells, the treatment of the cells with luteolin at a range of 1–4 µM revealed that viability was concentration-dependent. The senescent activity of luteolin was observed in its potential to regulate the SIRT6 gene. In another study by Zhu et al. [183], the potential of luteolin to attenuate senescence was also confirmed in hydrogen peroxide-induced oxidative cells.
4.13. Fisetin
Fisetin is a naturally occurring flavonoid found in fruits and vegetables, such as apples, grapes, onions, and cucumbers [184]. It has been recognized for its anticancer activities and its ability to prevent old-age-related conditions [185,186]. To examine the effect of fisetin on the neurodegenerative decline associated with Alzheimer’s disease, Currais et al. [187] investigated the role of the compound in SAMP8 models of aging. Feeding the models with approximately 25 mg/kg/day of fisetin for a period of 7 months, certain behavioral changes and protein expression were analyzed between the fisetin-fed mice and the control mice. The outcome of the experiment showed that fisetin had the potential to suppress changes associated with aging, which contribute greatly to the development of age-related disorders. Also, other observations, such as a decrease in brain inflammation and the alteration of specific brain and plasma metabolites, were also recorded.
Yousefzadeh et al. [188] investigated a list of flavonoids using senescent murine and fibroblast cells. These cells, which had undergone genotoxic and oxidative stress, were treated with different concentrations of the flavonoids ranging from 1 to 15 µM. The results from the experiment showed that at a concentration of 5 µM of fisetin was able to reduce senescent cells and also suppressed senescent markers without hindering cell proliferation. In vivo testing using a mice model also revealed that by supplementing mice diets with 500 mg/kg of fisetin for four weeks intermittently, senescent-induced markers were significantly reduced. In a similar experiment which investigated the role of fisetin in reducing senescence, Mahoney et al. [189] examined this in vascular cells and arteries so as to improve arterial function in old mice. An optimal concentration of 1 µM of fisetin was observed to reduce SA-β gal activity, which is associated with senescent cells. Further experimentation revealed that in human aortic endothelial cells, 1 µM of fisetin reduced the viability of senescent cells. In a more translational model such as sheep, the treatment of 6–7-year-old female sheep with 100 mg/kg of fisetin for 7 weeks led to a significant reduction in senescent cells [190].
Through its antioxidative potential, a concentration of 10 µM of fisetin delayed postovulatory oocyte aging in MII oocytes [191]. This was confirmed to occur through its elevation of SIRT1 expression levels. In a telomerase-deficient mouse model, the supplementation of a diet with 500 mg/kg of fisetin for a period of 7 weeks was observed to result in the reversal of premature aging signs [192]. This was observed through significant upregulation of aging markers and reduced collagen fiber deposition. In Caenorhabditis elegans, dietary supplementation with fisetin significantly improved the response of the cells to stress and aging [193]. Worms treated with 0.1 g/L of fisetin had an increase in both their mean and maximum lifespans. Furthermore, by examining the impact of fisetin on a Parkinson’s disease model, fisetin was found to inhibit the degeneration of dopaminergic neurons, thereby establishing its potential in delaying the onset of age-related diseases.
4.14. Kaempferol
Kaempferol, a flavonoid scientifically referred to as (3,4′,5,7-tetrahydroxyflavone), is abundantly found in various edible medicinal plants, such as broccoli, onion, tea, tomatoes, and grapes. Several scientific studies have highlighted the remarkable properties of Kaempferol, including its potent antioxidant, anticancer, anti-aging, and cardioprotective activities [194,195,196]. In a study conducted by Min et al. [197], it was observed that aged rats treated with 2 or 4 mg/kg/day of kaempferol for a period of 10 days experienced the suppression of the NF-κB cascade. This suppression was achieved through the modulation of nuclear factor-inducing kinase (NIK)/IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs).
4.15. β-Carotene
β-carotene, a provitamin A, is present in various fruits and vegetables. This provitamin A activity is specific to carotenoids derived from plants, which are responsible for the vibrant red, orange, and yellow hues observed in plants, fruits, and vegetables [198]. Notably, pumpkins, carrots, apricots, and mangos are rich sources of β-carotene [12]. Recent studies, conducted in vivo and in vitro, have shown that β-carotene possesses impressive anti-aging properties [199]. Exposure to β-carotene has been found to reduce the activity of SA-β-Gal, decrease the production of P21, P16, and P53, and lower the levels of pro-inflammatory factors such as IL-1β, IL-6, and tumor necrosis factor-β (TNF-β) in senescent mesenchymal stem cells (MSCs) induced by H2O2. These effects are accompanied by a decrease in the phosphorylation levels of nuclear factor-kappaB (NF-B). It seems that β-carotene exerts its anti-aging effects by regulating the lysine acetyltransferase 7 (KAT7)-P15 axis, which leads to a G1-phase cell-cycle arrest [199].
4.16. Diosgenin
Diosgenin, a steroid saponin present in various plant species, has been identified as a promising bioactive biomolecule with a wide range of significant medicinal properties. These properties include hypolipidemic and hypoglycemic effects, antioxidant and anti-inflammatory activities, and antiproliferative properties [2,200,201]. In a recent study by Song et al. [202], diosgenin was investigated for its anti-aging activity. This naturally occurring steroidal sapogenin is primarily found in the roots of yam Dioscorea villosa and the seeds of fenugreek Trigonella foenum-graecum. The researchers specifically examined its effects on the male Nothobranchius guentheri, an aged fish model. The results were remarkable, as diosgenin administration significantly extended both the average and maximum lifespans of these aged male fish. Moreover, the study revealed that diosgenin treatment led to a reduction in the accumulation of histological aging biomarkers, namely, LF and SA-β-Gal. In addition, diosgenin administration resulted in decreased levels of reactive oxygen species (ROS), protein oxidation, and lipid peroxidation in the aged fish.
4.17. Ellagic Acid
Ellagic acid, a dietary polyphenol abundant in fruits and vegetables, possesses various beneficial properties, including antioxidant, antiproliferative, chemo-preventive, and anti-atherogenic effects [203]. In a study conducted by Rahimi et al. [204], it was observed that a low dosage of ellagic acid (30 mg/kg) only induced a reduction in the detrimental effects of D-galactose-induced aging after a 10-week treatment period. However, a high dosage of ellagic acid (100 mg/kg) proved to be effective in both 6 and 10 weeks of treatment in the D-galactose-induced aging model. Another study by Rahimi et al. [205] demonstrated the anti-aging effects of ellagic acid on SH-SY5Y cells following D-galactose-induced aging. The researchers discovered that ellagic acid (0.01–10 μM) significantly enhanced cell proliferation and GSH levels, while reducing ROS, MDA, TNF-α, β−GAL, and AGEs levels in the presence of D-glactose-induced aging. Additionally, pre-incubation with ZnPP hindered the protective activities of ellagic acid (0.1 and 1 μM) on cell proliferation, ROS, MDA, GSH, and TNF-α levels during D-galactose-induced aging, except for the effects of EA (10 μM), which remained unaltered. The study also emphasized the significant protection provided by ellagic acid at a concentration of 10 μM in terms of cell proliferation and TNF-α levels. Xian et al. [206] reported that ellagic acid ameliorated cognitive impairment and hippocampal damage, elevated GABA and 5-HT levels, and suppressed inflammation and oxidative stress in a D-galactose-induced aging rat model. Furthermore, Naghibi et al. [207] discovered that ellagic acid treatment increased the levels of antioxidant enzymes and reduced malondialdehyde concentration. Moreover, ellagic acid administration upregulated the mRNA and protein levels of SIRT1 and NRF2, and deacetylated NRF2 protein in aged rats, suggesting that ellagic acid exerts protective effects on aged rats by activating SIRT1 and NRF2 signaling.

Table 1.
Bioactive compounds with anti-aging effect.
Table 1.
Bioactive compounds with anti-aging effect.
| Bioactive Compound | Experimental Models | Condition | Effective Dose | Mechanism | References |
|---|---|---|---|---|---|
| Chlorogenic acid | Caenorhabditis elegans | Aging | 50 µM | Extension of adult mean lifespan by 20.1% through modulation of the insulin/insulin-like signaling pathway through the DAF-16 transcription factor. | [52] |
| Chlorogenic acid | Hep3B and HeLa cell lines | Aging | 20–40 µM | Increase in lifespan through the induction of SKN-1. | [54] |
| Chlorogenic acid | Yeast | Aging | 25 µM | Increase in chronological lifespan through the activation of antioxidative stress response, activation of SOD2 and SIR2 levels. | [56] |
| Chlorogenic acid | Mice, HUVECs | Vascular damage | 40 mg/kg | Suppression of angiotensin- and H2O2-induced senescence in a dose-dependent manner through increased expression of Sirt1 and eNOS. | [57] |
| Chlorogenic acid | 28 elderly individuals | Dementia | 330 mg | Significant improvement in verbal memory, attention, cognitive flexibility, motor speed and executive function. | [58] |
| Chlorogenic acid | 26 participants between 50–65 years | Dementia | 270 mg | Improvement of CNS vital signs such as psychomotor speed and motor speed. | [59] |
| Chlorogenic acid | 38 healthy participants | Subjective memory loss | 300 mg | Delayed cognitive decline and improved cognitive function. | [60] |
| Chlorogenic acid | UV-induced fibroblast cells | Increase in ROS levels due to UV exposure | 25 µg/mL | Increase in COL-3 gene expression. | [61] |
| Chlorogenic acid | Human fibroblast and keratinocytes | Exposure to ultraviolet rays | 20 µg/mL | Increase in Hyaluronic acid and collagen content and a decrease in MMP-1. | [64] |
| Chlorogenic acid | Mouse | Retinal pathogenic neovascularisation | 25–50 mg/kg/day | Significant reduction in retina vascular changes. | [66] |
| Quercetin | Simocephalus vetulus | Short lifespan | 1 mg/L | Increase in lifespan of 22%. | [73] |
| Quercetin | Human erythrocyte | Aging | 10 µM | Protection of membrane lipids from oxidative damage. | [74] |
| Quercetin | Caenorhabditis elegans | Aging | 0.15 mg/mL | Extension18.53% in lifespan by the regulation of the SIRT1 signaling pathway. | [76] |
| Quercetin | Pre-adipocytes and adipocyte cells | Aging | 20 µM | Reduction in beta galactosidase activity through the downregulation of miR-155-5p expression and upregulation of the SIRT-1 pathway. | [81] |
| Quercetin | Human skin tissues | UV-mediated aging | 20 µM | Suppressed expression of MMP-1 and the attenuation of inflammatory responses in the skin tissues due to a reduction in COX-2 expression | [82] |
| Rutin | Rats | Aging | 2% | Significant inhibition of increases in fasting blood glucose, insulin levels, and blood pressure. | [84] |
| Rutin | Human dermal fibroblast | Skin aging | 50 µM | Increase in dermal density and elasticity. | [88] |
| Rutin | Human prostrate stromal cell line | Senescence | 100 µM | Reduce the expression of senescence-associated secretory phenotype. | [89] |
| Rutin | Drosophila melanogaster | Aging | 100 µM | Extension of lifespan via mediating hormesis. | [90] |
| Rutin | Aged mice | Aging | 0.2 mg/mL | Lifespan extension of 10%. | [91] |
| Anthocyanins | Female Drosophila melanogaster | Aging | 10 mg/mL | Prolonged lifespan through the regulation of the autophagy pathway. | [96] |
| Anthocyanins | Male Drosophila melanogaster | Aging | 2.0 mg/mL | Prolonged lifespan through the regulation of the autophagy pathway. | [98] |
| Anthocyanins | Fruitflies | Aging | 20 mg/mL | Increase in lifespan of 10% through the upregulation of SOD gene and downregulation of the MTH, InR, TOR, Hep and PEPCK genes. | [99] |
| Anthocyanins | Caenorhabditis elegans | Aging | 100 µg/mL | Prevent oxidative damage through the activation of Nrf2 signaling pathway. | [103] |
| Anthocyanins | Caenorhabditis elegans | Aging | 150 µL | Upregulation of the SOD gene which resulted in a 56.25% increase in lifespan of the organism and was also associated with other factors, such as improved resistance to stress. | [104] |
| Anthocyanins | Retinal pigment epithelial cells | Aging- and light-induced damage | 10 µg/mL | Decrease in the percentage of senescent cells in the retinal pigment and also reduced intracellular ROS levels. | [105] |
| Resveratrol | SAMP8 mice model | Aging | 1 g/kg | Increased life expectancy through an increase in SIRT1 and AMPK levels. | [107] |
| Resveratrol | Bombyx mori | Aging | 500 µM | Lifespan extension through the activation of the SIRT7 pathway. | [111] |
| Resveratrol | Drosophila melanogaster | Aging | 60 mg/kg | Increase in lifespan and improvement of behavioral deficit due to oxidative damage | [112] |
| Resveratrol | Zebra fish retina | Oculopathy | 20 mg/mL | Restoration of mitochondrial integrity. | [115] |
| Resveratrol Rice | Drosophila melanogaster | Aging | 31.54 µg/L | Significant extension median lifespan. | [117] |
| Epigallocatechin gallate | Human serum albumin | Aging | 6 mg/kg | Neuroprotective potential by preventing the deposition of lipofuscin. | [121] |
| Epigallocatechin gallate | 3T3-L1 Preadipocyte cells | Senescence | 100 µM | Induce senescence through modulation of the pro- and anti-apoptotic pathways. | [122] |
| Epigallocatechin gallate | Drosophila melanogaster | Aging | 10 mg/mL | Significant increase in lifespan and increased survival rate. | [124] |
| Epigallocatechin gallate | Human keratinocyte cells | Aging | 20 µL | Improved anti-aging potential and protection against skin damage. | [128] |
| Caffeic acid | Drosophila melanogaster | Aging | 0.2 mg/mL | Increase in lifespan by delaying the onset of intestinal stem cells and progenitor cells. | [135] |
| Caffeic acid + APPPKK | Fibroblast cells | Skin aging | 100 µM | Reducing stress in cells. | [136] |
| Caffeic acid | Caenorhabditis elegans | Aging | 100 µM | Increase in mean lifespan. | |
| Caffeic acid | Caenorhabditis elegans | Aging | 200 µM | Increase in maximum lifespan. | [138] |
| Lycopene | Mice | Cognitive impairment | 50 mg/kg | Improvement in age-induced neuronal degeneration and MDA and BDNF expression. | [146] |
| Lycopene | Aged chicken | Ovarian aging | 100 ng/mL | Reduce oxidative stress via the activation of the Nrf2/HO-1 pathway. | [21] |
| Lycopene | Oocytes | Post-ovulatory aging | 200 nm | Reduce oxidative stress. | [150] |
| Blakeslea trispora (1.9% Lycopene) | Mice | Aging | 1068 mg/kg | Improved antioxidant activities. | [152] |
| Lutein | Drosophila melanogaster | Aging | 0.1 mg/mL | Increased maximum lifespan via a decrease in malonyldialdehyde levels and the upregulation of antioxidant enzymes. | [157] |
| Lutein | Women | Photoprotection | 20 mg/day | Increase in photoprotective activity marked by a significant increase in MED. | [158] |
| Lutein | ARPE-19 cells | Senescence | 1 µg/mL | Significant reduction in SA-b-gal activity. | [159] |
| Lutein | ARPE-19 cells | Senescence | 20 µM | Decrease in SA-b-gal activity. | [160] |
| Β-sitosterol | Male mice | Age-related muscle loss | 200 mg/kg | Protection from muscle loss and the restoration of grip strength. | [164] |
| Β-sitosterol + vermicularin | Human skin fibroblasts and keratinocytes | Skin aging | 50 µM | Increased expression of hyaluronic acid synthases. | [166] |
| Curcumin | Drosophila melanogaster | Aging | 250 µM | Extension of adult lifespan through the modulation of age-related genes such as mth, thor, INR, and INK | [170] |
| Curcumin | Larval feed | Senescence | 20 µM | Extend the minimum and maximum lifespan of the adult fly by increasing the development time and delaying the onset of senescence. | [171] |
| Curcumin | Caenorhabditis elegans | Aging | 20 µM | Extend the maximum adult lifespan by 17 days and delay the process of aging. | [172] |
| Curcumin | Human Umbilical Vein Endothelial Cells | Senescence | 25 µM | Reduction in hydrogen peroxide-induced apoptosis in the cells. | [173] |
| Curcumin | Albino Wistar rats | Aging | 400 mg/kg | Significant reduction in anti-aging inflammatory markers. | [175] |
| Curcumin | Saccharomyces cerevisiae | Aging | 200 µM | Extension of minimum and maximum lifespan. | [176] |
| Luteolin | Mouse oocytes | Postovulatory oocyte aging | 5 µM | Protection of oocytes from post-ovulatory aging due to the activation of the SIRT1 gene. | [179] |
| Luteolin | Immortalized nucleus pulposus cells | Cellular senescence | 4 µM | Regulation of the SIRT6 gene. | [182] |
| Fisetin | SAMP8 | Neurodegenerative decline | 25 mg/kg | Suppression of changes associated with aging through the alteration of specific brain and plasma metabolites. | [187] |
| Fisetin | Murine and fibroblast cells | Senescence | 5 µM | Suppression of senescent cells. | [188] |
| Fisetin | Vascular cells | Senescence | 1 µM | Reduction in SA-β gal activity. | [189] |
| Fisetin | Sheep | Senescence | 100 mg/kg | Reduction in senescent cells. | [190] |
| Fisetin | MII oocytes | Post-ovulatory oocyte aging | 10 µM | Elevation of SIRT1 expression levels. | [191] |
| Fisetin | Mouse | Premature Aging | 500 mg/kg | Significant upregulation of aging markers and reduction in collagen fiber deposition. | [192] |
| Fisetin | Caenorhabditis elegans | Aging | 0.1 g/L | Increase in both their mean and maximum lifespan. | [193] |
| Kaempferol | Aged rats | Aging | 4 mg/kg | Suppression of the NF-κB cascade through the modulation of nuclear factor-inducing kinase (NIK)/IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs). | [197] |
| β-carotene | Mesenchymal stem cells | Senescence | 5 µM | Decrease the activity of SA-β-Gal, reduce the production of P21, P16, and P53, and lower the levels of pro-inflammatory factors like IL-1β, IL-6, and tumor necrosis factor-β (TNF-β). | [199] |
| Ellagic acid | Mice | Aging | 100 mg/kg | Reduction in ROS levels. | [204] |
| Ellagic acid | SH-SY-5Y | Aging | 10 µM | Significantly increase cell proliferation and GSH levels, while decreasing ROS, MDA, TNF-α, β−GAL, and AGEs levels. | [205] |
| Ellagic acid | Rats | Aging | 100 mg/kg | Improved cognitive impairment and hippocampal damage, increased GABA and 5-HT levels, and suppressed inflammation and oxidative stress. | [206] |
| Ellagic acid | Rats | Aging | 30 mg/kg | Increased the levels of antioxidant enzymes and reduced malondialdehyde concentrations. | [207] |
5. Conclusions
The bioactive phytoconstituents in edible medicinal plants, including chlorogenic acid, caffeic acid, quercetin, rutin, anthocyanins, resveratrol, β-carotene, lycopene, lutein, diosgenin, β-sitosterol, epigallocatechin gallate, curcumin, ellagic acid, luteolin, fisetin, and kaempferol, have been recognized for their potential to counteract the aging process and extend lifespans in various model organisms. These natural compounds, obtained from plant sources, have shown promising effects in combating age-related changes through various mechanisms. These bioactive phytoconstituents have the ability to slow down the aging process and improve overall health and longevity. The exploration of these natural compounds found in edible medicinal plants could provide an inexhaustible source for the development of anti-aging therapies and interventions. One limitation of this study is the absence of a clearly defined mechanism of action for the utilization of bioactive compounds as anti-aging agents. In addition, there is a scarcity of clinical trial studies available to support this approach. However, it is imperative to prioritize additional research efforts towards comprehending the underlying mechanisms of action of these bioactive compounds. Furthermore, it is crucial to conduct thorough investigations into the chronic and genotoxic effects of these compounds and optimize their usage for anti-aging purposes. This optimization process should encompass quality control analysis, the determination of safe doses, and rigorous clinical trials.
Author Contributions
The development and writing of this article have been significantly contributed by all the listed authors. The study was designed by E.J.I., O.E.A. and E.A.U., while the manuscript was drafted and proofread by E.A.U., O.E.A., E.J.I., U.I., C.A.O. and O.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Transhuman Coin Scientific Advisory Board whereas part of the Article Processing Charge (APC) was funded by Covenant University Centre for Research Innovation and Discovery (CUCRID).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Okoro, N.O.; Odiba, A.S.; Osadebe, P.O.; Omeje, E.O.; Liao, G.; Fang, W.; Jin, C.; Wang, B. Bioactive phytochemicals with anti-aging and lifespan extending potentials in Caenorhabditis elegans. Molecules 2021, 26, 7323. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-aging activity and modes of action of compounds from natural food sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef]
- Zhang, L.; Yousefzadeh, M.J.; Suh, Y.; Niedernhofer, L.J.; Robbins, P.D. Signal Transduction, Ageing and Disease, Subcellular Biochemistry; Springer: New York, NY, USA, 2019; Volume 91, pp. 227–247. [Google Scholar] [CrossRef]
- Kuppusamy, U.R.; Arumugam, B. Chapter 18—Novel plant bioactives, their antiaging potencies: Reality and promises. In Plant Bioactives as Natural Panacea against Age-Induced Diseases; Pandey, K.B., Suttajit, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 359–386. [Google Scholar] [CrossRef]
- World Population Prospects. 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 16 January 2024).
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive compounds of edible fruits with their anti-aging properties: A comprehensive review to prolong human life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2020, 173, 113719. [Google Scholar] [CrossRef]
- Sripanidkulchai, B.; Suttajit, M.; Ratanavalachai, T. Chapter 2—Anti-aging strategies, plant bioactives, and drug development: Current insights. In Plant Bioactives as Natural Panacea against Age-Induced Diseases; Pandey, K.B., Suttajit, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 23–48. [Google Scholar]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural compounds and products from an anti-aging perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Ukpai, O.M.; Ijioma, S.N.; Kanu, K.; Orieke, D.; Chinedu-Ndukwe, P.A.; Ugwuanyi, K.C.; Ugbogu, E.A. Phytochemical composition, toxicological profiling and effect on pup birth weight of Corchorus olitorius leaf extract in rats: Implications for fetal macrosomia control. J. Ethnopharmacol. 2024, 319, 117170. [Google Scholar] [CrossRef] [PubMed]
- Ugbogu, E.A.; Okoro, H.; Emmanuel, O.; Ugbogu, O.C.; Ekweogu, C.N.; Uche, M.; Dike, E.D.; Ijioma, S.N. Phytochemical characterization, anti-diarrhoeal, analgesic, anti-inflammatory activities and toxicity profile of Ananas comosus (L.) Merr (pineapple) leaf in albino rats. J. Ethnopharmacol. 2024, 319, 117224. [Google Scholar] [CrossRef] [PubMed]
- Ekweogu, C.N.; Akubugwo, E.I.; Emmanuel, O.; Nosiri, C.I.; Uche, M.E.; Adurosakin, O.E.; Ijioma, S.N.; Ugbogu, E.A. Phytochemical profiling, toxicity studies, wound healing, analgesic and anti-inflammatory activities of Musa paradisiaca L. Musaceae (Plantain) stem extract in rats. J. Ethnopharmacol. 2024, 322, 117639. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Natural. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Progress Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investigat. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef]
- Kim, S.G.; Sung, J.Y.; Kim, J.R.; Choi, H.C. Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway. Korean J. Physiol. Pharmacol. 2020, 24, 69–79. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Zhang, S.; Guo, C.; Li, J.; Mi, Y.; Zhang, C. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging 2018, 10, 2016–2036. [Google Scholar] [CrossRef]
- Ikonne, E.U.; Ikpeazu, V.O.; Ugbogu, E.A. The potential health benefits of dietary natural plant products in age related eye diseases. Heliyon 2020, 6, e04408. [Google Scholar] [CrossRef]
- Iweala, E.J.; Oluwapelumi, A.E.; Dania, O.E.; Ugbogu, E.A. Bioactive phytoconstituents and their therapeutic potentials in the treatment of haematological cancers: A Review. Life 2023, 13, 1422. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T. The search for DAF-16/FOXO transcriptional targets: Approaches and discoveries. Exp. Gerontol. 2006, 41, 910–921. [Google Scholar] [CrossRef]
- Morris, B.J.; Willcox, D.C.; Donlon, T.A.; Willcox, B.J. FOXO3: A major gene for human longevity—A mini-review. Gerontology 2015, 61, 515–525. [Google Scholar] [CrossRef]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef]
- Flachsbart, F.; Caliebe, A.; Kleindorp, R. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. USA 2009, 106, 2700–2705. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Menzel, R.; Stürzenbaum, S.R.; Steinberg, C.E.W. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 2009, 10, 565–578. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K.; et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Dröge, W.; Ffrench, M.; Terman, A. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Witczak, C.A.; Sharoff, C.G.; Goodyear, L.J. AMP-activated protein kinase in skeletal muscle: From structure and localization to its role as a master regulator of cellular metabolism. Cell Mol. Life Sci. 2008, 65, 3737–3755. [Google Scholar] [CrossRef]
- Hwang, J.T.; Park, I.J.; Shin, J.I. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef]
- Bruning, A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med. Chem. 2013, 13, 1025–1031. [Google Scholar] [CrossRef]
- Huang, S. Inhibition of PI3K/Akt/mTOR signaling by natural products. Anticancer Agents Med. Chem. 2013, 13, 967–970. [Google Scholar] [CrossRef]
- Syed, D.; Adhami, V.; Khan, M.; Mukhtar, H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med. Chem. 2013, 13, 995–1001. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Gerhart-Hines, Z.; Puigserver, P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008, 582, 46–53. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Chang, C.J.; Young, J.D. Antiaging effects of bioactive molecules isolated from plants and fungi. Med. Res. Rev. 2019, 39, 1515–1552. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Chang, C. Chlorogenic acid intake guidance: Sources, health benefits, and safety. Asia Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic acid: A dietary phenolic acid with promising pharmacotherapeutic potential. Cur. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Ding, A.J.; Wu, G.S.; Luo, H.R. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via insulin/IGF-1 signaling pathway. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar]
- Son, H.G.; Altintas, O.; Kim, E.J.E.; Kwon, S.; Lee, S.J.V. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 2019, 18, e12853. [Google Scholar] [CrossRef]
- Siswanto, F.M.; Sakuma, R.; Oguro, A.; Imaoka, S. Chlorogenic acid activates Nrf2/SKN-1 and prolongs the lifespan of Caenorhabditis elegans via the Akt-FOXO3/DAF16a-DDB1 pathway and activation of DAF16f. J. Gerontol. Ser. A. 2022, 77, 1503–1516. [Google Scholar] [CrossRef]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Abid, M.; Elamrani, A.; Drouet, S.; Addi, M.; Hano, C. Almond skin extracts and chlorogenic acid delay chronological aging and enhanced oxidative stress response in yeast. Life 2020, 10, 80. [Google Scholar] [CrossRef]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The protective effect of chlorogenic acid on vascular senescence via the Nrf2/HO-1 pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evid. Based Complement. Alternat. Med. 2018, 2018, 8608497. [Google Scholar] [CrossRef]
- Suzukamo, C.; Ochiai, R.; Mitsui, Y.; Osaki, N.; Ono, T. Short-Term Intake of Chlorogenic Acids Improves Psychomotor Speed and Motor Speed in Adults: A Randomized Crossover Trial. Brain Sci. 2022, 12, 370. [Google Scholar] [CrossRef]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of chlorogenic acids on cognitive function: A randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Lister, I.N.E.; yashfa Gunawan, K.; Widowati, W. Anti-inflammatory and antiaging properties of chlorogenic acid on UV-induced fibroblast cell. Peer J. 2021, 9, e11419. [Google Scholar] [CrossRef]
- Tanigawa, T.; Kanazawa, S.; Ichibori, R.; Fujiwara, T.; Magome, T.; Shingaki, K.; Miyata, S.; Hata, Y.; Tomita, K.; Matsuda, K.; et al. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement. Alternat. Med. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Ribitsch, I.; Gueltekin, S.; Keith, M.F.; Minichmair, K.; Peham, C.; Jenner, F.; Egerbacher, M. Age-related changes of tendon fibril micro-morphology and gene expression. J. Anat. 2020, 236, 688–700. [Google Scholar] [CrossRef]
- Alves, G.D.A.D.; Oliveira de Souza, R.; Ghislain Rogez, H.L.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa extract and chlorogenic acid exhibit antiaging effect in human fibroblasts and keratinocytes cells exposed to UV radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar] [CrossRef]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chemico-Biol. Interact. 2015, 234, 261–273. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.M.; Jung, W.; Park, S.B.; Kim, C.S.; Kim, J.S. Aster koraiensis extract and chlorogenic acid inhibit retinal angiogenesis in a mouse model of oxygen-induced retinopathy. Evid. Based Complement. Alternat. Med. 2018, 2018, 6402650. [Google Scholar] [CrossRef]
- Apte, R.S. Age-related macular degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef]
- Fiorani, M.; Guidarelli, A.; Blasa, M.; Azzolini, C.; Candiracci, M.; Piatti, E.; Cantoni, O. Mitochondria accumulate large amounts of quercetin: Prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010, 21, 397–404. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Choi, S.M.; Kim, B.C.; Cho, Y.H.; Choi, K.H.; Chang, J.; Park, M.S.; Kim, M.K.; Cho, K.H.; Kim, J.K. Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med. J. 2014, 50, 45–51. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.T.; Zhou, D.; Bai, X.Y.; Zhou, W.L.; Huang, C. Quercetin protects against the Aβ25–35-induced amnesic injury: Involvement of inactivation of RAGE-mediated pathway and conservation of the NVU. Neuropharmacology 2013, 67, 419–431. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Du, X.; Liu, Z.; Zhu, C.; Mao, W.; Liu, G.; Jiang, Q. Anti-aging effects of quercetin in Cladocera Simocephalus vetulus using proteomics. ACS Omega 2023, 8, 17609–17619. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Pusch, M.; Dossena, S.; Marino, A.; Morabito, R. D-Galactose induced early aging in human erythrocytes: Role of band 3 protein. J. Cell. Physiol. 2022, 237, 1586–1596. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation stress as a mechanism of aging in human erythrocytes: Protective effect of quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The composition and anti-aging activities of polyphenol extract from Phyllanthus emblica L. fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Abharzanjani, F.; Afshar, M.; Hemmati, M.; Moossavi, M. Short-term High Dose of Quercetin and Resveratrol Alters Aging Markers in Human Kidney Cells. Int. J. Prev. Med. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Forney, L.A.; Lenard, N.R.; Stewart, L.K.; Henagan, T.M. Dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int. J. Mol. Sci. 2018, 19, 895. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic effects of quercetin in an in vitro model of pre-adipocytes and adipocytes induced senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin directly targets JAK2 and PKCδ and prevents UV-induced photoaging in human skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharma. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Li, T.; Chen, S.; Feng, T.; Dong, J.; Li, Y.; Li, H. Rutin protects against aging-related metabolic dysfunction. Food Funct. 2016, 7, 1147–1154. [Google Scholar] [CrossRef]
- Makino, T.; Kanemaru, M.; Okuyama, S.; Shimizu, R.; Tanaka, H.; Mizukami, H. Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. J. Nat. Med. 2013, 67, 881–886. [Google Scholar] [CrossRef]
- Han, Y.; Ding, Y.; Xie, D.; Hu, D.; Li, P.; Li, X.; Xue, W.; Jin, L.; Song, B. Design, synthesis, and antiviral activity of novel rutin derivatives containing 1, 4-pentadien-3-one moiety. Europ. J. Med. Chem. 2015, 92, 732–737. [Google Scholar] [CrossRef]
- Perk, A.A.; Shatynska-Mytsyk, I.; Gerçek, Y.C.; Boztaş, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Chitnis, A.; Talekar, A.; Mulay, P.; Makkar, M.; James, J.; Thirumurugan, K. Hormetic efficacy of rutin to promote longevity in Drosophila melanogaster. Biogerontology 2017, 18, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Pan, R.; Cheng, J.; Cui, Q.; Chen, J.; Yuan, Z. Sodium rutin extends lifespan and health span in mice including positive impacts on liver health. Br. J. Pharmacol. 2022, 179, 1825–1838. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Georgousopoulou, E.N.; Dadigamuwage, L.; Kellett, J.; Panagiotakos, D.B.; Thomas, J.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br. J. Nutr. 2018, 119, 280–298. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Han, Y.; Pei, Y.; Guo, Y.; Cui, S.W. Purple sweet potato extract maintains intestinal homeostasis and extend lifespan through increasing autophagy in female Drosophila melanogaster. J. Food Biochem. 2021, 45, e13861. [Google Scholar] [CrossRef]
- Buszczak, M.; Krämer, H. Autophagy keeps the balance in tissue homeostasis. Dev. Cell 2019, 49, 499–500. [Google Scholar] [CrossRef]
- Han, Y.; Guo, Y.; Cui, S.W.; Li, H.; Shan, Y.; Wang, H. Purple sweet potato extract extends lifespan by activating autophagy pathway in male Drosophila melanogaster. Experiment. Gerontol. 2021, 144, 111190. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.M.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Chen, Z.Y. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Experiment. Gerontol. 2015, 69, 189–195. [Google Scholar] [CrossRef]
- Katewa, S.D.; Kapahi, P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Experiment. Gerontol. 2011, 46, 382–390. [Google Scholar] [CrossRef]
- Peng, C.; Zuo, Y.; Kwan, K.M.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.Y. Blueberry extract prolongs lifespan of Drosophila melanogaster. Experiment. Gerontol. 2012, 47, 170–178. [Google Scholar] [CrossRef]
- Sun, X.; Komatsu, T.; Lim, J.; Laslo, M.; Yolitz, J.; Wang, C.; Poirier, L.; Alberico, T.; Zou, S. Nutrient-dependent requirement for SOD1 in lifespan extension by protein restriction in Drosophila melanogaster. Aging Cell 2012, 11, 783–793. [Google Scholar] [CrossRef]
- Yan, F.; Chen, Y.; Azat, R.; Zheng, X. Mulberry anthocyanin extract ameliorates oxidative damage in hepg2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxid. Med. Cell Longev. 2017, 2017, 7956158. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Zhang, L.; Zheng, Y.; Ma, G.; Sun, X.; Yuan, J. Preparation of Dendrobium officinale flower anthocyanin and extended lifespan in Caenorhabditis elegans. Molecules 2022, 27, 8608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Zhang, D.; Zhou, F.; Wang, D.; Wei, Y.; Gao, F.; Xie, L.; Jia, G.; Wu, W.; et al. Blueberry anthocyanins: Protection against ageing and light-induced damage in retinal pigment epithelial cells. Br. J. Nutr. 2012, 108, 16–27. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef]
- D’Souza, Y.; Elharram, A.; Soon-Shiong, R.; Andrew, R.D.; Bennett, B.M. Characterization of Aldh2-/-mice as an age-related model of cognitive impairment and Alzheimer’s disease. Mol. Brain 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Bayod, S.; Del Valle, J.; Pelegri, C.; Vilaplana, J.; Canudas, A.M.; Camins, A.; Jimenez, A.; Sanchez-Roige, S.; Lalanza, J.F.; Escorihuela, R.M.; et al. Macroautophagic process was differentially modulated by long-term moderate exercise in rat brain and peripheral tissues. J. Physiol. Pharmacol. 2014, 65, 229–239. [Google Scholar]
- Donmez, G.; Wang, D.; Cohen, D.E.; Guarente, L. Retraction notice to: SIRT1 suppresses β-amyloid production by activating the α-secretase gene ADAM10. Cell 2014, 158, 959. [Google Scholar] [CrossRef]
- Song, J.; Liu, L.; Hao, K.; Mao, S.; Tang, Y.; Tong, X.; Dai, F. Resveratrol elongates the lifespan and improves antioxidant activity in the silkworm Bombyx mori. J. Pharmaceut. Anal. 2021, 11, 374–382. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Adedara, A.O.; Adie, M.A.; Vicente-Crespo, M.; Farombi, E.O. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2018, 503, 1042–1048. [Google Scholar] [CrossRef]
- Takihara, Y.; Inatani, M.; Eto, K.; Inoue, T.; Kreymerman, A.; Miyake, S.; Ueno, S.; Nagaya, M.; Nakanishi, A.; Iwao, K.; et al. In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS. Proc. Natl. Acad. Sci. USA 2015, 112, 10515–10520. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Reddy, T.P. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr. Alzheimer Res. 2011, 8, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117. [Google Scholar] [CrossRef]
- Baek, S.H.; Shin, W.C.; Ryu, H.S.; Lee, D.W.; Moon, E.; Seo, C.S.; Hwang, E.; Lee, H.S.; Ahn, M.H.; Jeon, Y.; et al. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PLoS ONE 2013, 8, e57930. [Google Scholar] [CrossRef]
- Islam, M.S.; Jin, Y.Y.; Chung, H.J.; Kim, H.J.; Baek, S.H.; Hong, S.T. Effect of the resveratrol rice DJ526 on longevity. Nutrients 2019, 11, 1804. [Google Scholar] [CrossRef]
- Khan, M.; Park, S.; Kim, H.J.; Lee, K.J.; Kim, D.H.; Baek, S.H.; Hong, S.T. The resveratrol rice DJ526 callus significantly increases the lifespan of drosophila (resveratrol rice DJ526 callus for longevity). Nutrients 2019, 11, 983. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Cai, S.X.; Huang, J.A.; Wang, L.L.; Dong, X.R.; Gong, Y.S.; Li, J.; Li, Q.; Liu, Z.; Luo, G. Inhibiting effects of epigallocatechin gallate (EGCG) on the formation of age pigment in vitro and in vivo. J. Med. Plants Res. 2011, 5, 5470–5478. [Google Scholar]
- Kumar, R.; Sharma, A.; Kumari, A.; Gulati, A.; Padwad, Y.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2019, 20, 171–189. [Google Scholar] [CrossRef]
- Lilja, S.; Oldenburg, J.; Pointner, A.; Dewald, L.; Lerch, M.; Hippe, B.; Switzeny, O.; Haslberger, A. Epigallocatechin gallate effectively affects senescence and anti-SASP via SIRT3 in 3T3-L1 preadipocytes in comparison with other bioactive substances. Oxid. Med. Cell Longev. 2020, 2020, 4793125. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Piegholdt, S.; Rabe, D.; Baenas, N.; Schloesser, A.; Eggersdorfer, M.; Stocker, A.; Rimbach, G. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget 2015, 6, 30568. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Na, L.; Feng, R.; Gong, L.; Zhao, Y.; Li, Q.; Li, Y.; Sun, C. The phytochemical, EGCG, extends lifespan by reducing liver and kidney function damage and improving age-associated inflammation and oxidative stress in healthy rats. Aging Cell 2013, 12, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef]
- Shoko, T.; Maharaj, V.J.; Naidoo, D.; Tselanyane, M.; Nthambeleni, R.; Khorombi, E.; Apostolides, Z. Anti-aging potential of extracts from Sclerocarya birrea (A. Rich.) Hochst and its chemical profiling by UPLC-Q-TOF-MS. BMC Complement. Alternat. Med. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Rusmana, D.; Wahyudianingsih, R.; Elisabeth, M.; Balqis, B.; Maesaroh, M.; Widowati, W. Antioxidant activity of Phyllanthus niruri extract, rutin and quercetin. Indones. Biomed. J. 2017, 9, 84–90. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic acid and diseases-mechanisms of action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.O.; Christensen, L.P.; Grevsen, K. Harvest strategies for optimization of the content of bioactive alkamides and caffeic acid derivatives in aerial parts and in roots of Echinacea purpurea. J. Agric. Food Chem. 2018, 66, 11630–11639. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- Bastianini, M.; Faffa, C.; Sisani, M.; Petracci, A. Caffeic acid-layered double hydroxide hybrid: A new raw material for cosmetic applications. Cosmetics 2018, 5, 51. [Google Scholar] [CrossRef]
- Sheng, X.; Zhu, Y.; Zhou, J.; Yan, L.; Du, G.; Liu, Z.; Chen, H. Antioxidant effects of caffeic acid lead to protection of drosophila intestinal stem cell aging. Front. Cell Develop. Biol. 2021, 9, 735483. [Google Scholar] [CrossRef]
- Lee, H.; Vilian, A.E.; Kim, J.Y.; Chun, M.H.; Suh, J.S.; Seo, H.H.; Cho, S.H.; Shin, I.S.; Kim, S.J.; Park, S.H.; et al. Design and development of caffeic acid conjugated with Bombyx mori derived peptide biomaterials for anti-aging skin care applications. RSC Adv. 2017, 7, 30205–30213. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M. Quercetin, caffeic acid and resveratrol regulate circadian clock genes and aging-related genes in young and old human lung fibroblast cells. Mol. Biol Rep. 2020, 47, 1021–1032. [Google Scholar] [CrossRef]
- Gutierrez-Zetina, S.M.; González-Manzano, S.; Ayuda-Durán, B.; Santos-Buelga, C.; González-Paramás, A.M. Caffeic and dihydrocaffeic acids promote longevity and increase stress resistance in Caenorhabditis elegans by modulating expression of stress-related genes. Molecules 2021, 26, 1517. [Google Scholar] [CrossRef]
- Ademosun, O.T.; Adebayo, A.H.; Ajanaku, K.O. Solanum lycopersicum and Daucus carota: Effective anticancer agents (a mini review). J. Phys. Conf. Ser. 2021, 1943, 012169. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Riccioni, G.; Mancini, B.; Di Ilio, E.; Bucciarelli, T.; D’orazio, N. Protective effect of lycopene in cardiovascular disease. Europ. Rev. Med. Pharmacol. Sci. 2008, 12, 183–190. [Google Scholar]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Bao, Q.; Pan, J.; Qi, H.; Wang, L.; Qian, H.; Jiang, F.; Hu, X. Aging and age-related diseases–from endocrine therapy to target therapy. Mol. Cell. Endocrinol. 2014, 394, 115–118. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, Y.; Xu, Q.; He, Y.; Tian, S.; Zhu, S.; Zhu, Y.; Dong, X. Mussel oligopeptides ameliorate cognition deficit and attenuate brain senescence in D-galactose-induced aging mice. Food Chem. Toxicol. 2013, 59, 412–420. [Google Scholar] [CrossRef]
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxid. Med. Cell. Longev. 2016, 2016, 3218605. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene supplementation attenuates oxidative stress, neuroinflammation, and cognitive impairment in aged CD-1 mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.W.; Bae, J.S. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem. Toxicol. 2012, 50, 1826–1833. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Dis. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Abir, M.H.; Mahamud, A.S.U.; Tonny, S.H.; Anu, M.S.; Hossain, K.S.; Protic, I.A.; Khan, M.S.U.; Baroi, A.; Moni, A.; Uddin, M.J. Pharmacological potentials of lycopene against aging and aging-related disorders: A review. Food Sci. Nutr. 2023, 11, 5701–5735. [Google Scholar] [CrossRef]
- Rakha, S.I.; Elmetwally, M.A.; El-Sheikh Ali, H.; Balboula, A.Z.; Mahmoud, A.M.; Zaabel, S.M. Lycopene reduces the in vitro aging phenotypes of mouse oocytes by improving their oxidative status. Vet. Sci. 2022, 9, 336. [Google Scholar] [CrossRef]
- López-Nieto, M.J.; Costa, J.; Peiro, E.; Méndez, E.; Rodríguez-Sáiz, M.; De la Fuente, J.L.; Cabri, W.; Barredo, J.L. Biotechnological lycopene production by mated fermentation of Blakeslea trispora. Appl. Microbiol. Biotechnol. 2004, 66, 153–159. [Google Scholar] [CrossRef]
- Hu, W.; Dai, D.; Li, W. Anti-aging effect of Blakeslea trispora powder on adult mice. Biotechnol. Lett. 2013, 35, 1309–1315. [Google Scholar] [CrossRef]
- Liu, X.; Dilxat, T.; Shi, Q.; Qiu, T.; Lin, J. The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene 2022, 822, 146348. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Non-nutritive bioactive constituents of plants: Lycopene, lutein and zeaxanthin. Int. J. Vit. Nutr. Res. 2003, 73, 95–100. [Google Scholar] [CrossRef]
- Zhao, C.; Cheng, H.; Jiang, P.; Yao, Y.; Han, J. Preparation of lutein-loaded particles for improving solubility and stability by Polyvinylpyrrolidone (PVP) as an emulsion-stabilizer. Food Chem. 2014, 156, 123–128. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Investigat. Ophthalmol. Vis. Sci. 2015, 56, 252–258. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, S.; Wang, H.; Wang, T. Lutein extends the lifespan of Drosophila melanogaster. Arch. Gerontol. Geriatr. 2014, 58, 153–159. [Google Scholar] [CrossRef]
- Žmitek, K.; Žmitek, J.; Butina, M.R.; Hristov, H.; Pogačnik, T.; Pravst, I. Dietary lutein supplementation protects against ultraviolet-radiation-induced erythema: Results of a randomized double-blind placebo-controlled study. J. Funct. Foods 2020, 75, 104265. [Google Scholar] [CrossRef]
- Hwang, J.S.; Han, S.G.; Lee, C.H.; Seo, H.G. Lutein suppresses hyperglycemia-induced premature senescence of retinal pigment epithelial cells by upregulating SIRT1. J. Food Biochem. 2018, 42, e12495. [Google Scholar] [CrossRef]
- Chae, S.Y.; Park, S.Y.; Park, G. Lutein protects human retinal pigment epithelial cells from oxidative stress induced cellular senescence. Mol. Med. Rep. 2018, 18, 5182–5190. [Google Scholar] [CrossRef]
- Bin Sayeed, M.S.; Karim, S.M.R.; Sharmin, T.; Morshed, M.M. Critical analysis on characterization, systemic effect, and therapeutic potential of beta-sitosterol: A plant-derived orphan phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. Biomed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Hah, Y.S.; Lee, W.K.; Lee, S.; Kim, E.J.; Lee, J.H.; Lee, S.J.; Ji, Y.H.; Kim, S.G.; Lee, H.H.; Hong, S.Y.; et al. β-Sitosterol attenuates dexamethasone-induced muscle atrophy via regulating FoxO1-dependent signaling in C2C12 cell and mice model. Nutrients 2022, 14, 2894. [Google Scholar] [CrossRef]
- Lomenick, B.; Shi, H.; Huang, J.; Chen, C. Identification and characterization of β-sitosterol target proteins. Bioorganic Med. Chem. Lett. 2015, 25, 4976–4979. [Google Scholar] [CrossRef]
- Haiyuan, Y.U.; Shen, X.; Liu, D.; Hong, M.; Lu, Y. The protective effects of β-sitosterol and vermicularin from Thamnolia vermicularis (Sw.) Ach. against skin aging in vitro. An. Acad. Bras. Cienc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.A.; Akinduti, P.A.; Ajanaku, K.O. Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The role of curcumin in the modulation of ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, B.S.; Semnani, S.; Avanesian, A.; Um, C.Y.; Jeon, H.J.; Seong, K.M.; Yu, K.; Min, K.J.; Jafari, M. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010, 13, 561–570. [Google Scholar] [CrossRef]
- Soh, J.W.; Marowsky, N.; Nichols, T.J.; Rahman, A.M.; Miah, T.; Sarao, P.; Khasawneh, R.; Unnikrishnan, A.; Heydari, A.R.; Silver, R.B.; et al. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila. Experiment. Gerontol. 2013, 48, 229–239. [Google Scholar] [CrossRef]
- Liao, V.H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biol. Pharmaceut. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef]
- Grabowska, W.; Suszek, M.; Wnuk, M.; Lewinska, A.; Wasiak, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin elevates sirtuin level but does not postpone in vitro senescence of human cells building the vasculature. Oncotarget 2016, 7, 19201. [Google Scholar] [CrossRef]
- Shailaja, M.; Gowda, K.D.; Vishakh, K.; Kumari, N.S. Anti-aging role of curcumin by modulating the inflammatory markers in albino wistar rats. J. Natl. Med. Assoc. 2017, 109, 9–13. [Google Scholar] [CrossRef]
- Stępień, K.; Wojdyła, D.; Nowak, K.; Mołoń, M. Impact of curcumin on replicative and chronological aging in the Saccharomyces cerevisiae yeast. Biogerontology 2020, 21, 109–123. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-inflammatory and active biological properties of the plant-derived bioactive compounds luteolin and luteolin 7-glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Baran, Y. Therapeutic potential of luteolin on cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef]
- Xing, X.; Peng, J.; Zhao, J.; Shi, R.; Wang, C.; Zhang, Z.; Wang, Z.; Li, Z.; Wu, Z. Luteolin regulates the distribution and function of organelles by controlling SIRT1 activity during postovulatory oocyte aging. Front. Nutr. 2023, 10, 1192758. [Google Scholar] [CrossRef]
- Hu, L.L.; Li, H.G.; Liao, B.Y.; Xu, Y.; Sun, S.C.; Wang, J.L. Exposure to nonylphenol impairs oocyte quality via the induction of organelle defects in mice. Ecotoxicol. Environ. Saf. 2022, 230, 113136. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Luteolin suppresses TNF α induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF κB pathway. Experiment. Ther. Med. 2022, 24, 1–11. [Google Scholar]
- Zhu, R.Z.; Li, B.S.; Gao, S.S.; Seo, J.H.; Choi, B.M. Luteolin inhibits H2O2-induced cellular senescence via modulation of SIRT1 and p53. Korean J. Physiol. Pharmacol. 2021, 25, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin reduces the impact of aging on behavior and physiology in the rapidly aging SAMP8 mouse. J. Gerontol. Ser. A 2018, 73, 299–307. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.I.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Mahoney, S.A.; Venkatasubramanian, R.; Darrah, M.A.; Ludwig, K.R.; VanDongen, N.S.; Greenberg, N.T.; Longtine, A.G.; Hutton, D.A.; Brunt, V.E.; Campisi, J.; et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell 2023, 23, e14060. [Google Scholar] [CrossRef]
- Huard, C.A.; Gao, X.; Dey Hazra, M.E.; Dey Hazra, R.O.; Lebsock, K.; Easley, J.T.; Millett, P.J.; Huard, J. Effects of Fisetin treatment on cellular senescence of various tissues and organs of old sheep. Antioxidants 2023, 12, 1646. [Google Scholar] [CrossRef]
- Xing, X.; Liang, Y.; Li, Y.; Zhao, Y.; Zhang, Y.; Li, Z.; Li, Z.; Wu, Z. Fisetin delays postovulatory oocyte aging by regulating oxidative stress and mitochondrial function through Sirt1 pathway. Molecules 2023, 28, 5533. [Google Scholar] [CrossRef]
- Zhao, R.; Kou, H.; Jiang, D.; Wang, F. Exploring the anti-aging effects of fisetin in telomerase-deficient progeria mouse model. PeerJ 2023, 11, e16463. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.K.; Park, S.K. Effects of fisetin, a plant-derived flavonoid, on response to oxidative stress, aging, and age-related diseases in Caenorhabditis elegans. Pharmaceuticals 2022, 15, 1528. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G. Discovery and Development of Anti-Breast Cancer Agents from Natural Products: An Overview. In Discovery and Development of Anti-Breast Cancer Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–6. [Google Scholar]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Qattan, M.Y.; Khan, M.I.; Alharbi, S.H.; Verma, A.K.; Al-Saeed, F.A.; Abduallah, A.M.; Al Areefy, A.A. therapeutic importance of kaempferol in the treatment of cancer through the modulation of cell signalling pathways. Molecules 2022, 27, 8864. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, E.K.; Heo, H.S.; Kim, M.S.; Sung, B.; Kim, M.K.; Lee, J.; Kim, N.D.; Anton, S.; Choi, J.S.; et al. The anti-inflammatory effect of kaempferol in aged kidney tissues: The involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinase pathways. J. Med. Food 2009, 12, 351–358. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- Zheng, W.V.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-aging effect of β-carotene through regulating the KAT7-P15 signaling axis, inflammation and oxidative stress process. Cell. Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef]
- Jesus, M.; Martins, A.P.; Gallardo, E.; Silvestre, S. Diosgenin: Recent highlights on pharmacology and analytical methodology. J. Anal. Methods Chem. 2016, 2016, 4156293. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An updated pharmacological review and therapeutic perspectives. Oxid. Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef]
- Song, L.; Li, C.; Wu, F.; Zhang, S. Dietary intake of diosgenin delays aging of male fish Nothobranchius guentheri through modulation of multiple pathways that play prominent roles in ROS production. Biogerontology 2022, 23, 201–213. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, Y.; Wang, X.; Nie, J.; Meng, X.; Zhang, Y. Ellagic acid: A dietary-derived phenolic compound for drug discovery in mild cognitive impairment. Front. Aging Neurosci. 2022, 14, 925855. [Google Scholar] [CrossRef]
- Rahimi, B.V.; Askari, V.R.; Mousavi, S.H. Ellagic acid dose and time-dependently abrogates d-galactose-induced animal model of aging: Investigating the role of PPAR-γ. Life Sci. 2019, 232, 116595. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Askari, V.R.; Mousavi, S.H. Ellagic acid reveals promising anti-aging effects against d-galactose-induced aging on human neuroblastoma cell line, SH-SY5Y: A mechanistic study. Biomed. Pharmacother. 2018, 108, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Deng, Y.; Yang, Y.; Tan, Z.; Chen, C.; Li, W.; Yang, R. Ameliorative effect of ellagic acid on aging in rats with the potential mechanism relying on the gut microbiota and urolithin a-producing ability. J. Agric. Food Chem. 2023, 71, 7396–7407. [Google Scholar] [CrossRef]
- Naghibi, N.; Sadeghi, A.; Movahedinia, S.; Rahimi Naiini, M.; Rajizadeh, M.A.; Bahri, F.; Nazari-Robati, M. Ellagic acid ameliorates aging-induced renal oxidative damage through upregulating SIRT1 and NRF2. BMC Complement. Med. Ther. 2023, 23, 77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).