Development of Tannic Acid Coated Polyvinylidene Fluoride Membrane for Filtration of River Water Containing High Natural Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
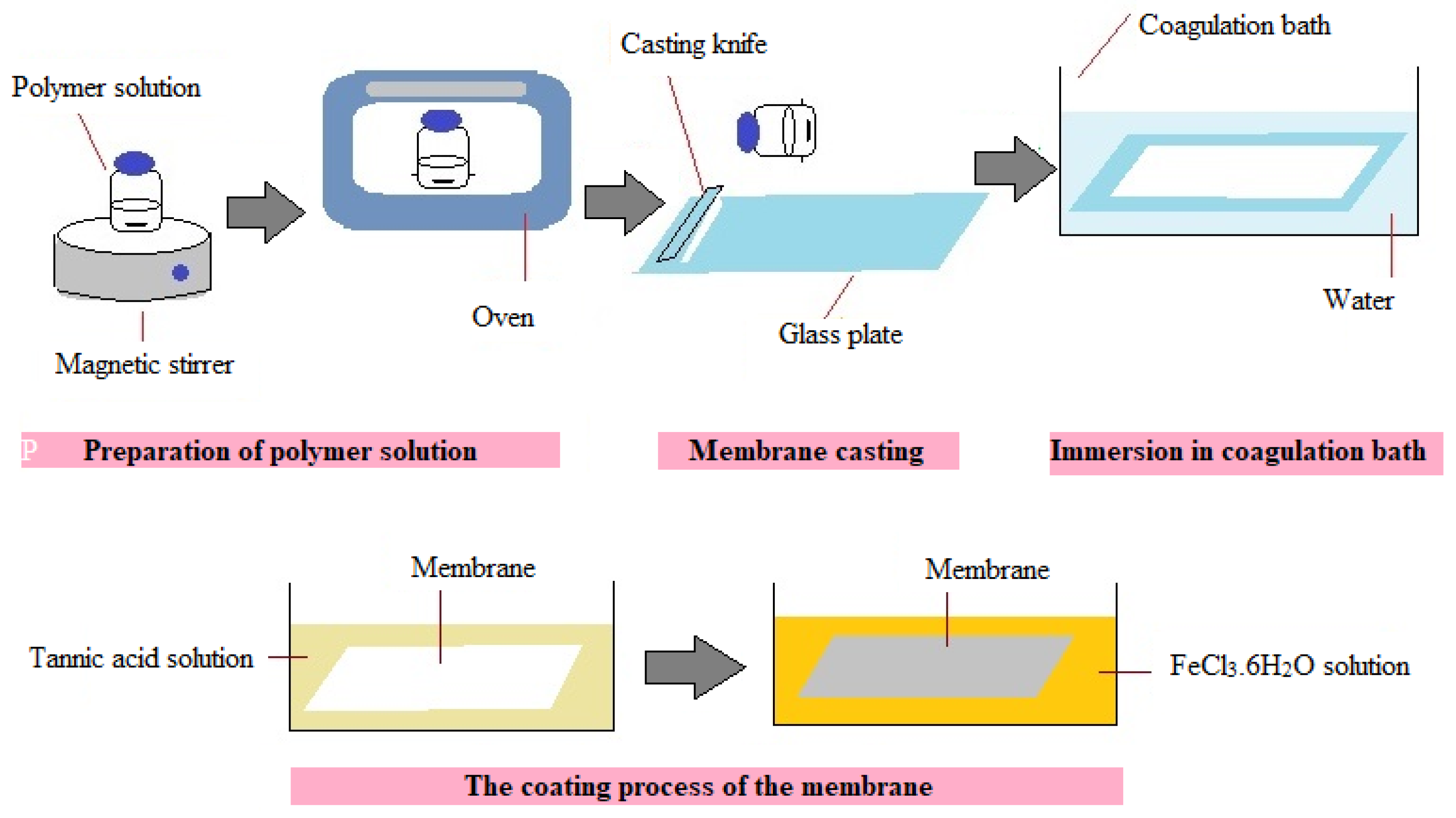
2.1. Membrane Preparation and Development
2.2. Membrane Properties
2.2.1. Morphology and Elemental Mapping
2.2.2. Surface Chemistry
2.2.3. Surface Hydrophilicity
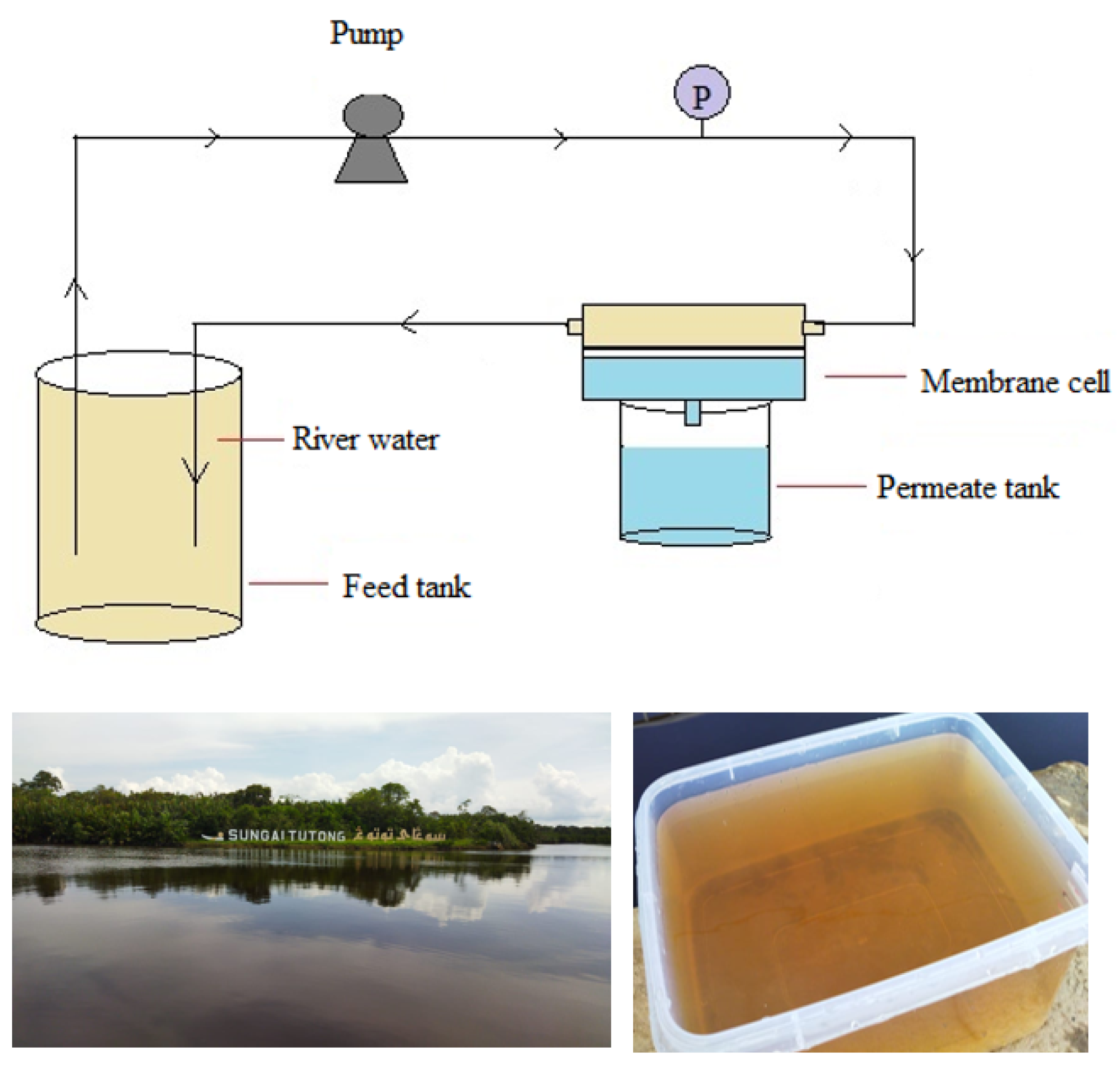
2.3. Filtration Test
2.4. Membrane Fouling Analysis
3. Results and Discussion
3.1. Membrane Properties
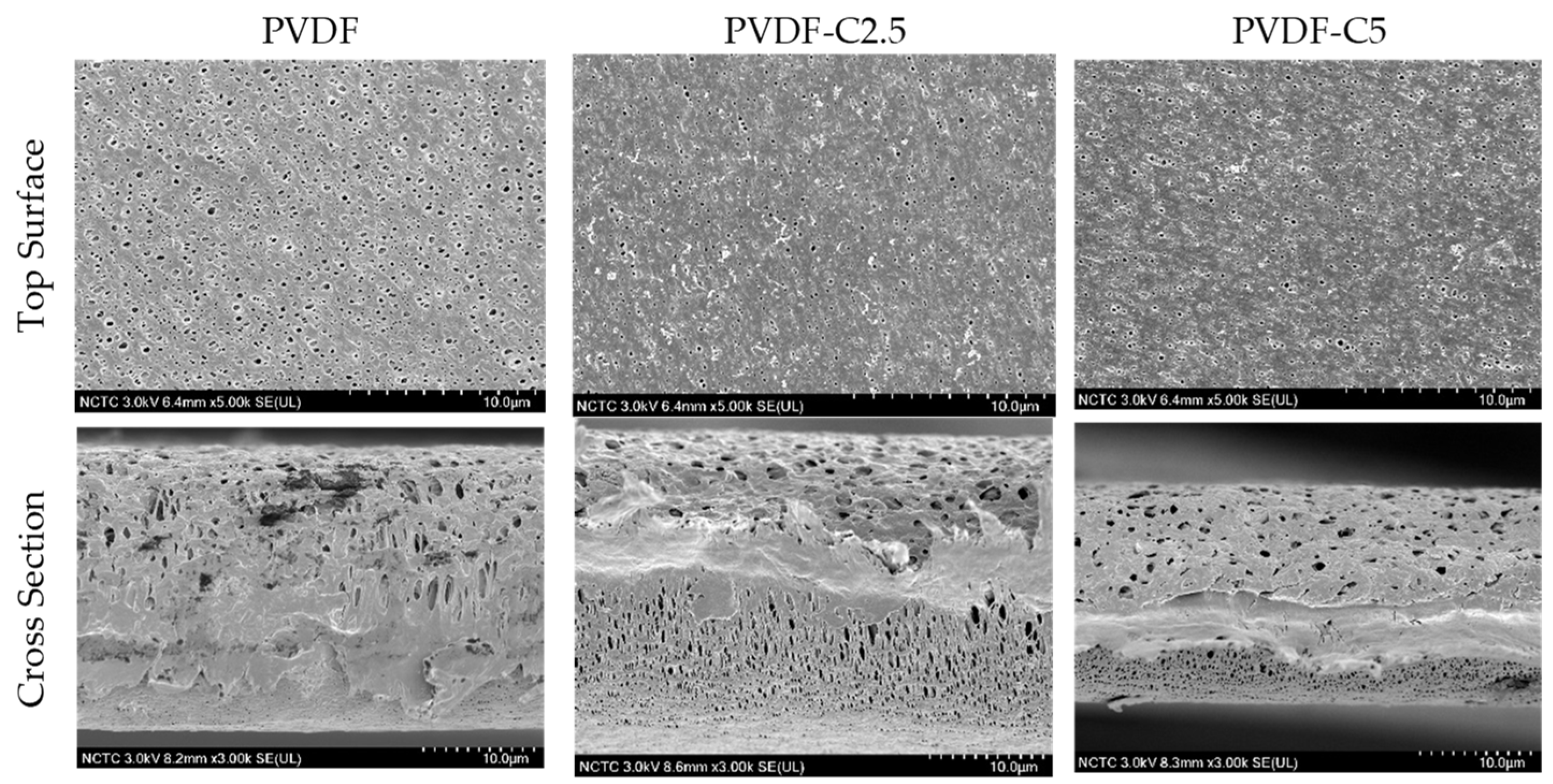
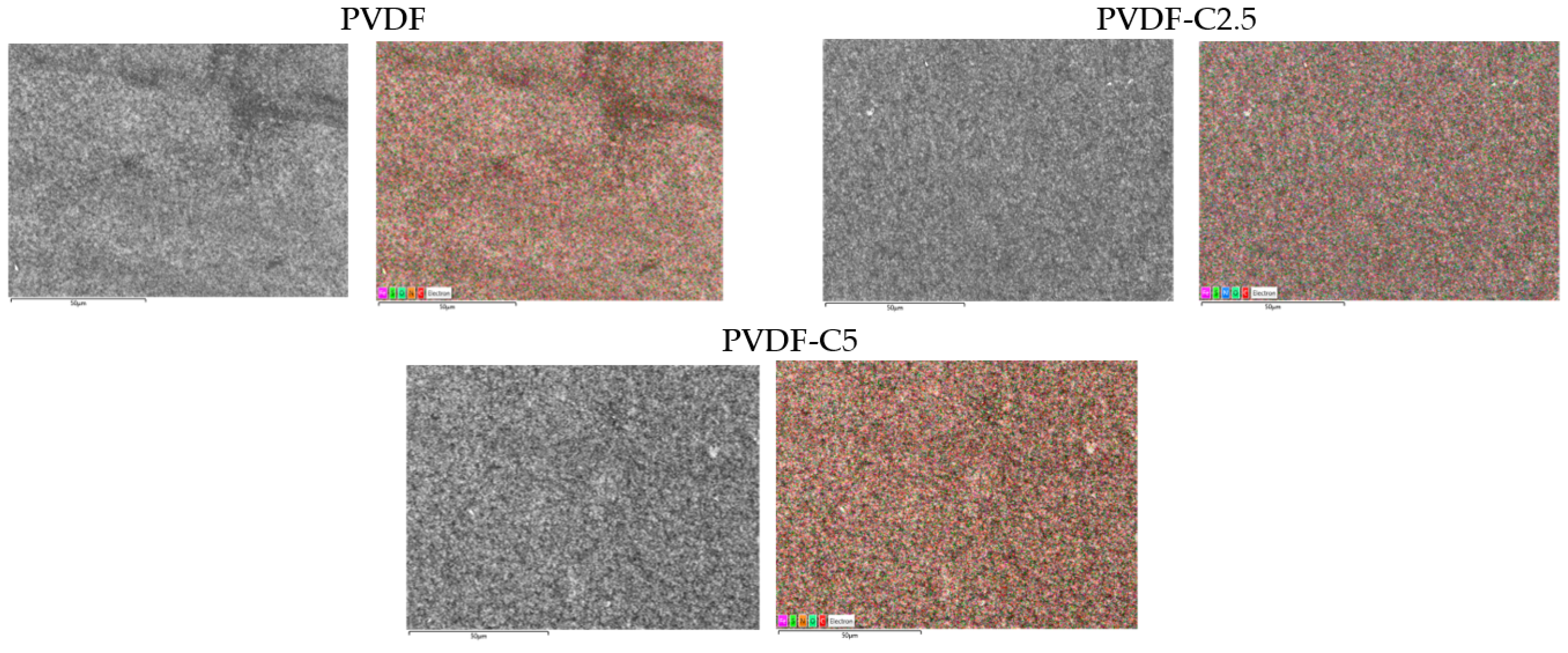
3.1.1. Membrane Morphology
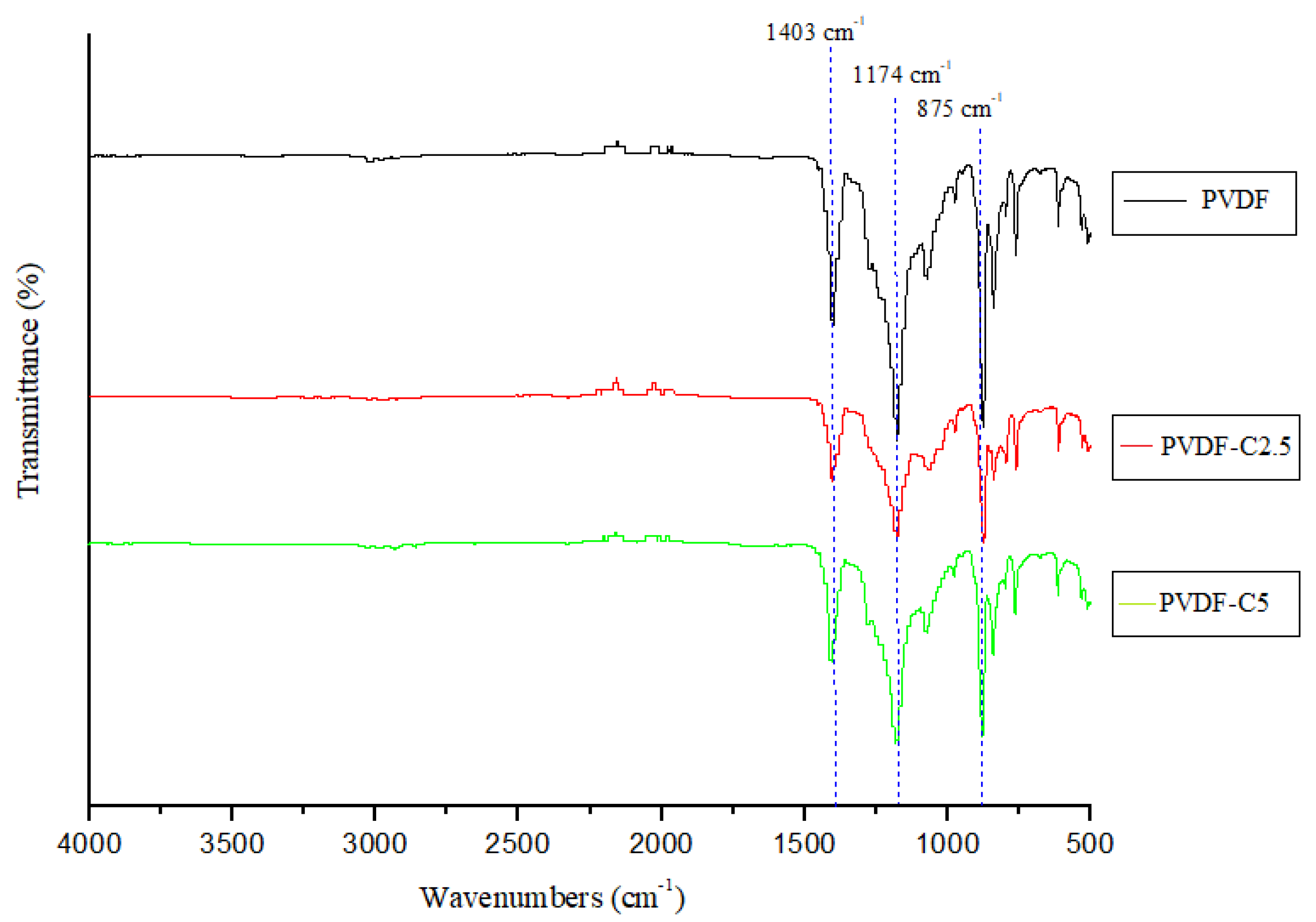
3.1.2. FTIR Spectra
3.1.3. Surface Elemental Composition
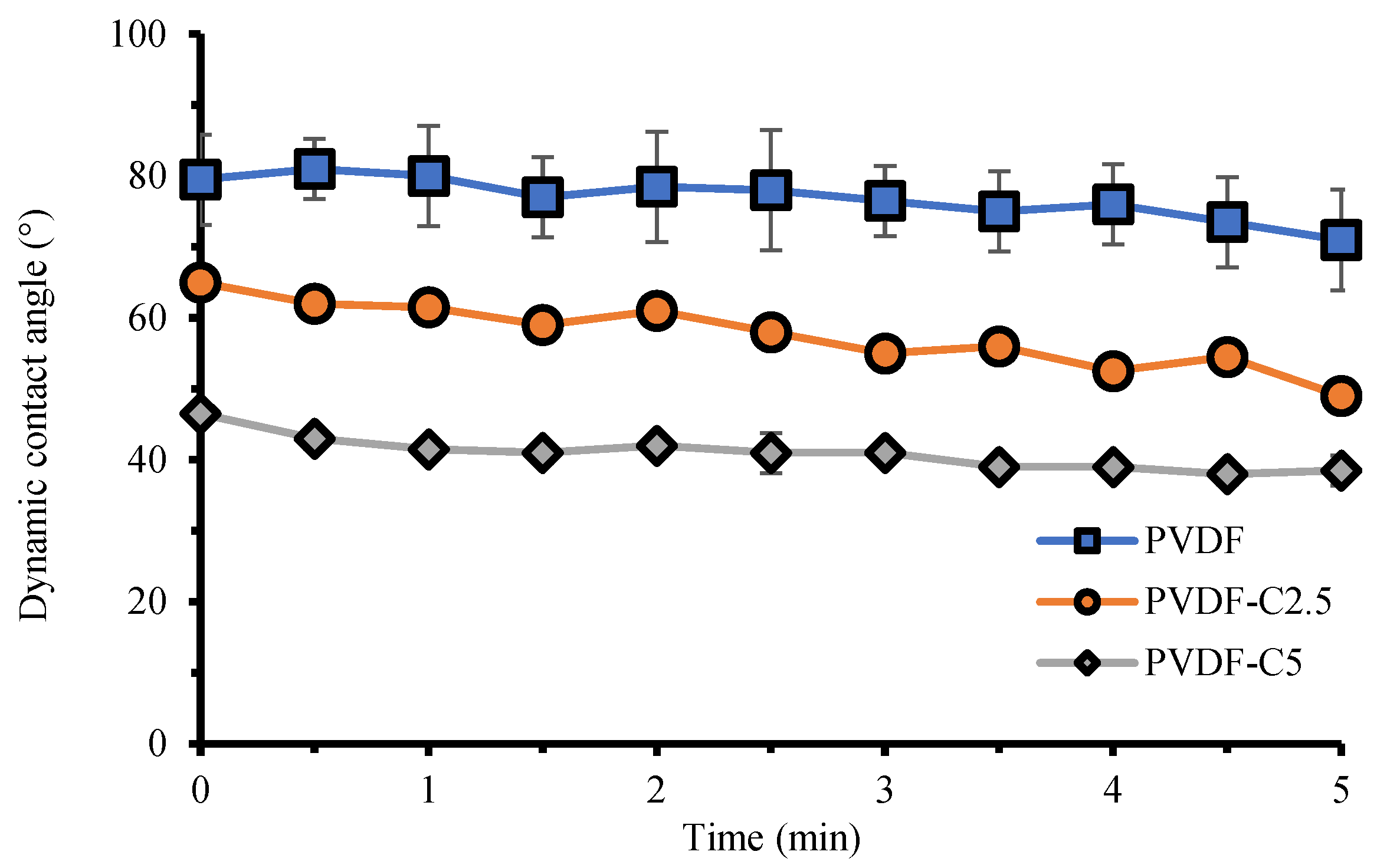
3.1.4. Surface Hydrophilicity
3.2. Membrane Filtration Performance
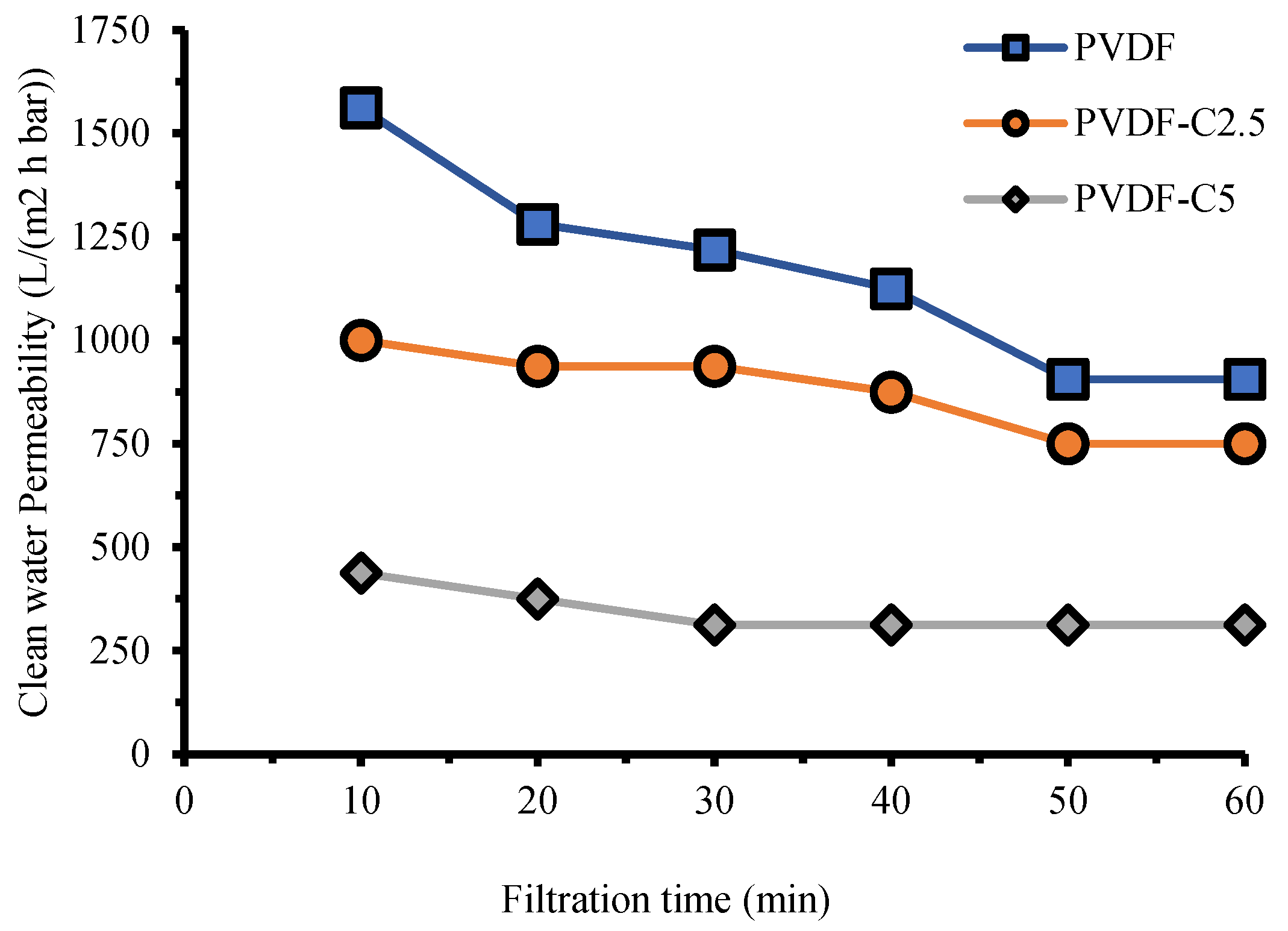
3.2.1. Clean Water Permeability
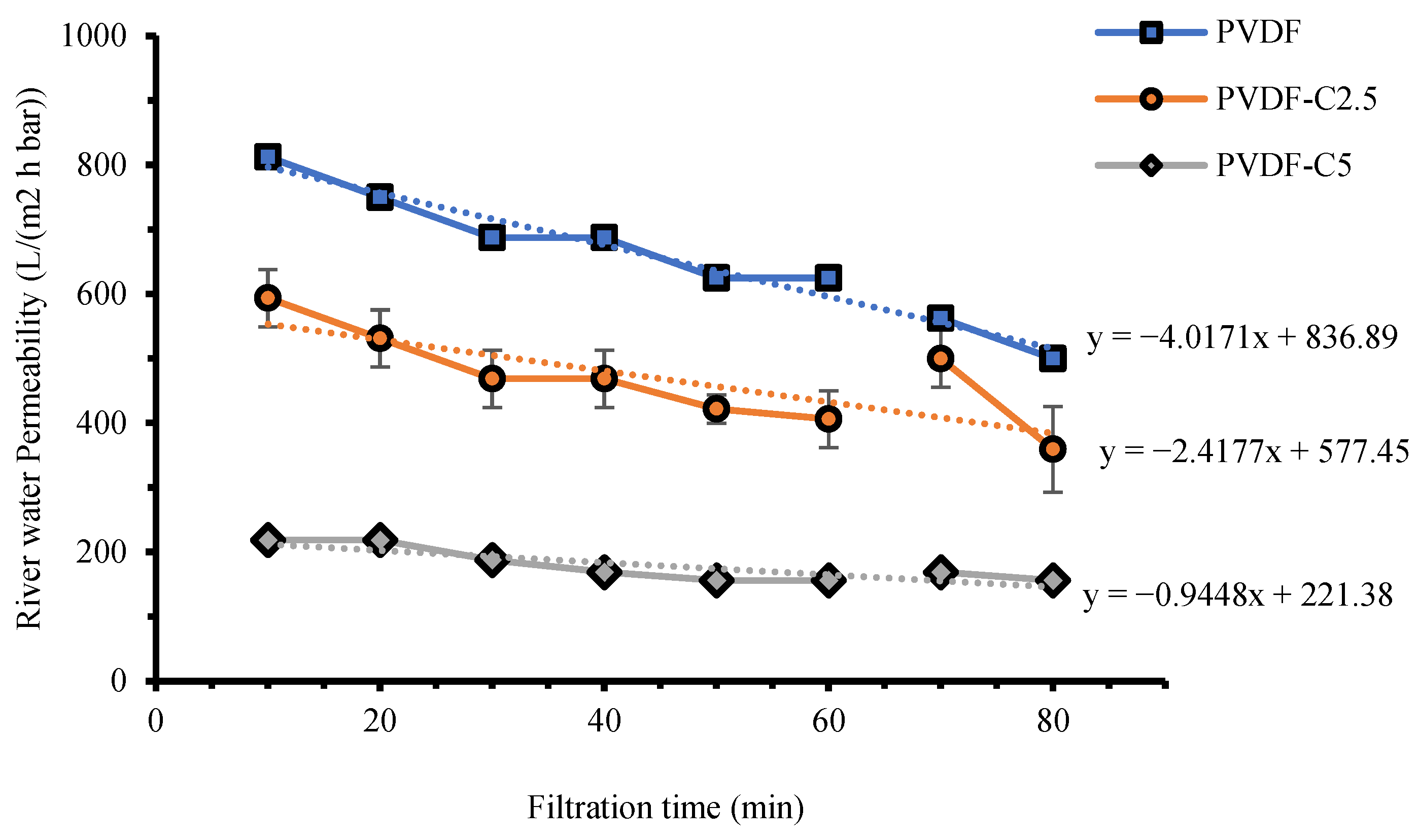
3.2.2. River Water Permeability
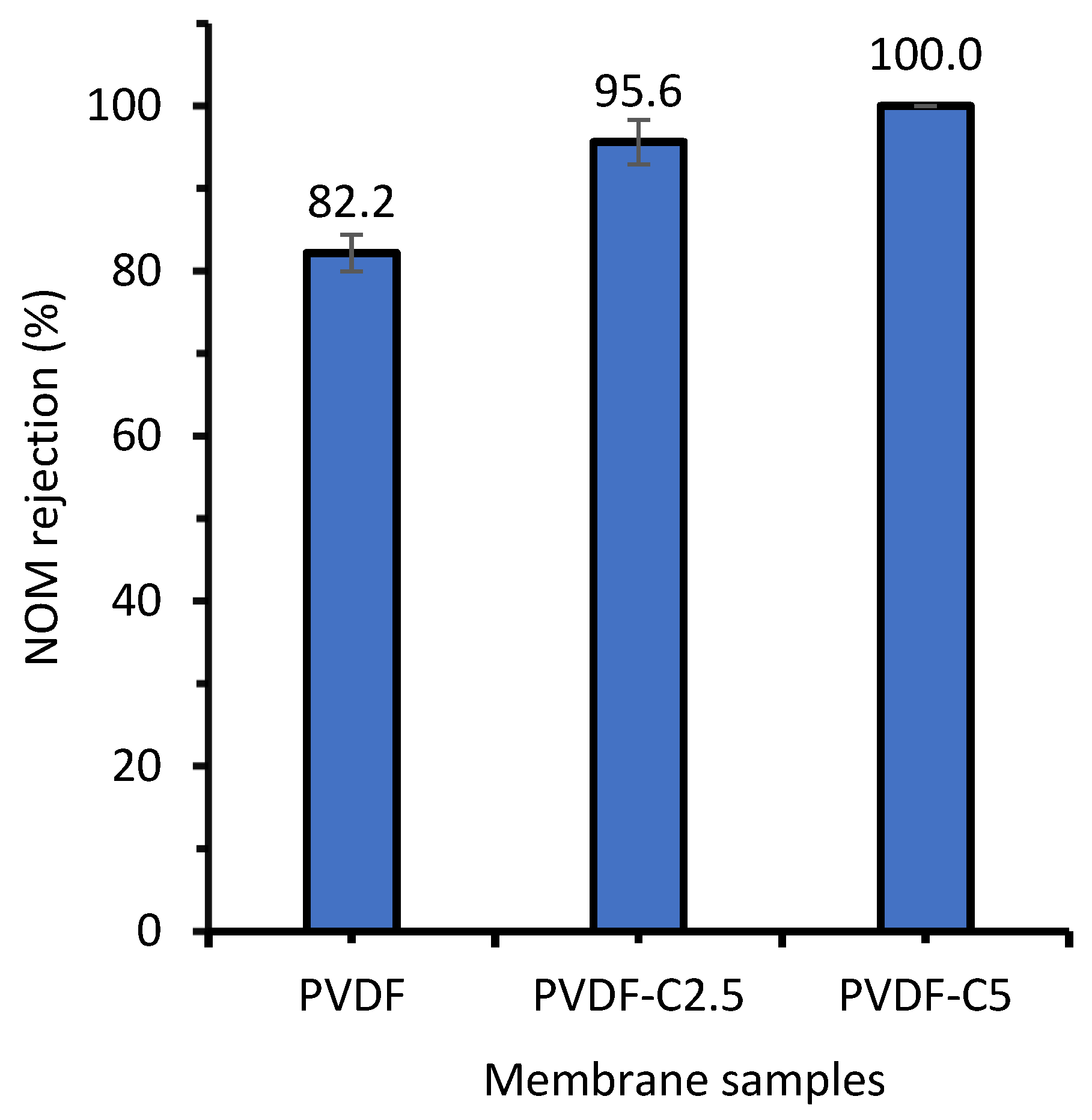
3.2.3. NOM Rejection
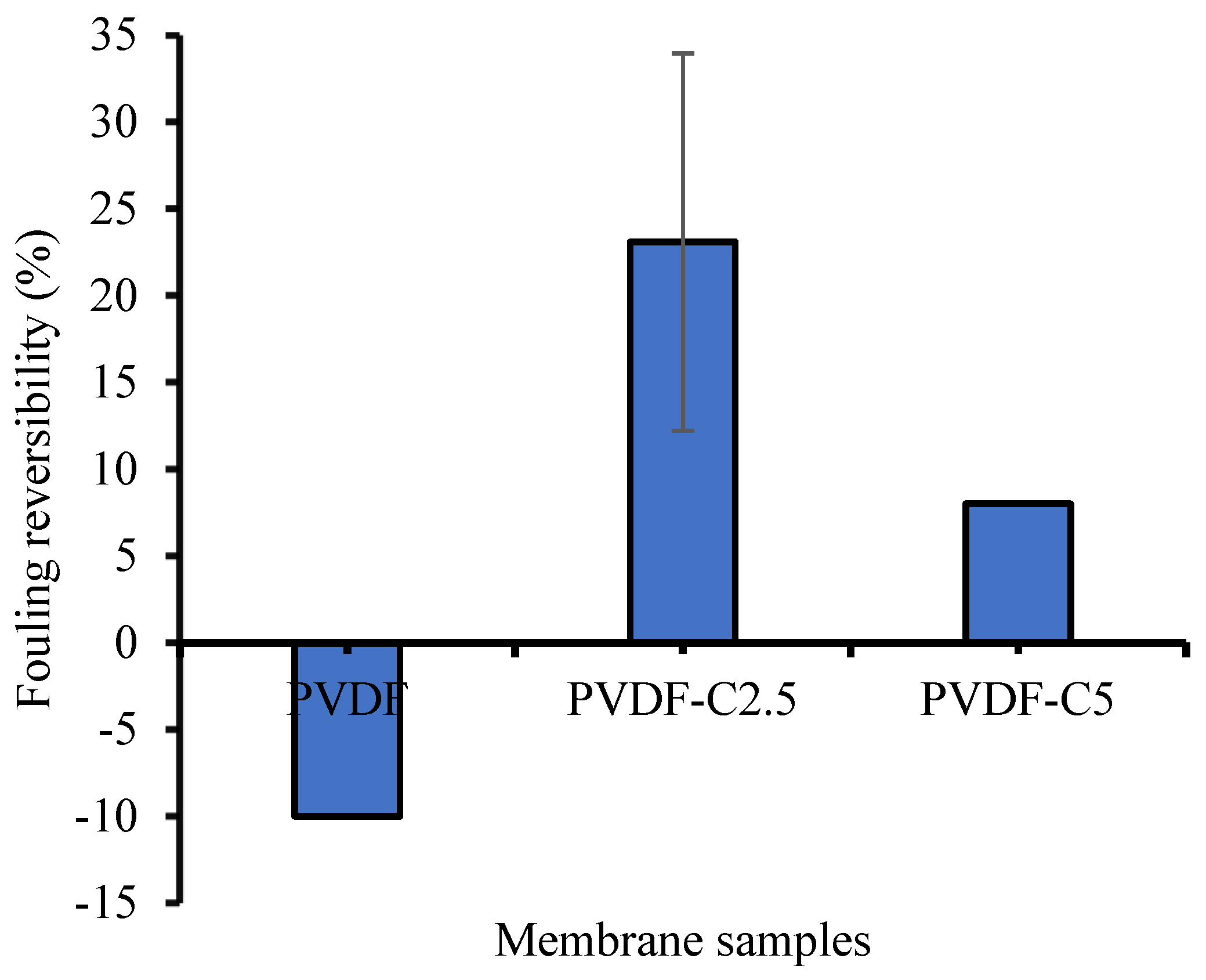
3.2.4. Fouling Resistance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramirez, L.; Gentile, R.S.; Zimmerman, S.; Stoll, S. Behavior of TiO2 and CeO2 Nanoparticles and Polystyrene Nanoplastics in Bottled Mineral, Drinking and Lake Geneva Waters. Impact of Water Hardness and Natural Organic Matter on Nanoparticle Surface Properties and Aggregation. Water 2019, 11, 2–14. [Google Scholar] [CrossRef]
- Health Canada, Guidance on Natural Organic Matter in Drinking Water, July 2020. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-natural-organic-matter-drinking-water.html (accessed on 14 November 2023).
- Moustafa, I.H.; Bream, A.S.; Awaad, A.M.E.-S.; Nader, A.M.Y. Assessment of Disinfection By-Products Levels in Aga Surfacewater Plant and Its Distribution System, Dakhlia, Egypt. Int. J. Adv. Res. Biol. Sci. 2017, 4, 37–43. [Google Scholar] [CrossRef]
- Xu, J.; Xie, A.; Sun, H.; Wu, Y.; Li, C.; Xue, C.; Cui, J.; Pan, J. Construction of Tannic Acid-Fe Complex Coated PVDF Membrane via Simple Spraying Method for Oil/Water Emulsion Separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131621. [Google Scholar] [CrossRef]
- Ali, I.; Bamaga, O.A.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.H.; Soliman, M.F.; Drioli, E.; Albeirutty, M. Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Yardimci, A.I.; Kayhan, M.; Durmus, A.; Ai, Y.; Kayhan, M.; Durmus, A.; Aksoy, M.; Synthesis, T.O.; Yardimci, A.I.; Kayhan, M.; et al. Synthesis and Air Permeability of Electrospun PAN / PVDF Nanofibrous Membranes To Cite This Article Synthesis and Air Permeability of Electrospun PAN/PVDF Nanofibrous Membranes. Res. Eng. Struct. Mater. 2022, 8, 223–231. [Google Scholar]
- Mulyati, S.; Aprilia, S.; Muchtar, S.; Syamsuddin, Y.; Rosnelly, C.M.; Bilad, M.R.; Samsuri, S.; Ismail, N.M. Fabrication of Polyvinylidene Difluoride Membrane with Enhanced Pore and Filtration Properties by Using Tannic Acid as an Additive. Polymers 2022, 14, 186. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Siekierka, A.; Bryjak, M. Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater. Polymers 2017, 9, 679. [Google Scholar] [CrossRef]
- Chen, F.; Ding, X.; Jiang, Y.; Guan, Y.; Wei, D.; Zheng, A.; Xu, X. Permanent Antimicrobial Poly(Vinylidene Fluoride) Prepared by Chemical Bonding with Poly(Hexamethylene Guanidine). ACS Omega 2020, 5, 10481–10488. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, G.; Jia, X.; Liu, D.; Wyman, I. Preparation of Multipurpose Polyvinylidene Fluoride Membranes via a Spray-Coating Strategy Using Waterborne Polymers. ACS Appl. Mater. Interfaces 2021, 13, 4485–4498. [Google Scholar] [CrossRef]
- Brinke, T.E.; Achterhuis, I.; Reurink, D.M.; De Grooth, J.; De Vos, W.M. Multiple Approaches to the Buildup of Asymmetric Polyelectrolyte Multilayer Membranes for Efficient Water Purification. ACS Appl. Polym. Mater. 2020, 2, 715–724. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of Tannic Acid in Membrane Technologies: A Review. Adv. Colloid Interface Sci. 2020, 284, 102267. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Chen, S.T.; Sivakumar, M.; Ang, M.B.M.Y.; Patra, T.; Almodovar, J.; Wickramasinghe, S.R.; Hung, W.S.; Lai, J.Y. Zwitterionic Polymer Brush Grafted on Polyvinylidene Difluoride Membrane Promoting Enhanced Ultrafiltration Performance with Augmented Antifouling Property. Polymers 2020, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, C. Efficient Preparation of Super Antifouling PVDF Ultrafiltration Membrane with One Step Fabricated Zwitterionic Surface. ACS Appl. Mater. Interfaces 2015, 7, 17947–17953. [Google Scholar] [CrossRef]
- Wu, L.; Lin, Q.; Liu, C.; Chen, W. A Stable Anti-Fouling Coating on PVDF Membrane Constructed of Polyphenol Tannic Acid, Polyethyleneimine and Metal Ion. Polymers 2019, 11, 1975. [Google Scholar] [CrossRef]
- Li, M.; Wu, L.; Zhang, C.; Chen, W.; Liu, C. Hydrophilic and Antifouling Modification of PVDF Membranes by One-Step Assembly of Tannic Acid and Polyvinylpyrrolidone. Appl. Surf. Sci. 2019, 483, 967–978. [Google Scholar] [CrossRef]
- Ghernaout, D. Natural Organic Matter Removal in the Context of the Performance of Drinking Water Treatment Processes—Technical Notes. OALib 2020, 7, 1–40. [Google Scholar] [CrossRef]
- Chheang, M.; Hongprasith, N.; Ratanatawanate, C.; Lohwacharin, J. Effects of Chemical Cleaning on the Ageing of Polyvinylidene Fluoride Microfiltration and Ultrafiltration Membranes Fouled with Organic and Inorganic Matter. Membranes 2022, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, S.; Wang, L.; Yang, Y.; Liang, J.; Cao, B. Preparation of Hydroxyapatite/Tannic Acid Coating to Enhance the Corrosion Resistance and Cytocompatibility of AZ31 Magnesium Alloys. Coatings 2017, 7, 105. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Amat, S.O.; Bilad, M.R.; Nordin, N.A.H.M.; Shamsuddin, N.; Prayogi, S.; Narkkun, T.; Faungnawakij, K. Development of Polyvinylidene Fluoride Membrane via Assembly of Tannic Acid and Polyvinylpyrrolidone for Filtration of Oil/Water Emulsion. Polymers 2021, 13, 976. [Google Scholar] [CrossRef]
- Tangarfa, M.; Semlali Aouragh Hassani, N.; Alaoui, A. Behavior and Mechanism of Tannic Acid Adsorption on the Calcite Surface: Isothermal, Kinetic, and Thermodynamic Studies. ACS Omega 2019, 4, 19647–19654. [Google Scholar] [CrossRef] [PubMed]
- Espina, A.; Cañamares, M.V.; Jurašeková, Z.; Sanchez-Cortes, S. Analysis of Iron Complexes of Tannic Acid and Other Related Polyphenols as Revealed by Spectroscopic Techniques: Implications in the Identification and Characterization of Iron Gall Inks in Historical Manuscripts. ACS Omega 2022, 7, 27937–27949. [Google Scholar] [CrossRef] [PubMed]
- Syawaliah, S.; Arahman, N.; Riza, M.; Mulyati, S. The Influences of Polydopamine Immersion Time on Characteristics and Performance of Polyvinylidene Fluoride Ultrafiltration Membrane. In Proceedings of the 3rd Annual Applied Science and Engineering Conference (AASEC 2018), Banda Aceh, Indonesia, 12 September 2018; Volume 197. [Google Scholar] [CrossRef]
- Hester, J.F.; Banerjee, P.; Won, Y.Y.; Akthakul, A.; Acar, M.H.; Mayes, A.M. ATRP of Amphiphilic Graft Copolymers Based on PVDF and Their Use as Membrane Additives. Macromolecules 2002, 35, 7652–7661. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, H.; He, C. Enhanced Separation and Antifouling Properties of PVDF Ultrafiltration Membranes with Surface Covalent Self-Assembly of Polyethylene Glycol. RSC Adv. 2015, 5, 81115–81122. [Google Scholar] [CrossRef]
- Xue, X.; Tan, G.; Zhu, Z. All-Polymer and Self-Roughened Superhydrophobic Pvdf Fibrous Membranes for Stably Concentrating Seawater by Membrane Distillation. ACS Appl. Mater. Interfaces 2021, 13, 45977–45986. [Google Scholar] [CrossRef]
- Kan, J.; Zhang, Z.; Ren, L.; Han, J.; Zhang, H.; Li, J.; Wu, H.; Chen, J. Hydrophilic Modification of PVDF Membranes for Oily Water Separation with Enhanced Anti-Fouling Performance. J. Appl. Polym. Sci. 2023, 140, e53538. [Google Scholar] [CrossRef]
- Bilad, M.R.; Junaeda, S.R.; Khery, Y.; Nufida, B.A.; Shamsuddin, N.; Usman, A.; Violet, V. Compaction of a Polymeric Membrane in Ultra-Low-Pressure Water Filtration. Polymers 2022, 14, 3254. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Sait, N.R.; Bilad, M.R.; Shamsuddin, N.; Jaafar, J.; Nordin, N.A.H.; Narkkun, T.; Faungnawakij, K.; Mohshim, D.F. Polyvinylidene Fluoride Membrane via Vapour Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers 2021, 13, 427. [Google Scholar] [CrossRef]
- Singhal, A.V.; George, R.; Sharma, A.K.; Malwal, D.; Lahiri, I. Development of Superhydrophillic Tannic Acid-Crosslinked Graphene Oxide Membranes for Efficient Treatment of Oil Contaminated Water with Enhanced Stability. Heliyon 2020, 6, e05127. [Google Scholar] [CrossRef]
| Membrane | Relative Composition (%) | ||
|---|---|---|---|
| C | O | O/C | |
| PVDF | 79.95 | 19.54 | 0.244 |
| PVDF-C2.5 | 77.11 | 22.51 | 0.292 |
| PVDF-C5 | 77.82 | 21.80 | 0.280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, R.; Shamsuddin, N.; Abu Bakar, M.S.; Thongratkaew, S.; Faungnawakij, K.; Bilad, M.R. Development of Tannic Acid Coated Polyvinylidene Fluoride Membrane for Filtration of River Water Containing High Natural Organic Matter. Sci 2023, 5, 42. https://doi.org/10.3390/sci5040042
Dewi R, Shamsuddin N, Abu Bakar MS, Thongratkaew S, Faungnawakij K, Bilad MR. Development of Tannic Acid Coated Polyvinylidene Fluoride Membrane for Filtration of River Water Containing High Natural Organic Matter. Sci. 2023; 5(4):42. https://doi.org/10.3390/sci5040042
Chicago/Turabian StyleDewi, Rosmaya, Norazanita Shamsuddin, Muhammad Saifullah Abu Bakar, Sutarat Thongratkaew, Kajornsak Faungnawakij, and Muhammad Roil Bilad. 2023. "Development of Tannic Acid Coated Polyvinylidene Fluoride Membrane for Filtration of River Water Containing High Natural Organic Matter" Sci 5, no. 4: 42. https://doi.org/10.3390/sci5040042
APA StyleDewi, R., Shamsuddin, N., Abu Bakar, M. S., Thongratkaew, S., Faungnawakij, K., & Bilad, M. R. (2023). Development of Tannic Acid Coated Polyvinylidene Fluoride Membrane for Filtration of River Water Containing High Natural Organic Matter. Sci, 5(4), 42. https://doi.org/10.3390/sci5040042







