Yield and Composition Variations of the Milk from Different Camel Breeds in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Locations
2.2. Milk Yield
2.3. Milk Composition
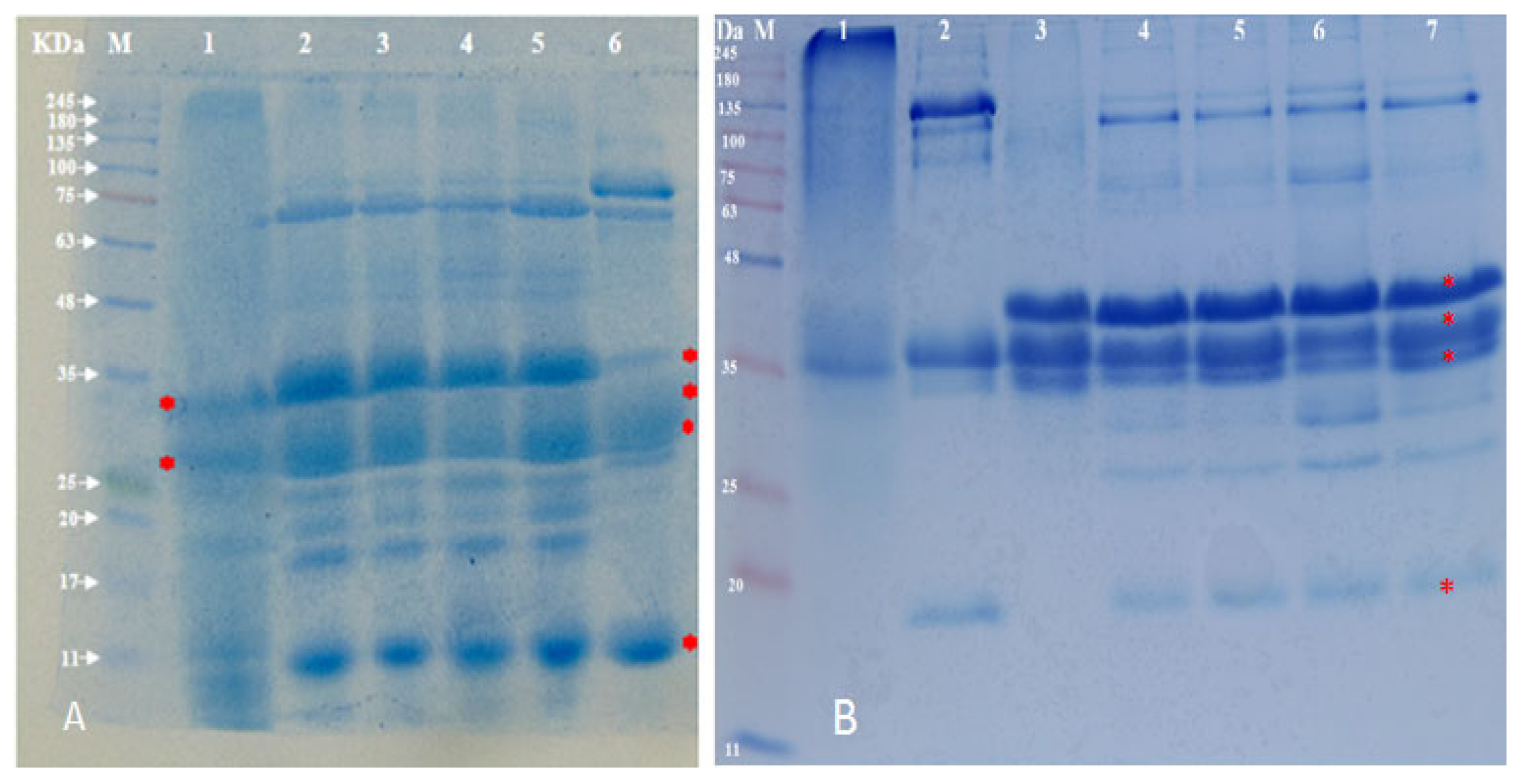
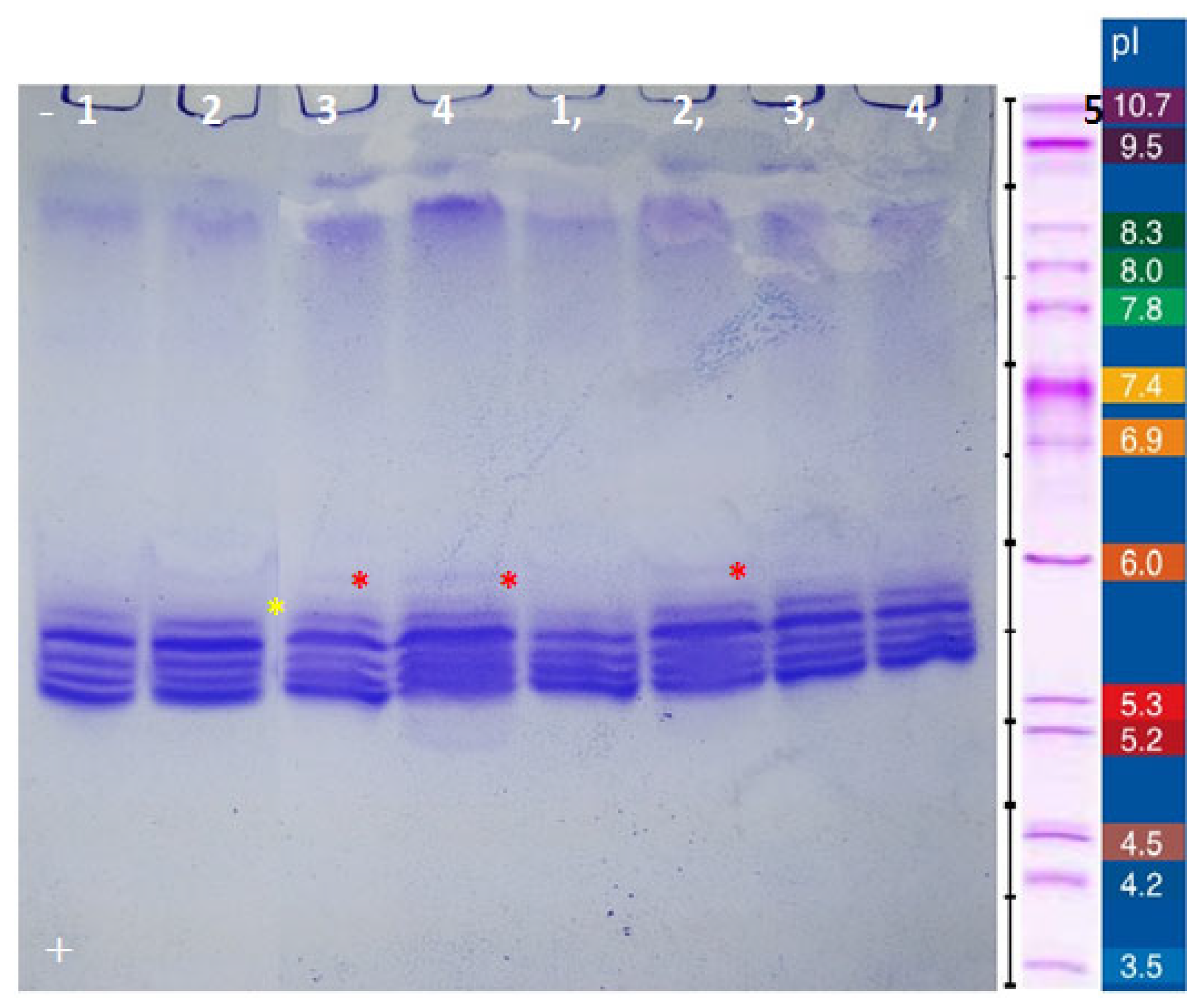
2.4. Purification and Electrophoresis of Casein from Different Camel Breeds
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Seasonal Variation in Milk Yield
3.2. Seasonable Variability in the Milk Composition of Saudi Camel Breeds
3.3. Fractionation of Camel Casein and Cream
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banaja, A.A.; Ghandour, A.M. A review of parasites of camels (Camelus dromedarius) in Saudi Arabia. Science 1994, 6, 75–86. [Google Scholar] [CrossRef]
- FAO. Available online: http://www.fao.org/ (accessed on 3 December 2021).
- El Agamy, E.I. Camel milk. In Handbook of Non-Bovine Mammals; Park, Y.W., Haenlein, F.W., Eds.; Blackwell Publisher Professional: Iowa, NJ, USA, 2006; pp. 297–344. [Google Scholar]
- Farah, Z.; Mollet, M.; Younan, M.; Dahir, R. Camel dairy in Somalia: Limiting factors and development potential. Livest. Sci. 2007, 110, 187–191. [Google Scholar] [CrossRef]
- Farah, Z. Camel Milk Properties and Products; Swiss Centre for Developments Cooperation in Technology and Management: St. Gallen, Switzerland, 1996. [Google Scholar]
- Zhang, H.; Yao, J.; Zhao, D.; Liu, H.; Li, J.; Guo, M. Changes in chemical composition of Alxa bactrian camel milk during lactation. J. Dairy Sci. 2005, 88, 3402–3410. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.; Inoue-Murayama, M. Advances in camel genomics and their applications: A review. J. Anim. Gen. 2017, 45, 49–58. [Google Scholar] [CrossRef]
- Kappeler, S.; Farah, Z.; Puhan, Z. Sequence analysis of Camelus dromedarius milk caseins. J. Dairy Res. 1998, 65, 209–222. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G. The composition of camel milk: A meta-analysis of the literature data. J. Food Compost. Anal. 2009, 22, 95–101. [Google Scholar] [CrossRef]
- Nikkah, A. Equidae, camel, and yak milks as functional foods: A review. J. Nutr. Food Sci. 2011, 1, 1000116. [Google Scholar]
- Ganzorig, K.; Urashima, T.; Fukuda, K. Exploring Potential Bioactive Peptides in Fermented Bactrian Camel’s Milk and Mare’s Milk Made by Mongolian Nomads. Foods 2020, 9, 1817. [Google Scholar] [CrossRef]
- Abdallah, H.R.; Faye, B. Phenotypic classification of Saudi Arabian camel (Camelus dromedarius) by their body measurements. Emir. J. Food Agric. 2012, 24, 272–280. [Google Scholar]
- Mahmoud, A.H.; Abu-Tarbush, F.M.; Alshaik, M.; Aljumaah, R.; Saleh, A. Genetic diversity and population genetic structure of six dromedary camel (camelus dromedarius) populations in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 1384–1389. [Google Scholar] [CrossRef]
- Al-Swailem, A.M.; Shehata, M.M.; Abu-Duhier, F.M.; Al-Yamani, E.J.; Al-Busadah, K.A.; Al-Arawi, M.S.; Al-Khider, A.Y.; Al-Muhaimeed, A.N.; Al-Qahtani, F.H.; Manee, M.M.; et al. Sequencing, analysis, and annotation of expressed sequence tags for Camelus dromedarius. PLoS ONE 2010, 5, e10720. [Google Scholar] [CrossRef]
- Almathen, F.; Mwaracharo, J.; Hanotte, O. Genetic diversity and relationships of indigenous Saudi Arabia camel Camelus dromedarius populations. In Proceedings of the 3rd Conference of the International Society of Camelid Research and Development (ISOCARD)., Mascat, Oman, 29 January–1 February 2012; pp. 40–41. [Google Scholar]
- AL-Haknah, M.M. Milk production and behaviour of Saudi camel. Sci. Technol. J. 2003, 17, 25–55. [Google Scholar]
- Nagy, P.; Thomas, S.; Marko, O.; Juhasz, J. Milk production, raw milk quality and fertility of dromedary camels (Camelus Dromedarius) under intensive management. Acta Vet. Hung. 2013, 61, 71–84. [Google Scholar] [CrossRef]
- Abdalla, E.B.; Ashmawy, A.A.; Farouk, M.H.; Salama, O.A.; Khalil, F.A.; Seioudy, A.F. Milk production potential in Maghrebi she-camels. Small Rumin. Res. 2015, 123, 129–135. [Google Scholar] [CrossRef]
- Mussad, A.M.; Faye, B.; AL-Mutairi, S.E. Seasonal and physiological variation of gross composition of camel milk in Saudi Arabia. Emir. J. Food Agric. 2013, 25, 618–624. [Google Scholar]
- Shah, N.P. Effects of milk-derived bioactives: An overview. Br. J. Nutr. 2000, 84 (Suppl. S1), 3–10. [Google Scholar] [CrossRef]
- Cohen, M.S.; Britigan, B.E.; French, M.; Bean, K. Preliminary observations on lactoferrin secretion in human vaginal mucus: Variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am. J. Obstet. Gynecol. 1987, 157, 1122–1125. [Google Scholar] [CrossRef]
- Harmsen, M.C.; Swart, P.J.; de Bethune, M.P.; Pauwels, R.; De Clercq, E.; The, T.H.; Meijer, D.K. Antiviral effects of plasma and milk proteins: Lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J. Infect. Dis. 1995, 172, 380–388. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin--a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Sanchez, L.; Calvo, M.; Brock, J.H. Biological role of lactoferrin. Arch. Dis. Child. 1992, 67, 657–661. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, A. Lactoferrin research, technology and applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Abedelbaky, N.; Haroun, B.M.; Sanchez, L.; Redwan, N.A.; Redwan, E.M. Anti-infectivity of camel polyclonal antibodies against hepatitis C virus in Huh7.5 hepatoma. Virol. J. 2012, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; El-Fakkarany, E.; Lonnerdal, B.; Redwan, E.M. Inhibitory effects of native and recombinant full-length camel lactoferrin and its N and C lobes on hepatitis C virus infection of Huh7.5 cells. J. Med. Microbiol. 2012, 61, 375–383. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, E.M.; Sanchez, L.; Al-Mehdar, H.A.; Redwan, E.M. Effectiveness of human, camel, bovine and sheep lactoferrin on the hepatitis C virus cellular infectivity: Comparison study. Virol. J. 2013, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Szymanowska, M.; Zwierzchowski, L.; Leroux, C. The impact of genetic polymorphisms on the protein composition of ruminant milks. Reprod. Nutr. Dev. 2002, 42, 433–459. [Google Scholar] [CrossRef]
- Scott, F.W. Cow milk and insulin-dependent diabetes mellitus: Is there a relationship? Am. J. Clin. Nutr. 1990, 51, 489–491. [Google Scholar] [CrossRef]
- Dahl-Jorgensen, K.; Joner, G.; Hanssen, K.F. Relationship between cows’ milk consumption and incidence of IDDM in childhood. Diabetes Care 1991, 14, 1081–1083. [Google Scholar] [CrossRef]
- Shori, A.B. Camel milk as a potential therapy for controlling diabetes and its complications: A review of in vivo studies. J. Food Drug Anal. 2015, 23, 609–618. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Tantia, P.; Jain, S.; Agrawal, R.; Agrawal, V. Camel milk: A possible boon for type 1 diabetic patients. Cell. Mol. Biol. 2013, 59, 99–107. [Google Scholar]
- Agrawal, R.P.; Jain, S.; Shah, S.; Chopra, A.; Agarwal, V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 1048–1052. [Google Scholar] [CrossRef]
- Mohamad, R.H.; Zekry, Z.K.; Al-Mehdar, H.A.; Salama, O.; El-Shaieb, S.E.; El-Basmy, A.A.; Al-said, M.G.; Sharawy, S.M. Camel milk as an adjuvant therapy for the treatment of type 1 diabetes: Verification of a traditional ethnomedical practice. J. Med. Food 2009, 12, 461–465. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Beniwal, R.; Kochar, D.K.; Tuteja, F.C.; Ghorui, S.K.; Sahani, M.S.; Sharma, S. Camel milk as an adjunct to insulin therapy improves long-term glycemic control and reduction in doses of insulin in patients with type-1 diabetes A 1 year randomized controlled trial. Diabetes Res. Clin. Pract. 2005, 68, 176–177. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.; Sharma, R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014, 35, 153–156. [Google Scholar] [CrossRef]
- Bakry, I.A.; Yang, L.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Cacciotti, I.; Ziedan, N.I.; Jin, J.; Jin, Q.; Wei, W.; et al. A Comprehensive Review of the Composition, Nutritional Value, and Functional Properties of Camel Milk Fat. Foods 2021, 10, 2158. [Google Scholar] [CrossRef]
- Bakry, I.A.; Ali, A.H.; Abdeen, E.-S.M.; Ghazal, A.F.; Wei, W.; Wang, X. Comparative characterisation of fat fractions extracted from Egyptian and Chinese camel milk. Int. Dairy J. 2020, 105, 104691. [Google Scholar] [CrossRef]
- Bakry, I.A.; Korma, S.A.; Wei, W.; Nafea, A.E.; Mahdi, A.A.; Ziedan, N.I.; Wang, X. Changes in the fatty acid content of Egyptian human milk across the lactation stages and in comparison with Chinese human milk. Eur. Food Res. Technol. 2021, 247, 1035–1048. [Google Scholar] [CrossRef]
- Nyuar, K.; Min, Y.; Ghebremeskel, K.; Khalil, A.; Elbashir, M.; Cawford, M. Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Paediatr. 2010, 99, 1824–1827. [Google Scholar] [CrossRef]
- Cardak, A.; Yetismeyen, A.; Bruckner, H. Quantitative comparison of camel, goat and cow milk fatty acids. Milchwissenschaft 2003, 58, 34–36. [Google Scholar]
- Maqsood, S.; Al-Dowaila, A.; Mudgil, P.; Kamal, H.; Jobe, B.; Hassan, H.M. Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2019, 279, 328–338. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef]
- Akbar, N. Science of camel and yak milks: Human nutrition and health perspectives. Food Nutr. Sci. 2011, 2, 6626. [Google Scholar]
- Mann, J.I. Diet and risk of coronary heart disease and type 2 diabetes. Lancet 2002, 360, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Gorban, A.M.; Izzeldin, O.M. Study on cholesteryl ester fatty acids in camel and cow milk lipid. Int. J. Food Sci. Technol. 1999, 34, 229–234. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Lemarie, É.; Faye, B.; Loiseau, G.; Montet, D. Fatty acid and cholesterol composition of camel’s (Camelus bactrianus, Camelus dromedarius and hybrids) milk in Kazakhstan. Dairy Sci. Technol. 2008, 88, 327–340. [Google Scholar] [CrossRef]
- Smiddy, M.A.; Huppertz, T.; van Ruth, S.M. Triacylglycerol and melting profiles of milk fat from several species. Int. Dairy J. 2012, 24, 64–69. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M.; Al-Masaud, A.; Konuspayeva, G. Comparative milk and serum cholesterol content in dairy cow and camel. J. King Saud Univ.-Sci. 2015, 27, 168–175. [Google Scholar] [CrossRef]
- Badriah, A. Effect of camel milk on blood glucose, cholesterol, triglyceride and liver enzymes activities in female albino rats. World Appl. Sci. J. 2012, 17, 1394–1397. [Google Scholar]
- Elayan, A.; Sulieman, A.; Saleh, F. The hypocholesterolemic effect of Gariss and Gariss containing bifidobacteria in rats fed on a cholesterol-enriched diet. Asian J. Biochem. 2008, 3, 43–47. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.S.; Sharma, R.; Singh, R. Effect of goat and camel milk vis a vis cow milk on cholesterol homeostasis in hypercholesterolemic rats. Small Rumin. Res. 2019, 171, 8–12. [Google Scholar] [CrossRef]
- Nagy, P.; Fábri, Z.N.; Varga, L.; Reiczigel, J.; Juhász, J. Effect of genetic and nongenetic factors on chemical composition of individual milk samples from dromedary camels (Camelus dromedarius) under intensive management. J. Dairy Sci. 2017, 100, 8680–8693. [Google Scholar] [CrossRef]
- Nagy, P.; Juhász, J.; Reiczigel, J.; Császár, G.; Kocsis, R.; Varga, L. Circannual changes in major chemical composition of bulk dromedary camel milk as determined by FT-MIR spectroscopy, and factors of variation. Food Chem. 2019, 278, 248–253. [Google Scholar] [CrossRef]
- Deibel, C.; Deibel, R. Laboratory analysis of milk and dairy products. In Dairy Processing and Quality Assurance; Chandan, R.C., Kilara, A., Shah, N.P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 600–646. [Google Scholar]
- Almahdy, O.; El-Fakharany, E.M.; El-Dabaa, E.; Ng, T.B.; Redwan, E.M. Examination of the activity of camel milk casein against hepatitis C virus (genotype-4a) and its apoptotic potential in hepatoma and hela cell lines. Hepat. Mon. 2011, 11, 724–730. [Google Scholar] [CrossRef]
- Redwan, E.M. Simple, sensitive, and quick protocol to detect less than 1 ng of bacterial lipopolysaccharide. Prep. Biochem. Biotechnol. 2012, 42, 171–182. [Google Scholar] [CrossRef]
- Almehdar, H.A.; Adel-Sadek, M.A.; Redwan, E.M. Immunoreactivity and two-dimensional gel-electrophoresis characterization of Egyptian cobra venom proteome. Pak. J. Pharm. Sci. 2015, 28, 59–64. [Google Scholar]
- O’Farrell, P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975, 250, 4007–4021. [Google Scholar] [CrossRef]
- Musaad, A.; Faye, B.; Nikhela, A.A. Lactation curves of dairy camels in an intensive system. Trop. Anim. Health Prod. 2013, 45, 1039–1046. [Google Scholar] [CrossRef]
- Mehaia, M.A.; Hablas, M.A.; Abdel-Rahman, K.M.; El-Mougy, S.A. Milk composition of Majaheim, Wadah and Hamra camels in Saudi Arabia. Food Chem. 1995, 52, 115–122. [Google Scholar] [CrossRef]
- Haddadin, M.S.; Gammoh, S.I.; Robinson, R.K. Seasonal variations in the chemical composition of camel milk in Jordan. J. Dairy Res. 2008, 75, 8–12. [Google Scholar] [CrossRef]
- Alhaj, O.A.; Altooq, N.J.; Alenezi, A.F.; Janahi, A.I.; Janahi, M.I.; Humood, A.M.; AlRasheed, M.M.; Bragazzi, N.L.; Jahrami, H.A.; Faye, B. Camel milk composition by breed, season, publication year, and country: A global systematic review, meta-analysis, and meta-regression. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2520–2559. [Google Scholar] [CrossRef]
- Hanganu, A.; Chira, N.A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Ivanova, M.; Hanganu, A.; Dumitriu, R.; Tociu, M.; Ivanov, G.; Stavarache, C.; Popescu, L.; Ghendov-Mosanu, A.; Sturza, R.; Deleanu, C.; et al. Saponification Value of Fats and Oils as Determined from (1)H-NMR Data: The Case of Dairy Fats. Foods 2022, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- Khaskheli, M.; Arain, M.A.; Chaudhry, S.; Soomro, A.H.; Qureshi, T.A. Physico-chemical quality of camel milk. J. Agricult. Soc. Sci. 2005, 2, 164–166. [Google Scholar]
- Mohamed, A. Characterization of Camel Milk Beta-Casein; University of Karachi: Karachi, Pakistan, 1993. [Google Scholar]
- Restani, P.; Gaiaschi, A.; Plebani, A.; Beretta, B.; Cavagni, G.; Fiocchi, A.; Poiesi, C.; Velona, T.; Ugazio, A.G.; Galli, C.L. Cross-reactivity between milk proteins from different animal species. J. Br. Soc. Allergy Clin. Immunol. 1999, 29, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.O.; Ismael, M.A.; Al-Hosaini, K.; Rame, C.; Al-Senaidy, A.M.; Dupont, J.; Ayoub, M.A. Differential Effects of Camel Milk on Insulin Receptor Signaling—Toward Understanding the Insulin-Like Properties of Camel Milk. Front. Endocrinol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Salmen, S.H.; Abu-Tarboush, H.M.; Al-Saleh, A.A.; Metwalli, A.A. Amino acids content and electrophoretic profile of camel milk casein from different camel breeds in Saudi Arabia. Saudi J. Biol. Sci. 2012, 19, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Al Haj, O.A.; Kanhal, H.A. Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J. 2010, 20, 811–821. [Google Scholar] [CrossRef]
- Erhardt, G.; Shuiep, E.T.S.; Lisson, M.; Weimann, C.; Wang, Z.; El Zubeir Iel, Y.; Pauciullo, A. Alpha S1-casein polymorphisms in camel (Camelus dromedarius) and descriptions of biological active peptides and allergenic epitopes. Trop. Anim. Health Prod. 2016, 48, 879–887. [Google Scholar] [CrossRef]
- Redwan, E.M.; Alkarim, S.A.; El-Hanafy, A.A.; Saad, Y.M.; Almehdar, H.A.; Uversky, V.N. Variability of Some Milk-Associated Genes and Proteins in Several Breeds of Saudi Arabian Camels. Protein J. 2018, 37, 333–352. [Google Scholar] [CrossRef]
- Mudgil, P.; Baba, W.N.; Alneyadi, M.; Redha, A.A.; Maqsood, S. Production, characterization, and bioactivity of novel camel milk-based infant formula in comparison to bovine and commercial sources. LWT 2022, 154, 112813. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bogh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. [Google Scholar] [CrossRef]
- Baba, W.N.; Baby, B.; Mudgil, P.; Gan, C.-Y.; Vijayan, R.; Maqsood, S. Pepsin generated camel whey protein hydrolysates with potential antihypertensive properties: Identification and molecular docking of antihypertensive peptides. Lwt 2021, 143, 111135. [Google Scholar] [CrossRef]
| Breeds | Milk Yield (kg/Week) | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| Majahem | 45.0 ± 4.1 a | 41.9 ± 2.4 a | 31.33 ± 0.77 a | 34.0 ± 3.1 a |
| Safra | 39.3 ± 5.1 b | 30.7 ± 3.4 b | 30.07 ± 0.85 a | 31.1 ± 6.2 a,b |
| Wadha | 41.9 ± 5.6 a,b | 39.1 ± 2.0 a | 25.3 ± 1.2 b | 27.1 ± 4.6 b |
| Hamra | 39.9 ± 4.7 b | 40.1 ± 1.3 a | 30.5 ± 0.52 a | 34.7 ± 6.6 a |
| Breed | Saudi Camel Clan Milk Composition | |||||||||||
| Fat% | Protein% | Lactose% | ||||||||||
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| Majahem | 3.65 ± 0.34 | 1.91 ± 0.06 b | 2.81 ± 0.52 | 3.47 ± 0.18 a | 3.15 ± 0.04 a | 2.61 ± 0.05 b | 2.72 ± 0.08 | 3.10 ± 0.03 | 4.73 ± 0.06 a,d | 3.92 ± 0.07 b | 4.08 ± 0.12 | 4.65±0.04 a |
| Safra | 4.00 ± 0.12 | 1.77 ± 0.20 b | 2.68 ± 0.62 | 3.91 ± 0.41 a | 2.93 ± 0.05 b | 2.90 ± 0.09 a | 2.76 ± 0.25 | 3.05 ± 0.06 | 4.40 ± 0.07 b,c | 4.23 ± 0.04 b | 4.14 ± 0.37 | 4.63 ± 0.06 a |
| Wadha | 3.78 ± 0.27 | 2.26 ± 0.11 b | 1.53 ± 0.22 | 3.43 ± 0.18 a | 3.18 ± 0.05 a | 2.67 ± 0.10 b | 2.78 ± 0.10 | 3.12 ± 0.09 | 4.77 ± 0.07 d | 3.89 ± 0.11 b | 4.18 ± 0.15 | 4.67 ± 0.07 a |
| Hamra | 4.04 ± 0.14 | 3.87 ± 0.26 a | 2.47 ± 0.45 | 2.25 ± 0.23 b | 3.05 ± 0.05 a,b | 2.95 ± 0.02 a | 2.89 ± 0.09 | 2.93 ± 0.04 | 4.56 ± 0.04 a,c | 4.64 ± 0.20 a | 4.35 ± 0.14 | 4.40 ± 0.06 b |
| Breed | Saudi Camel Clans’ Milk Composition | |||||||||||
| Salt% | SNF% | pH | ||||||||||
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| Majahem | 0.71 ± 0.01 a,d | 0.58 ± 0.01 b | 0.61 ± 0.01 | 0.70 ± 0.01 a | 8.60 ± 0.11 a | 7.14 ± 0.13 | 7.43 ± 0.23 | 8.74 ± 0.08 a | 6.71 ± 0.015 a | 6.37 ± 0.02 b | 6.71 ± 0.07 | 6.83 ± 0.01 a |
| Safra | 0.66 ± 0.11 b,c | 0.63 ± 0.01 a | 0.63 ± 0.05 | 0.69 ± 0.01 a | 8.00 ± 0.14 b | 7.70 ± 0.08 | 7.53 ± 0.68 | 8.42 ± 0.12 a | 6.61 ± 0.023 b | 6.50 ± 0.01 a | 7.01 ± 0.10 | 6.82 ± 0.02 a |
| Wadha | 0.71 ± 0.01 d | 0.58 ± 0.01 b | 0.62 ± 0.02 | 0.70 ± 0.011 a | 8.55 ± 0.19 a | 7.08 ± 0.20 | 7.60 ± 0.28 | 8.52 ± 0.14 a | 6.79 ± 0.021 c | 6.40 ± 0.02 b | 6.49 ± 0.09 | 6.87 ± 0.03 a |
| Hamra | 0.68 ± 0.01 a,c | 0.66 ± 0.01 a | 0.65 ± 0.02 | 0.66 ± 0.01 b | 8.33 ± 0.08 a,b | 7.70 ± 0.39 | 7.88 ± 0.24 | 7.97 ± 0.11 b | 6.77 ± 0.02 c,d | 6.48 ± 0.01 a | 6.50 ± 0.15 | 6.72 ± 0.28 b |
| From 100 mL of Camel Milk | ||||
|---|---|---|---|---|
| Safra | Wadha | Hamra | Majahem | |
| Cream | 6.76 ± 1.09 a | 2.69 ± 0.76 b | 3.33 ± 0.87 b | 4.35 ± 0.73 b |
| Casein | 1.73 ± 0.45 b | 2.29 ± 0.56 a | 2.07 ± 0.42 | 1.86 ± 0.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hanafy, A.A.; Saad, Y.M.; Alkarim, S.A.; Almehdar, H.A.; Alzahrani, F.M.; Almatry, M.A.; Uversky, V.N.; Redwan, E.M. Yield and Composition Variations of the Milk from Different Camel Breeds in Saudi Arabia. Sci 2023, 5, 2. https://doi.org/10.3390/sci5010002
El-Hanafy AA, Saad YM, Alkarim SA, Almehdar HA, Alzahrani FM, Almatry MA, Uversky VN, Redwan EM. Yield and Composition Variations of the Milk from Different Camel Breeds in Saudi Arabia. Sci. 2023; 5(1):2. https://doi.org/10.3390/sci5010002
Chicago/Turabian StyleEl-Hanafy, Amr A., Yasser M. Saad, Saleh A. Alkarim, Hussein A. Almehdar, Fuad M. Alzahrani, Mohammed A. Almatry, Vladimir N. Uversky, and Elrashdy M. Redwan. 2023. "Yield and Composition Variations of the Milk from Different Camel Breeds in Saudi Arabia" Sci 5, no. 1: 2. https://doi.org/10.3390/sci5010002
APA StyleEl-Hanafy, A. A., Saad, Y. M., Alkarim, S. A., Almehdar, H. A., Alzahrani, F. M., Almatry, M. A., Uversky, V. N., & Redwan, E. M. (2023). Yield and Composition Variations of the Milk from Different Camel Breeds in Saudi Arabia. Sci, 5(1), 2. https://doi.org/10.3390/sci5010002






