Synthesis and Structural Characterization of an Amorphous and Photoluminescent Mixed Eu/Zr Coordination Compound, a Potential Marker for Gunshot Residues
Abstract
1. Introduction
2. Materials and Methods
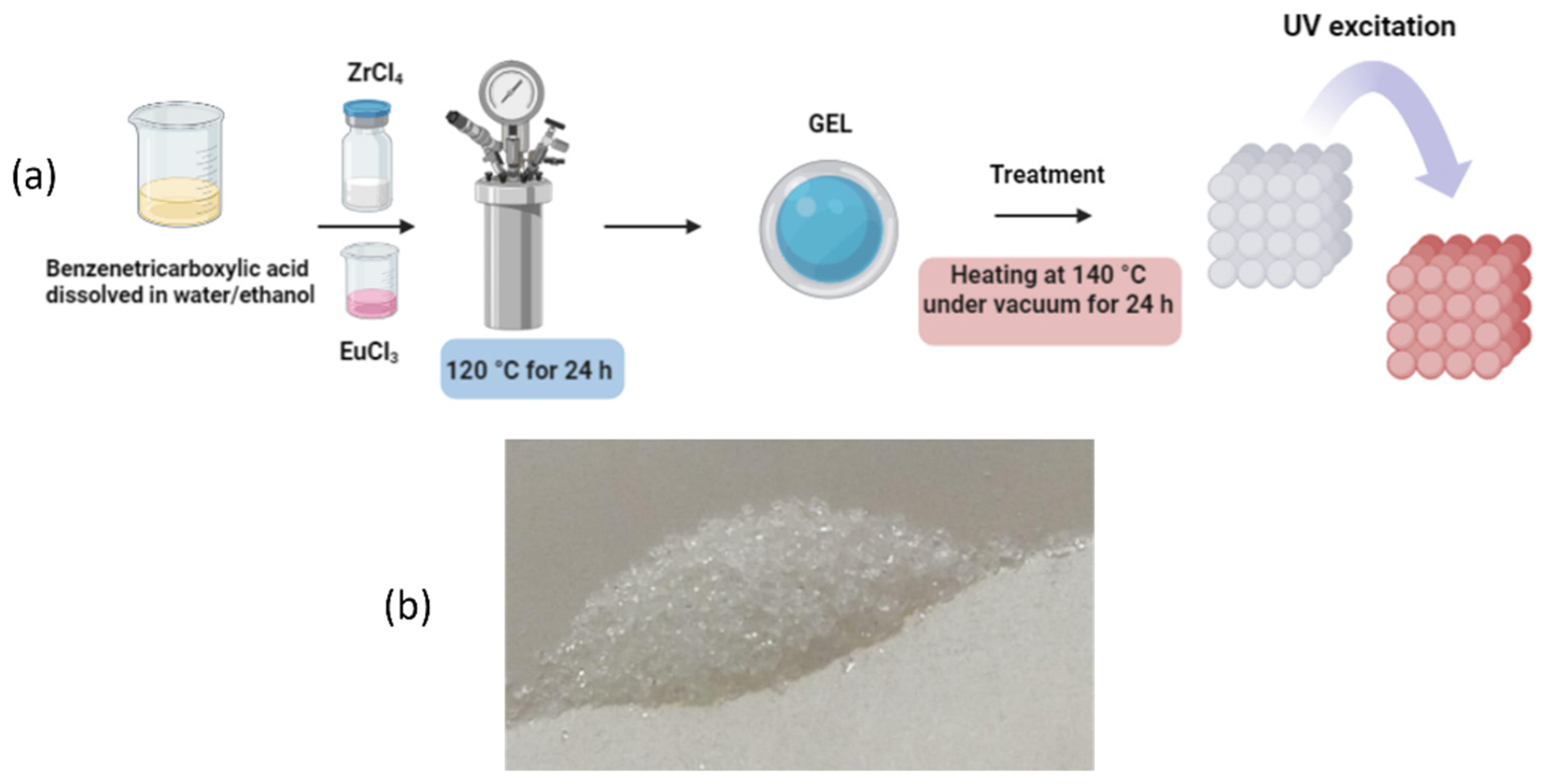
2.1. Synthesis of the [(Eu2Zr)(btc)3(Hbtc)0.5·6H2O)]
2.2. Physical Characterization
2.3. Solid State Nuclear Magnetic Resonance (NMR)
2.4. Gunshot Residue (GSR)
3. Results
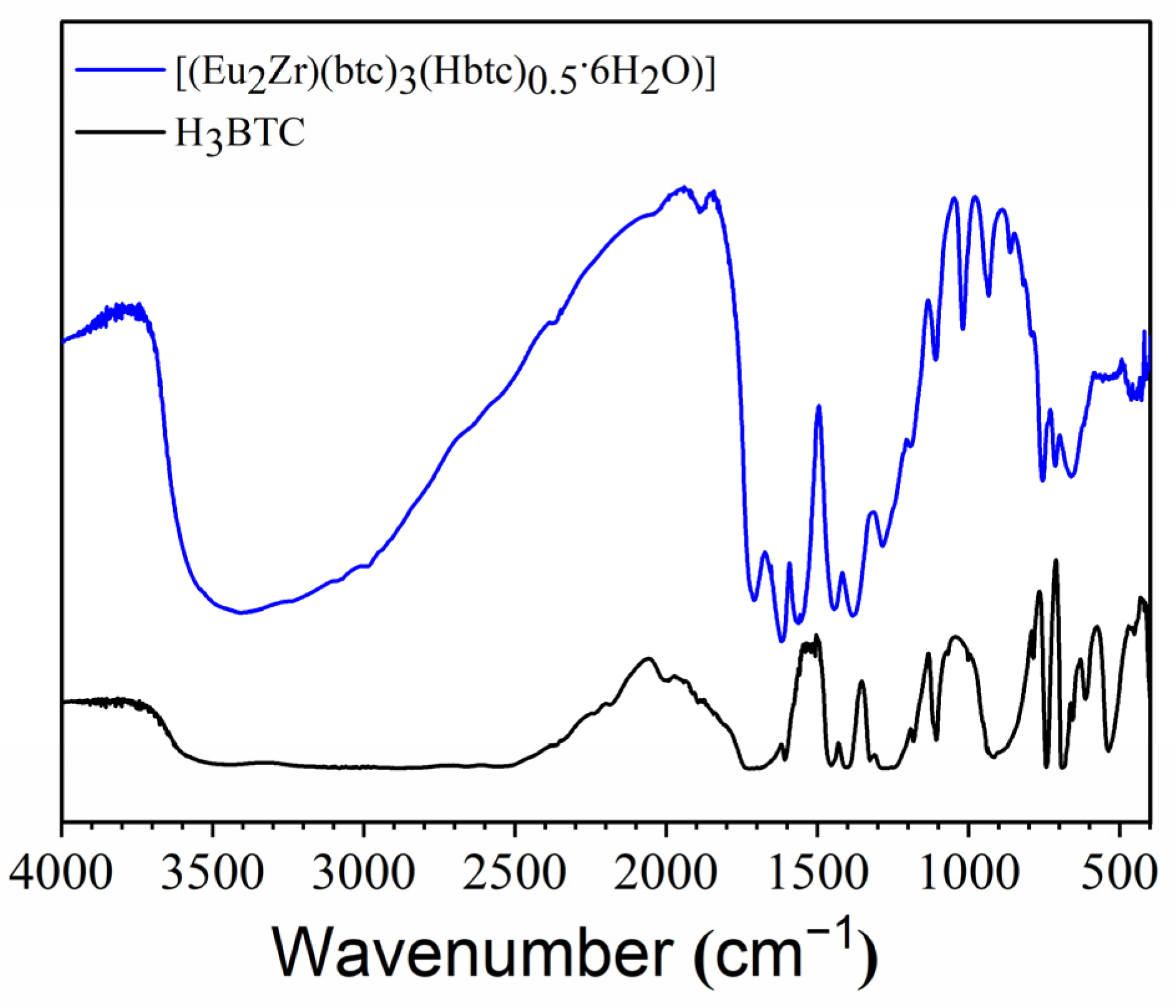
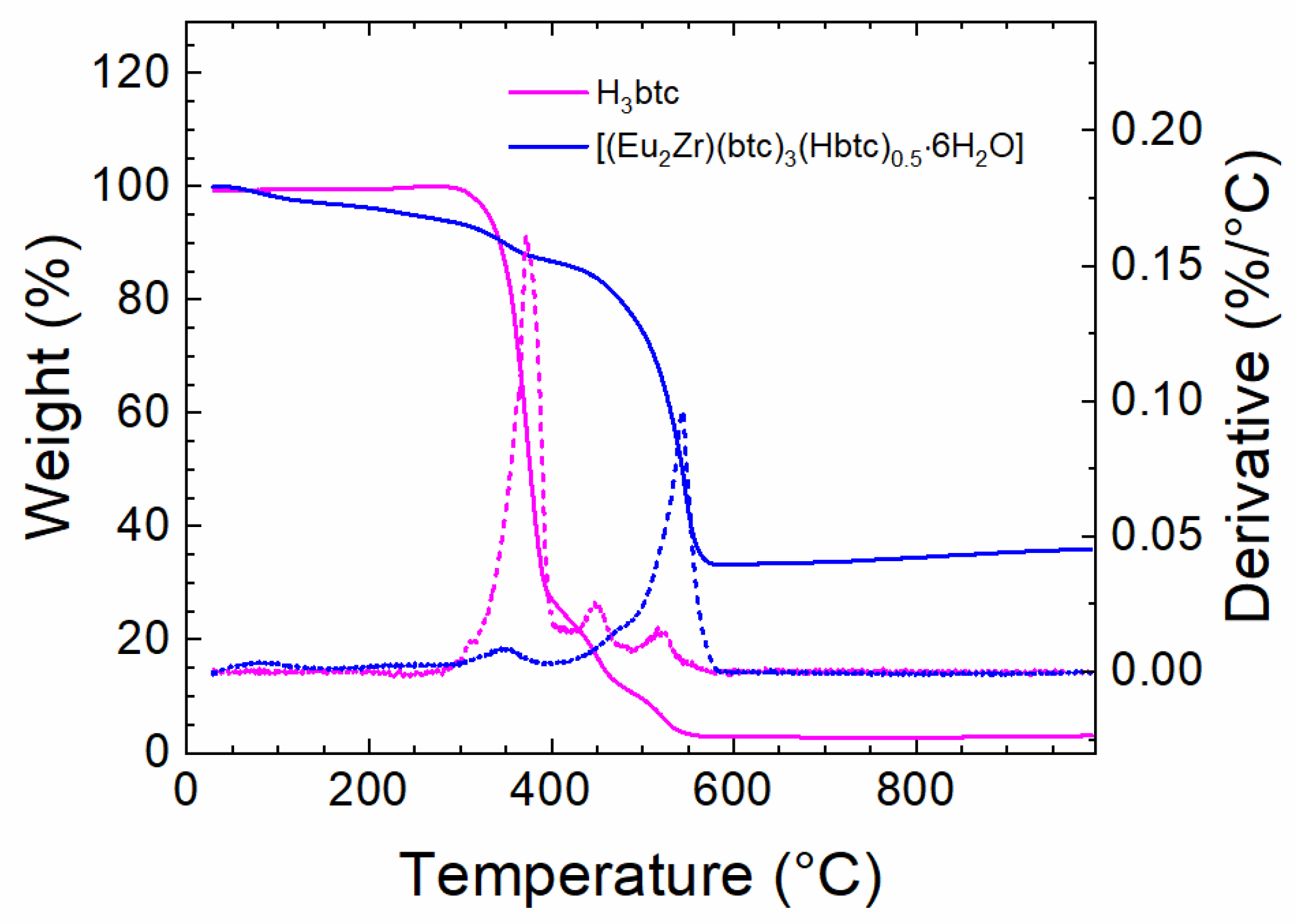
3.1. Vibrational Spectroscopy (Infrared) and Thermal Analysis
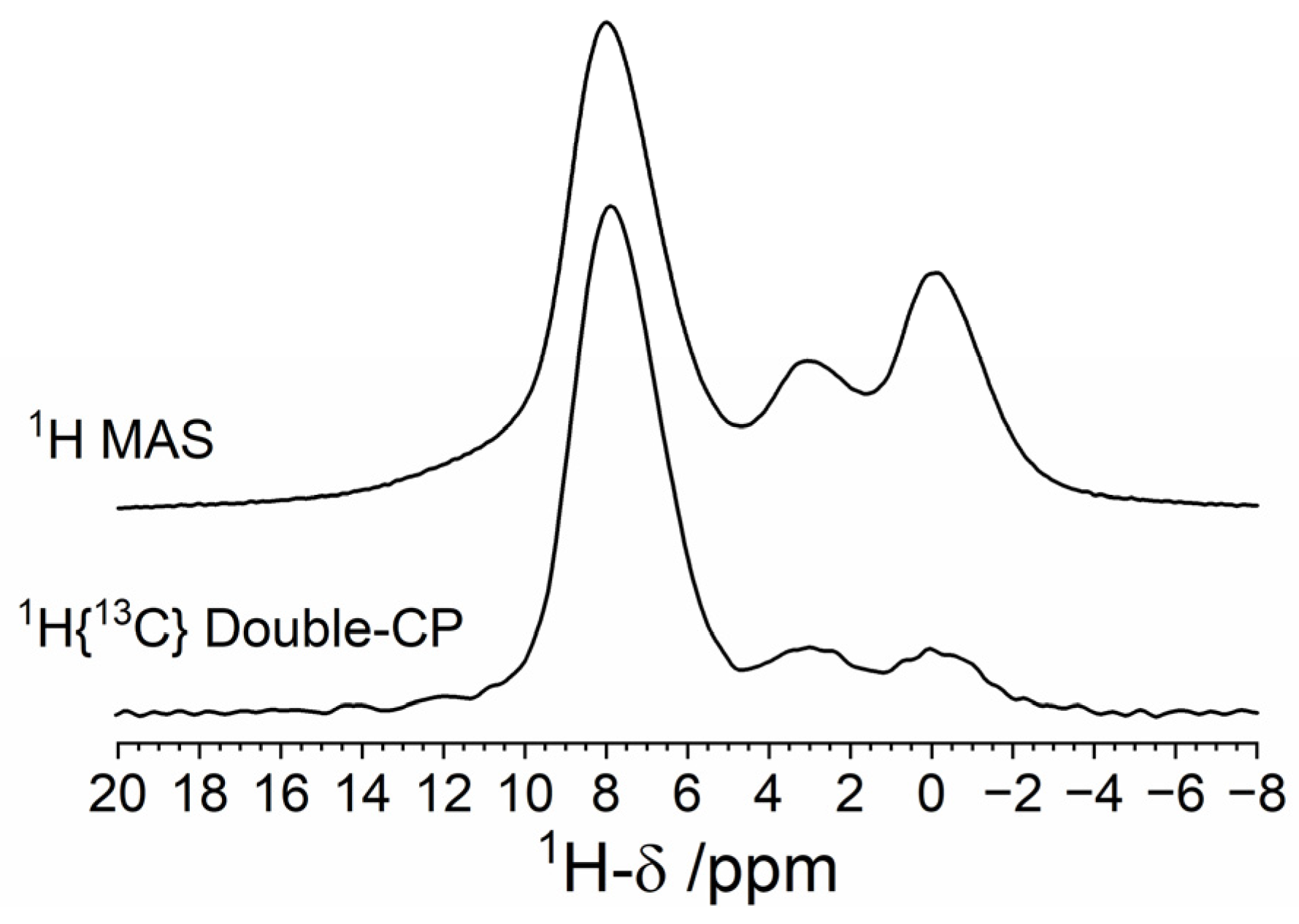
3.2. Solid-State NMR Characterization
3.3. Photoluminescence Studies
3.4. Experimental test of [(Eu2Zr)(btc)3(Hbtc)0.5·6H2O)] Compound as a Red Luminescent Marker for Ammunition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eliseeva, S.V.; Bünzli, J.-C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165. [Google Scholar] [CrossRef]
- Filho, P.C.S.; Larquet, E.; Dragoe, D.; Serra, O.A.; Gacoin, T. Lanthanoid-doped phosphate/vanadate mixed hollow particles as ratiometric luminescent sensors. ACS Appl. Mater. Interfaces 2017, 9, 1635. [Google Scholar] [CrossRef]
- Nascimento, L.F.; Lima, J.F.; de Filho, P.C.; Serra, O.A. Control of diesel particulate emission based on Ag/CeOx/FeOy catalysts supported on cordierite. Chem. Eng. J. 2016, 290, 454. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173. [Google Scholar] [CrossRef]
- Nascimento, L.F.; Serra, O.A. Washcoating of cordierite honeycomb with ceria-copper mixed oxides for catalytic diesel soot combustion. Process Saf. Environ. Prot. 2016, 101, 134. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanoid and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Bünzli, J.-C.G.; Moret, E.; Foiret, V.; Schen, K.J.; Mingzhao, W.; Linpei, J. Structural and photophysical properties of europium(III) mixed complexes with β-diketonates and o-phenanthroline. J. Alloys Compd. 1994, 208, 107. [Google Scholar] [CrossRef]
- Filho, E.V.; de Filho, P.C.; Serra, O.A.; Weber, I.T.; Lucena, M.A.M.; Luz, P.P. New luminescent lanthanoid-based coordination compounds: Synthesis, studies of optical properties and application as marker for gunshot residues. J. Lumin. 2018, 202, 89. [Google Scholar] [CrossRef]
- Júnior, J.C.A.; Santos, G.L.; Colaço, M.V.; Barroso, R.C.; Ferreira, F.F.; Santos, M.V.; de Campos, N.R.; Marinho, M.V.; Jesus, L.T.; Freire, R.O.; et al. New EuIII pyromellitic Metal–Organic Framework of intense red-orange luminescence and high thermal stability for marking in gunshot residues. J. Phys. Chem. C 2020, 124, 9996. [Google Scholar] [CrossRef]
- Talhari, A.L.R.; Lucena, M.A.M.; Mauricio, F.G.M.; Oliveira, M.F.L.; Veiga-Souza, F.H.; Alves, S., Jr.; Weber, I.T. Luminescent marker for GSR: Evaluation of the acute oral and inhalation toxicity of the MOF [Eu(DPA)(HDPA)]. ACS Appl. Bio Mater. 2020, 3, 3049. [Google Scholar] [CrossRef]
- Weber, I.T.; Melo, A.J.; Lucena, M.A.; Consoli, E.F.; Rodrigues, M.O.; de Sá, G.F.; Maldaner, A.O.; Talhavini, M.; Alves, S., Jr. Use of luminescent gunshot residues markers in forensic contex. Forensic Sci. Int. 2014, 244, 276. [Google Scholar] [CrossRef]
- Lima, P.P.; Nobre, S.S.; Freire, R.O.; Júnior, S.A.; Ferreira, R.A.S.; Pischel, U.; Malta, O.L.; Carlos, L.D. Energy transfer mechanisms in organic−inorganic hybrids incorporating europium(iii): A quantitative assessment by light emission spectroscopy. J. Phys. Chem. C 2007, 111, 17627–17634. [Google Scholar] [CrossRef]
- Liu, K.; Jia, G.; Zheng, Y.H.; Song, Y.H.; Yang, M.; Huang, Y.J.; Zhang, L.H.; You, H.P. Room-temperature synthesis and luminescence properties of Eu3+/Tb3+-doped La(1,3,5-btc)(H2O)(6). Inorg. Chem. Comm. 2009, 12, 1246. [Google Scholar] [CrossRef]
- de Roo, J.; Baquero, E.A.; Coppel, Y.; de Keukeleere, K.; van Driessche, I.; Nayral, C.; Hens, Z.; Delpech, F. Insights into the Ligand Shell, Coordination Mode, and Reactivity of Carboxylic Acid Capped Metal Oxide Nanocrystals. ChemPlusChem 2016, 81, 1216. [Google Scholar] [CrossRef]
- Ye, B.H.; Li, X.Y.; Williams, I.D.; Chen, X.M. Synthesis and structural characterization of di- and tetranuclear zinc complexes with phenolate and carboxylate bridges. Correlations between 13C NMR chemical shifts and carboxylate binding modes. Inorg. Chem. 2002, 41, 6426. [Google Scholar] [CrossRef] [PubMed]
- Faccini, F.; Fric, H.; Schubert, U.; Wendel, E.; Tsetsgee, O.; Müller, K.; Bertagnolli, H.; Venzo, A.; Gross, S. ω-Mercapto-functionalized hafnium- and zirconium-oxoclusters as nanosized building blocks for inorganic-organic hybrid materials: Synthesis, characterization and photothiol-ene polymerization. J. Mater. Chem. 2007, 17, 3297. [Google Scholar] [CrossRef]
- Dutra, J.D.L.; Bispo, T.D.; Freire, R.O. LUMPAC lanthanoid luminescence software: Efficient and user friendly. J. Comput. Chem. 2014, 35, 772. [Google Scholar] [CrossRef]
- Jaeger, C.; Hemmann, F. EASY: A simple tool for simultaneously removing background, deadtime and acoustic ringing in quantitative NMR spectroscopy—Part I: Basic principle and applications. Solid State Nucl. Magn. Reson. 2014, 57, 22. [Google Scholar] [CrossRef]
- Cory, D.G.; Ritchey, W.M. Suppression of signals from the probe in Bloch decay spectra. J. Magn. Reson. 1988, 80, 128. [Google Scholar] [CrossRef]
- Bennett, A.E.; Rienstra, C.M.; Auger, M.; Lakshmi, K.V.; Griffin, R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995, 103, 6951. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Bonhomme, C.; Innocenzi, P.; Babonneau, F. Solid-state NMR characterization of the surfactant-silica interface in templated silicas: Acidic versus basic conditions. Chem. Mater. 2007, 19, 1343. [Google Scholar] [CrossRef]
- Christiansen, S.C.; Hedin, N.; Epping, J.D.; Janicke, M.T.; del Amo, Y.; Demarest, M.; Brzezinski, M.; Chmelka, B.F. Sensitivity considerations in polarization transfer and filtering using dipole-dipole couplings: Implications for biomineral systems. Solid State Nucl. Magn. Reson. 2006, 29, 170. [Google Scholar] [CrossRef] [PubMed]
- Wiench, J.W.; Bronnimann, C.E.; Lin, V.S.-Y.; Pruski, M. Chemical shift correlation NMR spectroscopy with indirect detection in fast rotating solids: Studies of organically functionalized mesoporous silicas. J. Am. Chem. Soc. 2007, 129, 12076. [Google Scholar] [CrossRef] [PubMed]
- Mafra, L.; Siegel, R.; Fernandez, C.; Schneider, D.; Aussenac, F.; Rocha, J. High-resolution 1H homonuclear dipolar recoupling NMR spectra of biological solids at MAS rates up to 67 KHz. J. Magn. Reson. 2009, 199, 111–114. [Google Scholar] [CrossRef]
- Kimura, H.; Nakamura, K.; Eguchi, A.; Sugisawa, H.; Deguchi, K.; Ebisawa, K.; Suzuki, E.I.; Shoji, A. Structural study of α-amino-acid crystals by 1H CRAMPS NMR spectroscopy. J. Mol. Struct. 1998, 447, 247. [Google Scholar] [CrossRef]
- Potrzebowski, M.J.; Tekely, P.; Dusausoy, Y. Comment to 13C-NMR studies of α and γ polymorphs of glycine. Solid State Nucl. Magn. Reson. 1998, 11, 253. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR chemical shifts of trace impurities: Industrially preferred solvents used in process and green chemistry. Org. Process Res. Dev. 2016, 20, 661. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, C.; Sun, B.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Luminescent europium(III) complexes based on tridentate isoquinoline ligands with extremely high quantum yield. Inorg. Chem. Front. 2021, 8, 41. [Google Scholar] [CrossRef]
- Barbosa, C.D.; Da Luz, L.L.; Paz, F.A.; Malta, O.L.; Rodrigues, M.; Júnior, S.A.; Ferreira, R.A.; Carlos, L.D. Site-selective Eu(iii) spectroscopy of highly efficient luminescent mixed-metal Pb(ii)/Eu(iii) coordination polymers. RSC Adv. 2017, 7, 6093. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Proulx, P.P.; Bettinelli, M.; Negrisolo, F. Absorption and emission spectroscopy of Eu3+ in metaphosphate glasses. Phys. Rev. B Condens. Matter. 1990, 42, 5936. [Google Scholar] [CrossRef]
- Nogami, M.; Umehara, N.; Hayakawa, T. Effect of hydroxyl bonds on persistent spectral hole burning in Eu3+-doped BaO−P2O5 glasses. Phys. Rev. B 1998, 58, 6166. [Google Scholar] [CrossRef]
- de Oliveira, M., Jr.; Gonçalves, T.S.; Ferrari, C.; Magon, C.J.; Pizani, P.S.; de Camargo, A.S.S.; Eckert, H. Structure–Property Relations in Fluorophosphate Glasses: An Integrated Spectroscopic Strategy. J. Phys. Chem. C 2017, 121, 2968. [Google Scholar] [CrossRef]
- Galaço, A.R.B.S.; Freire, R.O.; de Jesus, L.T.; Serra, O.A. Experimental and Theoretical study of isoreticular lanthanoid organic framework (LOF): Structure and luminescence. J. Lumin. 2020, 223, 179. [Google Scholar]

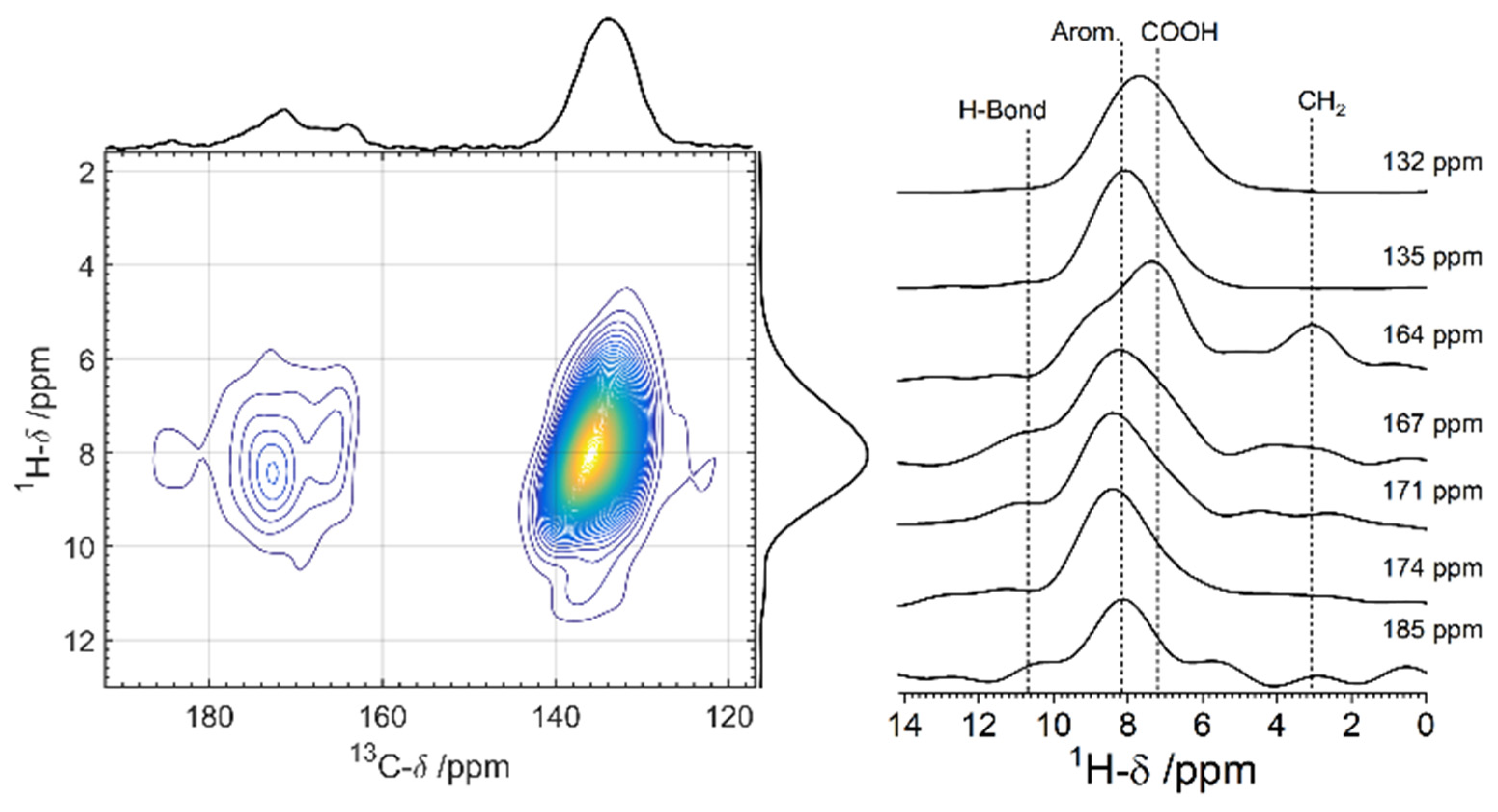
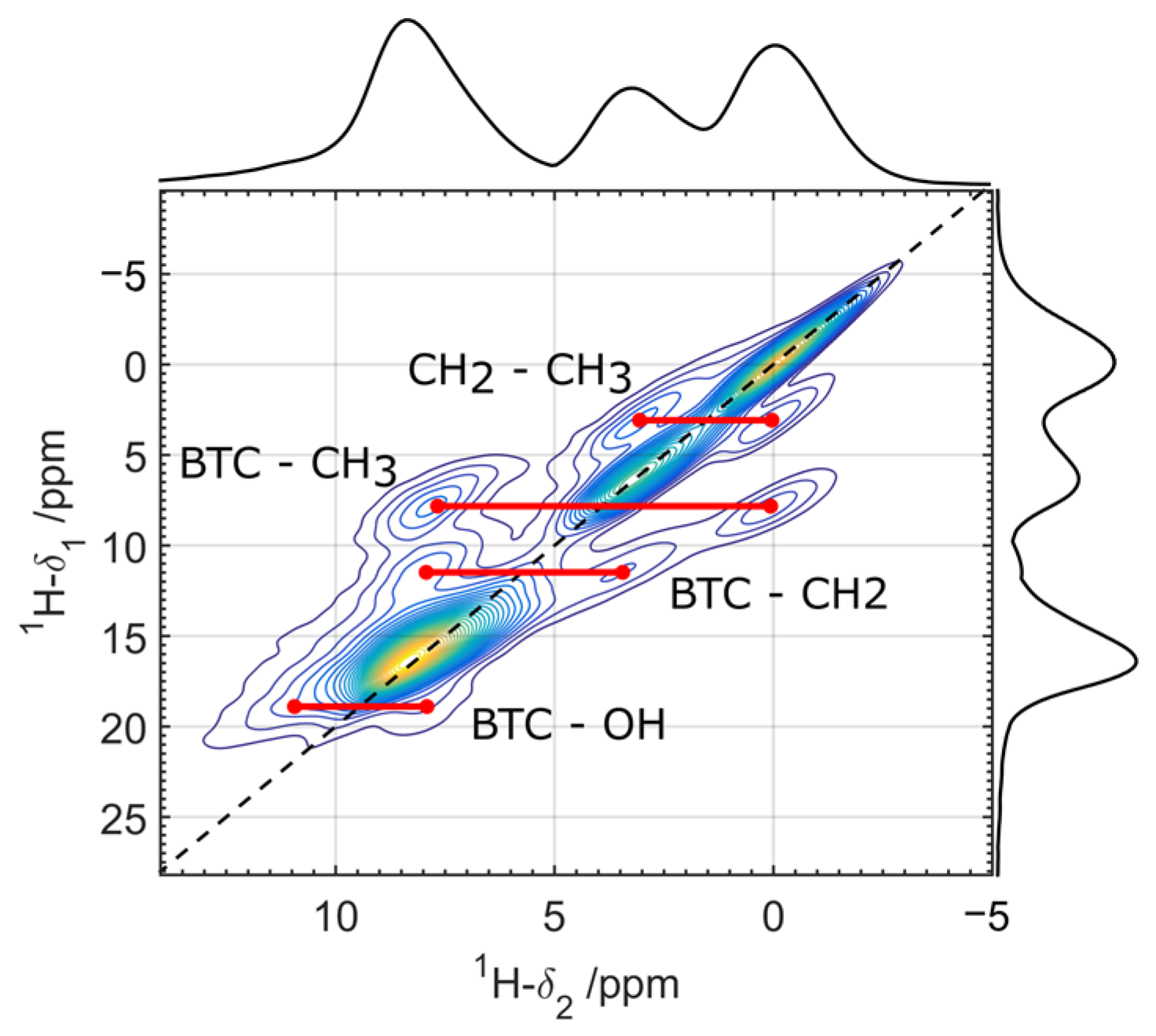
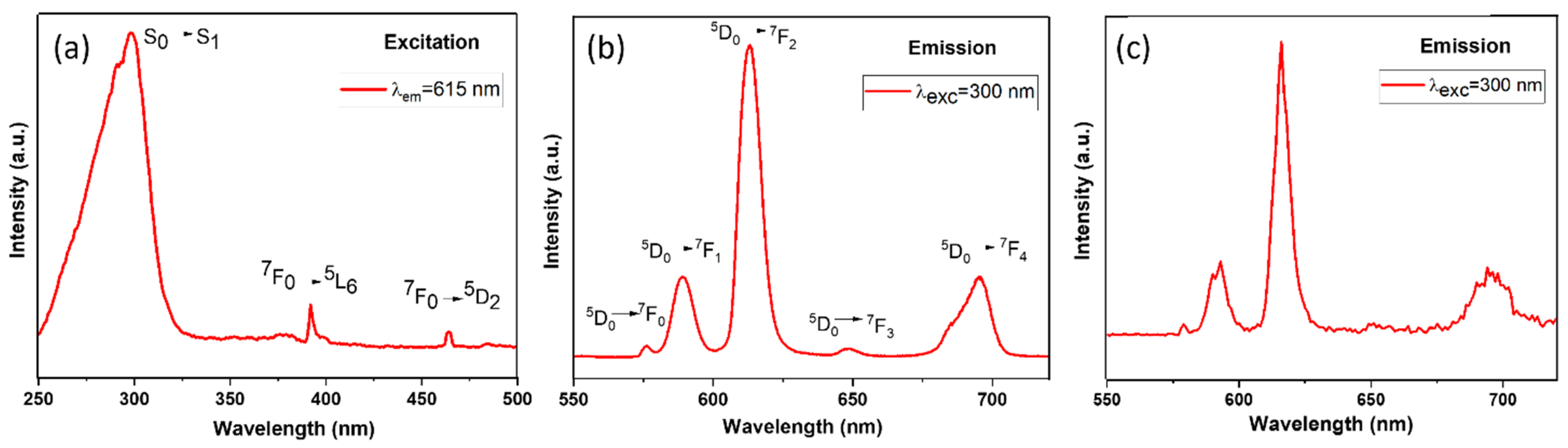

| Attribution | δiso (±1 ppm) | FWHM (±0.5 ppm) | I (±2%) |
|---|---|---|---|
| C1 | 132 | 5.6 | 31 |
| C2 | 135 | 5.8 | 33 |
| I | 164 | 3.6 | 5 |
| II, III a | 167/171 | 3.9/3.6 | 15/8 |
| IV | 174 | 7.1 | 7 |
| V | 185 | 3.1 | 1 |
| Arad | Anrad | Ω2 (10−20 s) | Ω4 (10−20 s) | η | τ (ms) |
|---|---|---|---|---|---|
| 354.08 | 4191.37 | 7.0 | 5.73 | 7.79 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, A.R.B.; Casagrande, T.R.; de Lima, J.F.; de Oliveira, M.F.; Júnior, S.A.; de Oliveira Junior, M.; Serra, O.A. Synthesis and Structural Characterization of an Amorphous and Photoluminescent Mixed Eu/Zr Coordination Compound, a Potential Marker for Gunshot Residues. Sci 2022, 4, 43. https://doi.org/10.3390/sci4040043
Serra ARB, Casagrande TR, de Lima JF, de Oliveira MF, Júnior SA, de Oliveira Junior M, Serra OA. Synthesis and Structural Characterization of an Amorphous and Photoluminescent Mixed Eu/Zr Coordination Compound, a Potential Marker for Gunshot Residues. Sci. 2022; 4(4):43. https://doi.org/10.3390/sci4040043
Chicago/Turabian StyleSerra, Ayla Roberta Borges, Thiago Rui Casagrande, Juliana Fonseca de Lima, Marcelo Firmino de Oliveira, Severino Alves Júnior, Marcos de Oliveira Junior, and Osvaldo Antonio Serra. 2022. "Synthesis and Structural Characterization of an Amorphous and Photoluminescent Mixed Eu/Zr Coordination Compound, a Potential Marker for Gunshot Residues" Sci 4, no. 4: 43. https://doi.org/10.3390/sci4040043
APA StyleSerra, A. R. B., Casagrande, T. R., de Lima, J. F., de Oliveira, M. F., Júnior, S. A., de Oliveira Junior, M., & Serra, O. A. (2022). Synthesis and Structural Characterization of an Amorphous and Photoluminescent Mixed Eu/Zr Coordination Compound, a Potential Marker for Gunshot Residues. Sci, 4(4), 43. https://doi.org/10.3390/sci4040043








