Synthesis, Structural Characterization, and Biological Activities of Organically Templated Cobalt Phosphite (H2DAB)[Co(H2PO3)4]·2H2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis
2.3. Crystal Structure Determination
2.4. Biological Assays
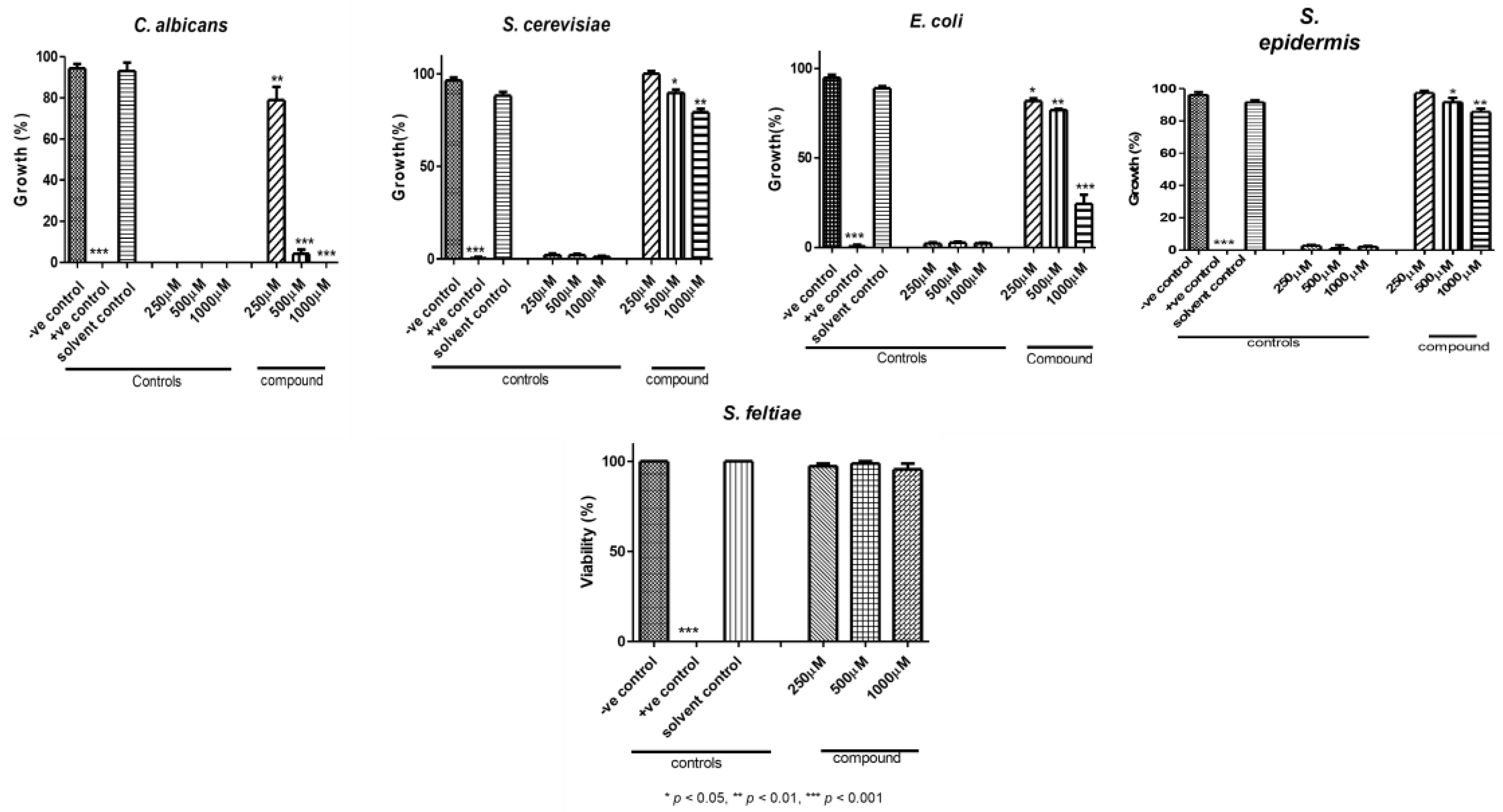
2.4.1. The Antimicrobial Activity
2.4.2. Nematocidal Activity
3. Results and Discussion
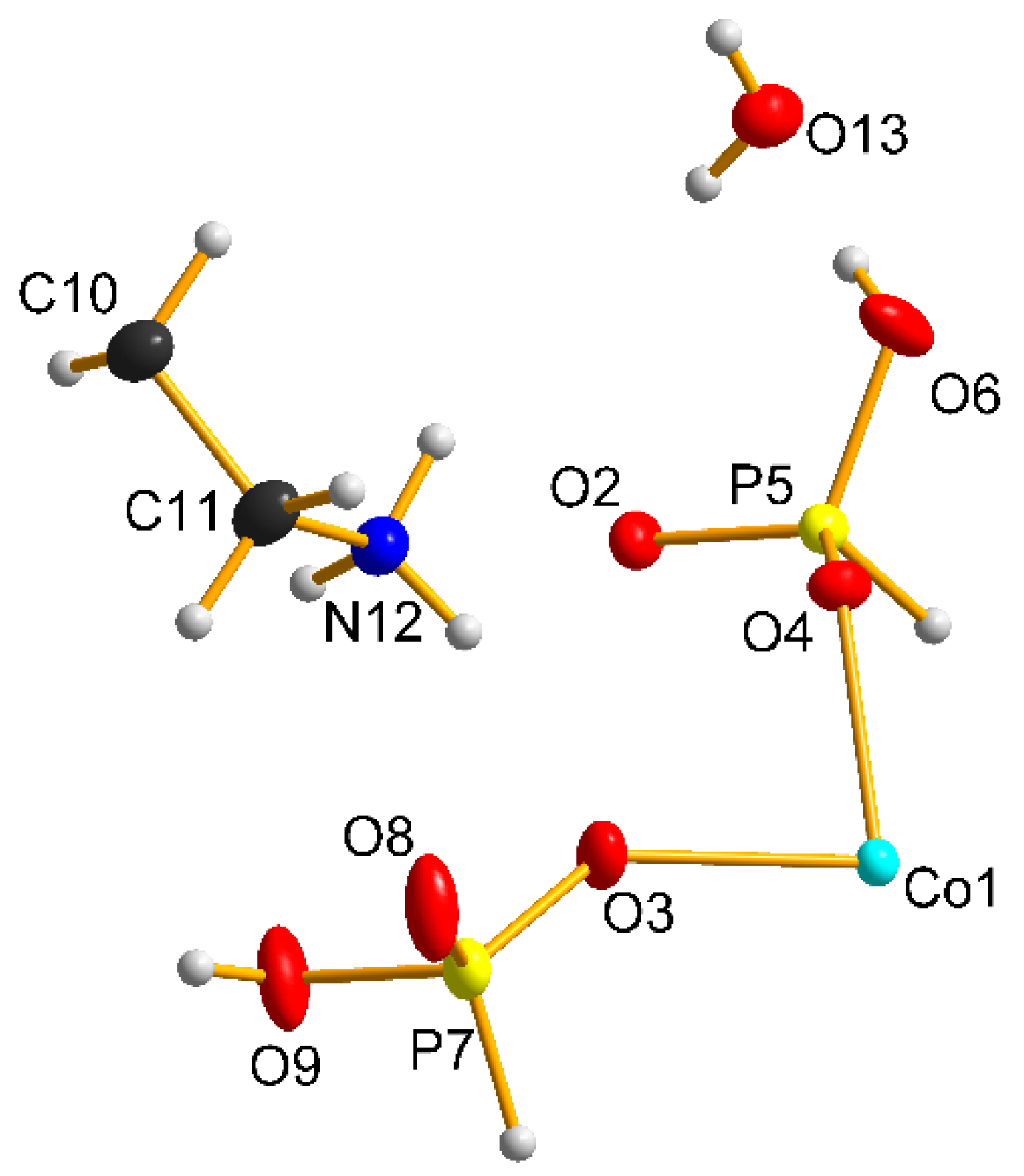
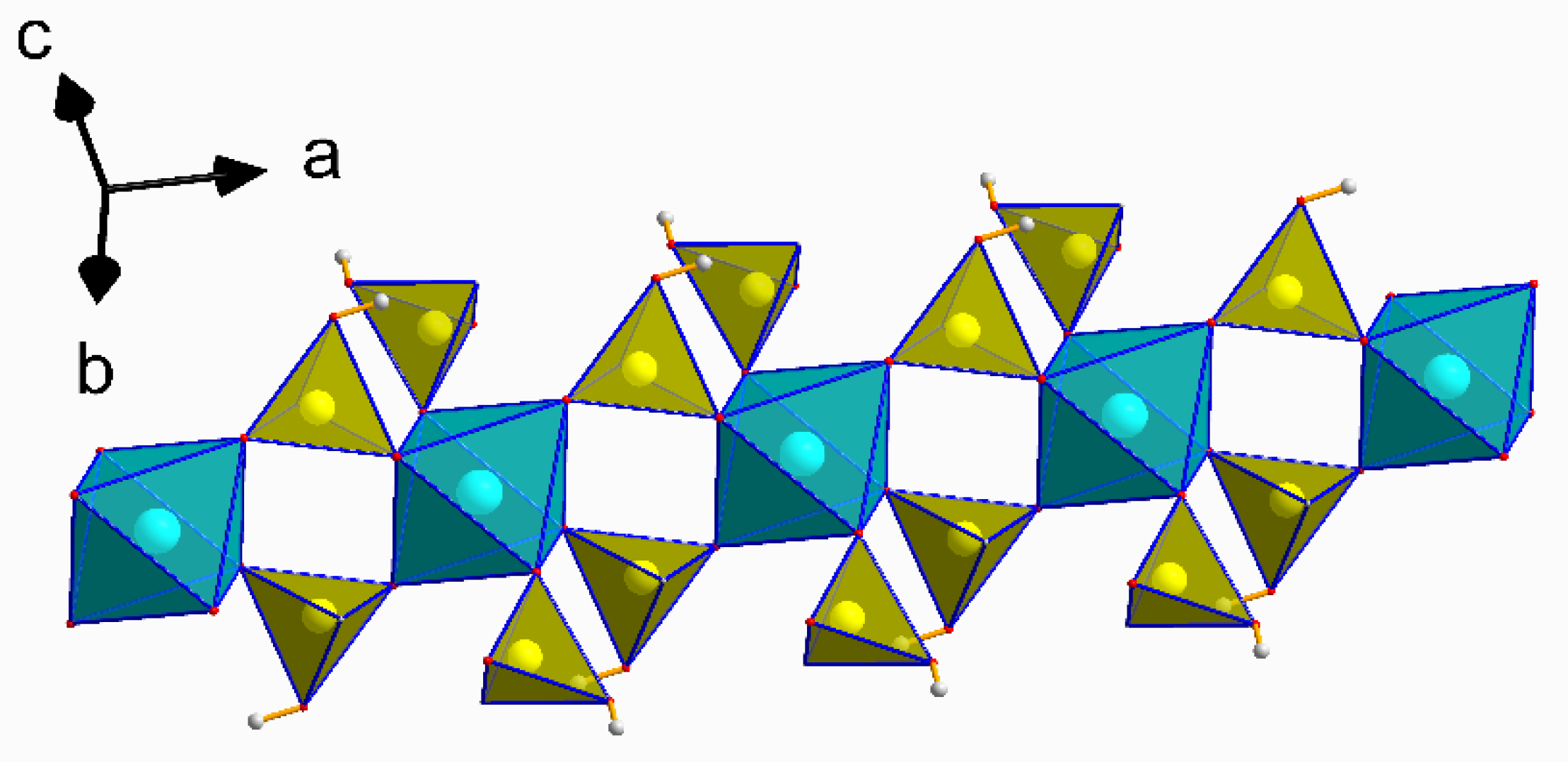
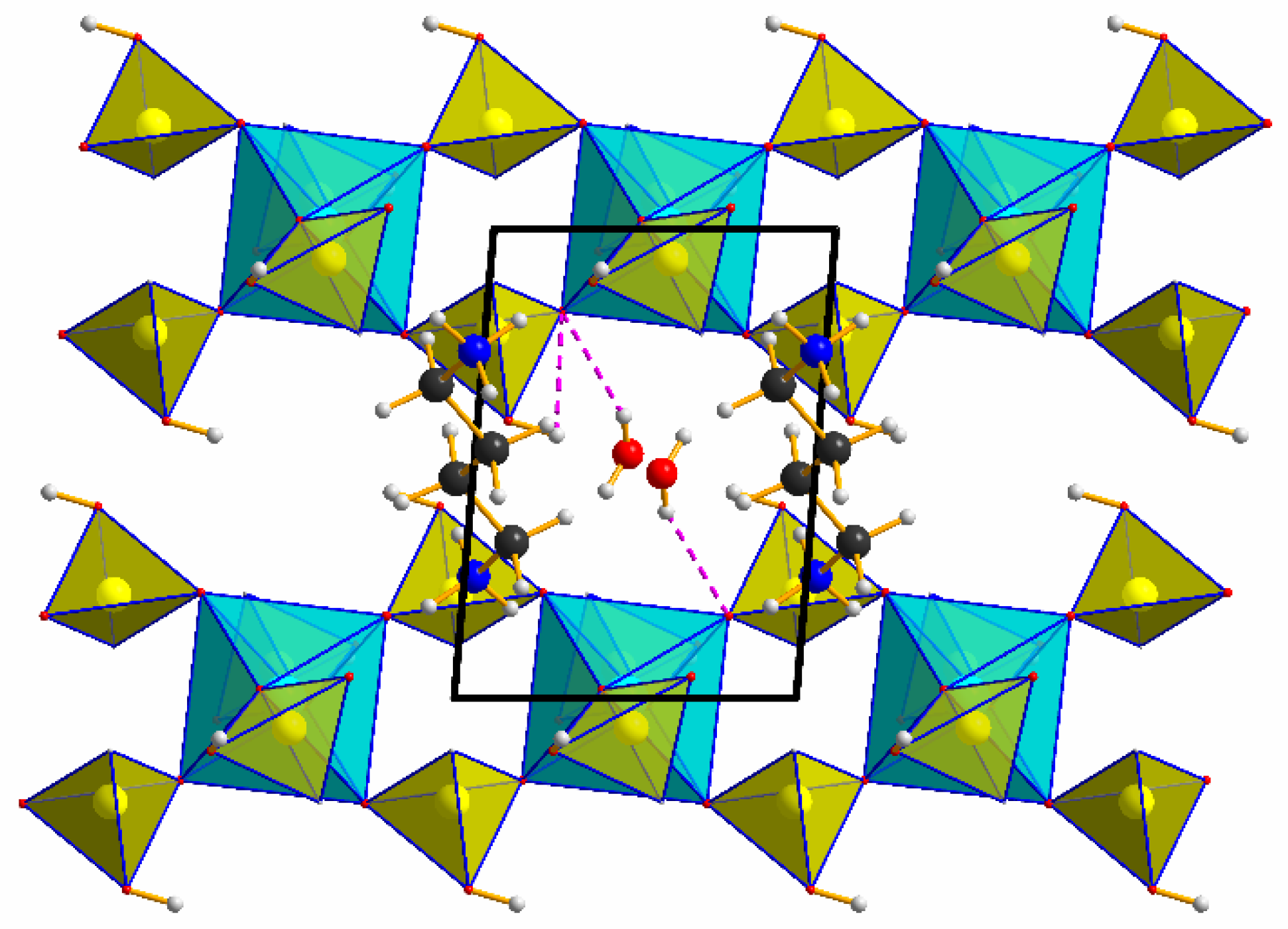
3.1. Structural Description
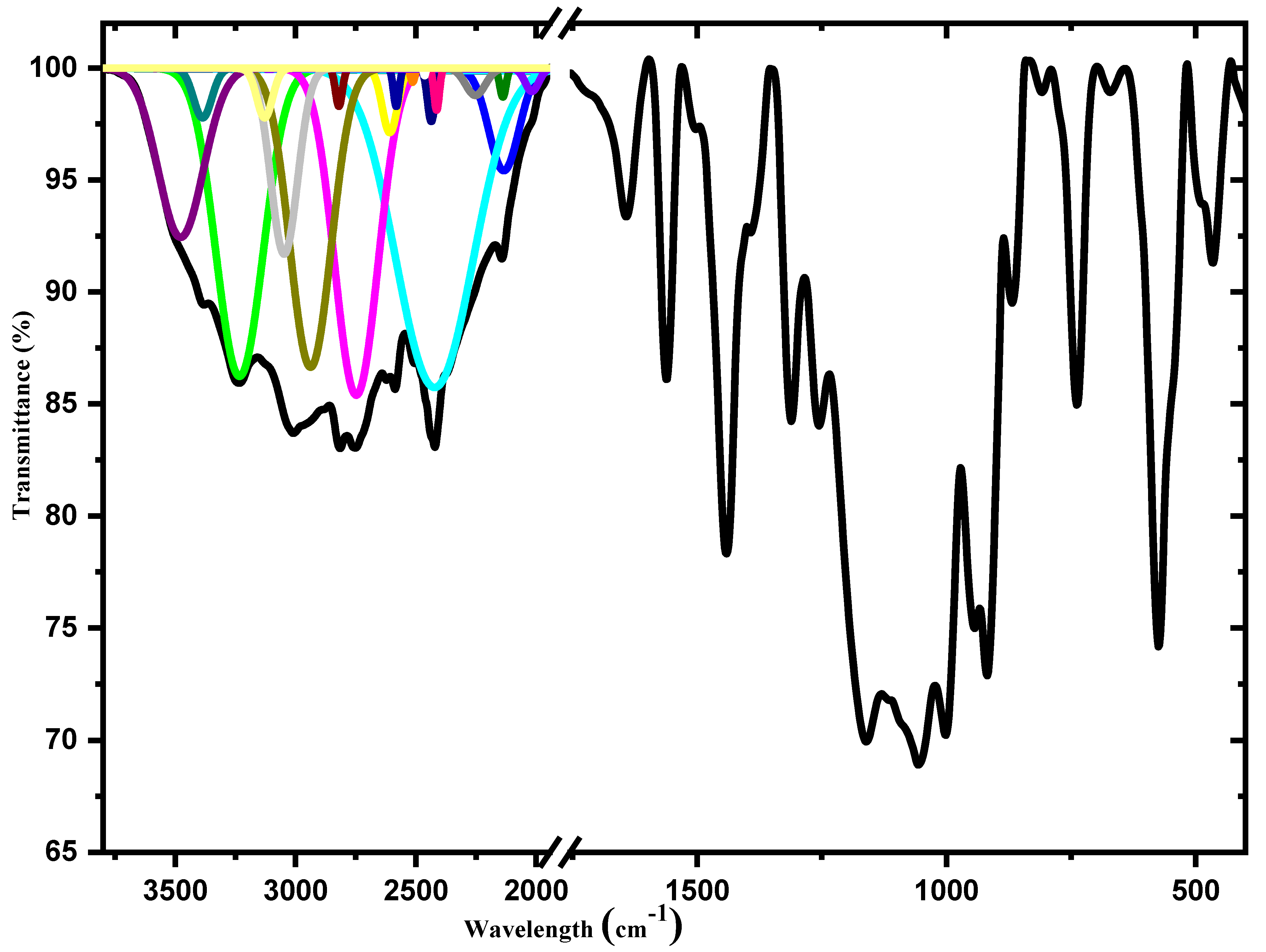
3.2. Infrared Spectroscopy
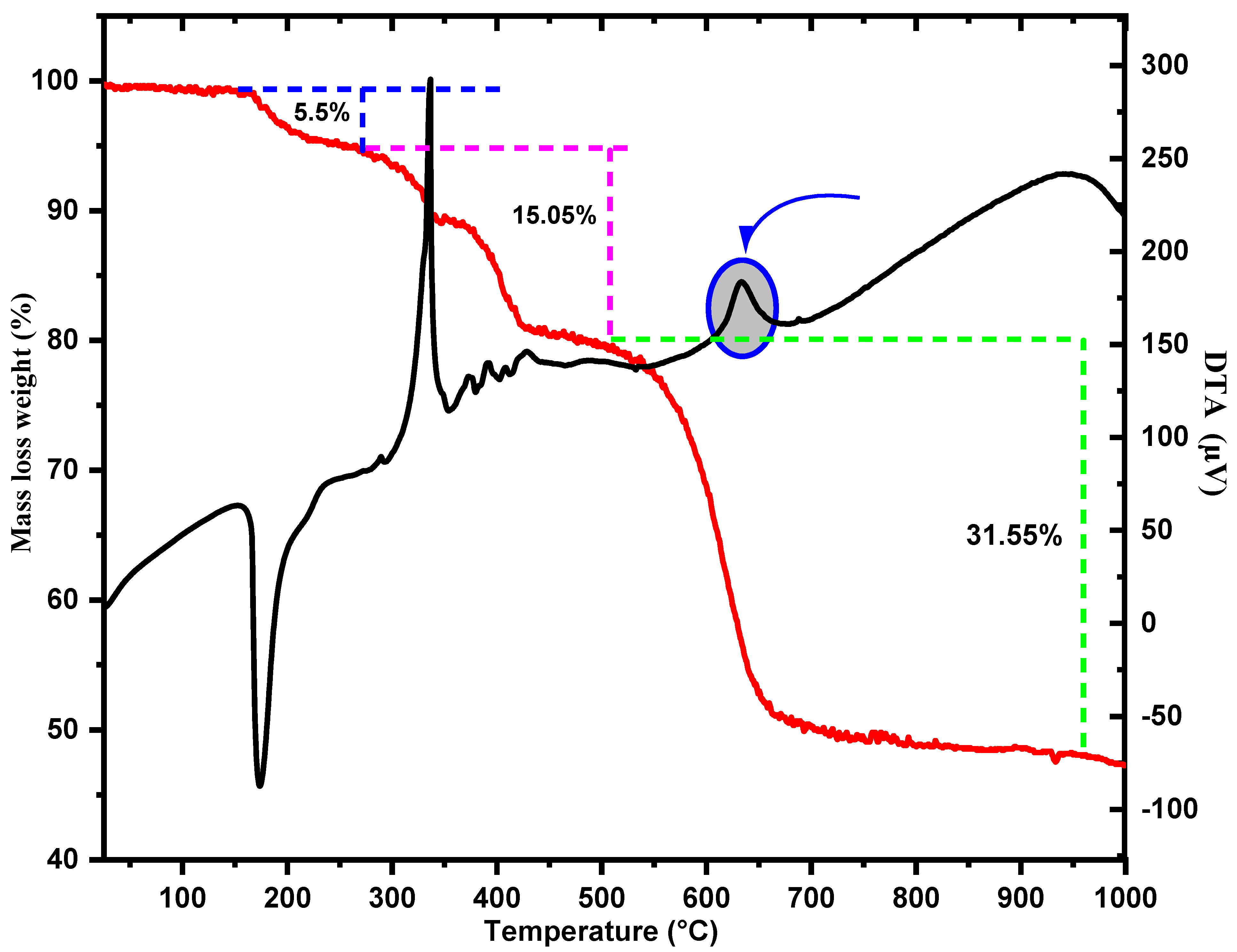
3.3. Thermal Behavior
3.4. Biological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline Metal-Organic Frameworks (MOFs): Synthesis, Structure and Function. Acta Cryst B 2014, 70, 3–10. [Google Scholar] [CrossRef]
- García-Fernández, A.; Bermúdez-García, J.M.; Castro-García, S.; Artiaga, R.; López-Beceiro, J.; Señarís-Rodríguez, M.A.; Sánchez-Andújar, M. Dielectric Properties Induced by the Framework in the Hybrid Organic–Inorganic Compounds M(Dca)2pyz M=Fe, Co and Zn. Polyhedron 2016, 114, 249–255. [Google Scholar] [CrossRef]
- Xiong, D.; Li, M.; Liu, W.; Chen, H.; Yang, X.; Zhao, J. Synthesis, Structure and Luminescence Property of Two Lanthanum Phosphite Hydrates: La2(H2O)x(HPO3)3 (x = 1,2). J. Solid State Chem. 2006, 179, 2571–2577. [Google Scholar] [CrossRef]
- Hamdi, N.; Hidaoui, S.; Hassani, H.O.; Lachkar, M.; Dusek, M.; Morley, N.; El Bali, B. Synthesis, Crystal Structure, Physical and Catalytic Oxidation Studies of a New Hybrid Phosphate [(N2H5)2Co(HPO4)2]. J. Mol. Struct. 2020, 1217, 128317. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial Applications of Metal–Organic Frameworks and Their Composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [Green Version]
- Karimi Alavijeh, R.; Beheshti, S.; Akhbari, K.; Morsali, A. Investigation of Reasons for Metal–Organic Framework’s Antibacterial Activities. Polyhedron 2018, 156, 257–278. [Google Scholar] [CrossRef]
- Havlin, J.L.; Schlegel, A.J. Review of Phosphite as a Plant Nutrient and Fungicide. Soil Syst. 2021, 5, 52. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered Porous Materials for Emerging Applications. Nature 2002, 417, 813–821. Available online: https://www.nature.com/articles/nature00785 (accessed on 10 October 2021). [CrossRef]
- Agarwal, R.A.; Mukherjee, S. One Dimensional Coordination Polymers of Cd (II) and Zn (II): Synthesis, Structure, Polar Packing through Strong Inter-Chain Hydrogen Bonding and Gas Adsorption Studies. Polyhedron 2016, 106, 163–170. [Google Scholar] [CrossRef]
- Bakuru, V.R.; DMello, M.E.; Kalidindi, S.B. Metal-Organic Frameworks for Hydrogen Energy Applications: Advances and Challenges. ChemPhysChem 2019, 20, 1177–1215. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Yang, C.; Cheng, K.; Tan, T.; Lv, Y.; Liu, Y. Hybrid Porous Crystalline Materials from Metal Organic Frameworks and Covalent Organic Frameworks. Adv. Sci. 2021, 8, 2101883. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Pettinari, R.; di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0010854521003957 (accessed on 10 October 2021). [CrossRef]
- Aguilar, C.A.H.; Jiménez, A.B.P.; Silva, A.R.; Kaur, N.; Thangarasu, P.; Ramos, J.M.V.; Singh, N. Organic-Inorganic Hybrid Nanoparticles for Bacterial Inhibition: Synthesis and Characterization of Doped and Undoped ONPs with Ag/Au NPs. Molecules 2015, 20, 6002–6021. [Google Scholar] [CrossRef] [PubMed]
- Ouarsal, R.; Tahiri, A.A.; Bali, B.E.; Lachkar, M.; Bolte, M. Strontium Di hydrogenphosphite. Acta Crystallogr. Sect. E 2002, 58, i19–i20. [Google Scholar] [CrossRef]
- Ouarsal, R.; Alaoui Tahiri, A.; Lachkar, M.; Slimani, Z.; El Bali, B.; Bolte, M. Barium Di hydrogen Phosphite Hemihydrate. Acta Crystallogr. E 2002, 58, i72–i73. [Google Scholar] [CrossRef]
- Chaouche, S.; Ouarsal, R.; Bali, B.E.; Lachkar, M.; Bolte, M.; Dusek, M. Li2HPO3, H2O: Crystal Structure and IR Spectrum. J. Chem. Cryst. 2010, 40, 526–530. [Google Scholar] [CrossRef]
- Chaouch, S.; Ouarsal, R.; Akouibaa, M.; Rakib, S.; Lachkar, M.; Bali, B.E.; Dusek, M. Cs2[M(H2O)6]3(HPO3)4, M = Co, Ni: Crystal Structures, IR and Thermal Studies. J. Phys. Conf. Ser. 2018, 984, 012015. [Google Scholar] [CrossRef]
- Ouarsal, R.; Tahiri, A.A.; El Bali, B.; Lachkar, M.; Harrison, W.T.A. Sodium Zinc Tris (Di hydrogenphosphite) Hydrate, NaZn(H2PO3)3·H2O. Acta Crystallogr. E 2002, 58, i23–i25. [Google Scholar] [CrossRef]
- Ouarsal, R.; Alaoui Tahiri, A.; Lachkar, M.; Dusek, M.; Fejfarová, K.; El Bali, B. Sodium Magnesium Tris (Di hydrogen phosphite) Monohydrate, NaMg(H2PO3)3·H2O. Acta Crystallogr. Sect. E 2003, 59, i33–i35. [Google Scholar] [CrossRef]
- Ouarsal, R.; Essehli, R.; Lachkar, M.; Zenkouar, M.; Dusek, M.; Fejfarova, K.; El Bali, B. Dipotassium Cobalt(II) Bis (Hydrogenphosphite) Dihydrate, K2Co(HPO3)2·2H2O. Acta Crystallogr. E 2004, 60, i66–i68. [Google Scholar] [CrossRef] [Green Version]
- Messouri, I.; El Bali, B.; Capitelli, F.; Piniella, J.F.; Lachkar, M.; Slimani, Z. Diammonium Tris [Hexa aqua magnesium(II)] Tetra kis [Hydrogenphosphate(III)], (NH4)2[Mg(H2O)6]3(HPO3)4. Acta Crystallogr. E 2005, 61, i129–i131. [Google Scholar] [CrossRef] [Green Version]
- Ouarsal, R.; El Bali, B.; Lachkar, M.; Dusek, M.; Fejfarova, K. Diammonium Tris [Hexa aqua nickel(II)] Tetra kis [Hydrogenphosphate(III)], (NH4)2[Ni(H2O)6]3(HPO3)4. Acta Crystallogr. Sect. E 2005, 61, i171–i173. [Google Scholar] [CrossRef]
- Ouarsal, R.; El Bali, B.; Lachkar, M.; Dusek, M.; Fejfarova, K. Diammonium Tris [Hexa aqua cobalt(II)] Tetra kis [Hydrogenphosphate(III)], (NH4)2[Co(H2O)6]3(HPO3)4. Acta Crystallogr. E 2005, 61, i168–i170. [Google Scholar] [CrossRef] [Green Version]
- Ouarsal, R.; Lachkar, M.; Dusek, M.; Albert, E.B.; Carda Castelló, J.B.; El Bali, B. Crystal Structure of NaCd(H2PO3)3·H2O and Spectroscopic Study of NaM(H2PO3)3·H2O, M = Mn, Co, Ni, Zn, Mg and Cd. Polyhedron 2016, 106, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Chaouche, S.; Ouarsal, R.; Lachkar, M.; Capitelli, F.; El Bali, B. Crystal Structure and IR Study of (C6H5NH3)[ZnCl(HPO3)]. J Chem. Cryst. 2010, 40, 486–490. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Xia, Q.; Yuan, G.; He, Q.; Cui, Y. Multiple Topological Isomerism of Three-Connected Networks in Silver-Based Metal–Organoboron Frameworks. Chem. Commun. 2010, 46, 2608–2610. [Google Scholar] [CrossRef]
- Zhuang, W.; Yuan, D.; Li, J.-R.; Luo, Z.; Zhou, H.-C.; Bashir, S.; Liu, J. Highly Potent Bactericidal Activity of Porous Metal-Organic Frameworks. Adv. Healthc. Mater. 2012, 1, 225–238. [Google Scholar] [CrossRef]
- Rigaku Corporation. CrysAlisPro Software System; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment–Olex2 Dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [Green Version]
- Diamond-Crystal and Molecular Structure Visualization, Crystal Impact-Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 10 October 2021).
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.V.D.; Wood, P.A. Mercury CSD 2.0–New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S. Inorganic–organic hybrid structure: Synthesis, structure and magnetic properties of a cobalt phosphite–oxalate, [C4N2H12][Co4(HPO3)2(C2O4)3]. J. Solid State Chem. 2005, 178, 2376–2382. [Google Scholar] [CrossRef]
- Fernández, S.; Pizarro, J.L.; Mesa, J.L.; Lezama, L.; Arriortua, M.I.; Rojo, T. Hydrothermal Synthesis of a New Layered Inorganic–Organic Hybrid Cobalt(II) Phosphite: (C2H10N2)[Co3(HPO3)4]: Crystal Structure and Spectroscopic and Magnetic Properties. Int. J. Inorg. Mater. 2001, 3, 331–336. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Liu, X. Solvothermal Synthesis and Magnetic Properties of Cobalt (II) Phosphite Structures of Varying Dimensionality. CrystEngComm 2010, 12, 383–386. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Zeng, H.; Zou, G.; Lin, Z. Solvent-Free Synthesis of Inorganic-Organic Hybrid Solids with Chainlike, Layered, and Open-Framework Structures. Inorg. Chem. Commun. 2018, 95, 8–11. [Google Scholar] [CrossRef]
- Kantarcı, Z.; Sağlam, S.; Kasap, E. Vibrational Spectroscopic Studies On The 1,4-Diaminobutane-Td-Type Clathrates: Cd(Dabn)M(CN)4 1,5C6H6 (M = Cd or Hg). Spectrosc. Lett. 2002, 35, 811–819. [Google Scholar] [CrossRef]
- Aguado, S.; Quirós, J.; Canivet, J.; Farrusseng, D.; Boltes, K.; Rosal, R. Antimicrobial Activity of Cobalt Imidazolate Metal–Organic Frameworks. Chemosphere 2014, 113, 188–192. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0045653514006638 (accessed on 10 October 2021). [CrossRef] [PubMed]
- Xie, B.-P.; Chai, J.-W.; Fan, C.; Ouyang, J.-H.; Duan, W.-J.; Sun, B.; Chen, J.; Yuan, L.-X.; Xu, X.-Q.; Chen, J.-X. Water-Stable Silver-Based Metal–Organic Frameworks of Quaternized Carboxylates and Their Antimicrobial Activity. ACS Appl. Bio Mater. 2020, 3, 8525–8531. Available online: https://pubs.acs.org/doi/abs/10.1021/acsabm.0c00896 (accessed on 10 October 2021). [CrossRef] [PubMed]
- Rodríguez, H.S.; Hinestroza, J.P.; Ochoa-Puentes, C.; Sierra, C.A.; Soto, C.Y. Antibacterial Activity against Escherichia Coli of Cu-BTC (MOF-199) Metal-organic Framework Immobilized onto Cellulosic Fibers. J. Appl. Polym. Sci. 2014, 131. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.40815 (accessed on 10 October 2021). [CrossRef]
- Mao, K.; Zhu, Y.; Rong, J.; Qiu, F.; Chen, H.; Xu, J.; Yang, D.; Zhang, T.; Zhong, L. Rugby-Ball like Ag Modified Zirconium Porphyrin Metal–Organic Frameworks Nanohybrid for Antimicrobial Activity: Synergistic Effect for Significantly Enhancing Photoactivation Capacity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125888. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Oveisi, E.; Bulut, S.; Sun, D.T.; Asgari, M.; Trukhina, O.; Queen, W.L. MOF-Derived Cobalt Phosphide/Carbon Nanocubes for Selective Hydrogenation of Nitroarenes to Anilines. Chem. A Eur. J. 2018, 24, 4234–4238. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.201705400 (accessed on 10 October 2021). [CrossRef] [PubMed]
- Xia, G.; Su, J.; Li, M.; Jiang, P.; Yang, Y.; Chen, Q. A MOF-Derived Self-Template Strategy toward Cobalt Phosphide Electrodes with Ultralong Cycle Life and High Capacity. J. Mater. Chem. A 2017, 5, 10321–10327. [Google Scholar] [CrossRef]
- Hou, C.-C.; Zou, L.; Wang, Y.; Xu, Q. MOF-Mediated Fabrication of a Porous 3D Superstructure of Carbon Nanosheets Decorated with Ultrafine Cobalt Phosphide Nanoparticles for Efficient Electrocatalysis and Zinc–Air Batteries. Angew. Chem. 2020, 132, 21544–21550. [Google Scholar] [CrossRef]
- Irgi, E.P.; Geromichalos, G.D.; Balala, S.; Kljun, J.; Kalogiannis, S.; Papadopoulos, A.; Turel, I.; Psomas, G. Cobalt(II) Complexes with the Quinolone Antimicrobial Drug Oxolinic Acid: Structure and Biological Perspectives. RSC Adv. 2015, 5, 36353–36367. [Google Scholar] [CrossRef]
- Wu, R.; Wang, D.P.; Zhou, K.; Srikanth, N.; Wei, J.; Chen, Z. Porous Cobalt Phosphide/Graphitic Carbon Polyhedral Hybrid Composites for Efficient Oxygen Evolution Reactions. J. Mater. Chem. A 2016, 4, 13742–13745. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt Complexes as Antiviral and Antibacterial Agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef] [Green Version]
- Hatamie, S.; Nouri, M.; Karandikar, S.K.; Kulkarni, A.; Dhole, S.D.; Phase, D.M.; Kale, S.N. Complexes of Cobalt Nanoparticles and Polyfunctional Curcumin as Antimicrobial Agents. Mater. Sci. Eng. C 2012, 32, 92–97. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, Structure and Antimicrobial Activity of Manganese(II) and Cobalt(II) Complexes of the Polyether Ionophore Antibiotic Sodium Monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef]
- Alahmadi, N.S.; Betts, J.W.; Cheng, F.; Francesconi, M.G.; Kelly, S.M.; Kornherr, A.; Prior, T.J.; Wadhawan, J.D. Synthesis and Antibacterial Effects of Cobalt–Cellulose Magnetic Nanocomposites. RSC Adv. 2017, 7, 20020–20026. [Google Scholar] [CrossRef] [Green Version]
- Dey, K.; Maiti, R.K.; Bhar, J.K.; Banerjee, R.D.; Sarkar, G.M.; Malakar, A.; Datta, S.; Banerjee, P. Antimicrobial, Insect Sterilizing and Ovicidal Activity of Some Cobalt(II) and Cobalt(III) Complexes. Agents Actions 1981, 11, 762–769. Available online: https://link.springer.com/article/10.1007/BF01978802 (accessed on 10 October 2021). [CrossRef]
| Chemical Formula | (C4H14N2)[Co(H2PO3)4]·2H2O |
|---|---|
| Mr (g/mol) | 509.08 |
| F(000) | 263.9 |
| Symmetry, S.G. | Triclinic P-1 (n. 2) |
| Cell parameters/V | A = 5.4814 (3) Å, b = 7.5515 (4) Å, c = 10.8548 (6)Å, α = 88.001 (4)°, β = 88.707 (5)°, γ = 85.126 (5)°/447.33 (4) Å3 |
| Z | 1 |
| λ (Mo Kα radiation) (Å) | 0.71073 |
| T(K)/µ(mm−1) | 298/1.39 |
| Crystal size (mm) | 0.25 × 0.25 × 0.3 |
| Measured reflections/independent reflections (reflections with I ≥ 2u(I))/parameters | 9480/2005 (1878)/137 |
| Θmin—θmax (°)/Rint | 1.9–27.8/0.024 |
| Reciprocal space limiting indices | h: −6–7, k: −9–9, l: −13–14 |
| R[F2 > 2σ(F2)]/wR(F2)/G.O.F. | 0.026/0.072/1.03 |
| D-H⋯A | D-H/Å | H⋯A/Å | D⋯A/Å | DHA/° |
|---|---|---|---|---|
| O6-H6⋯O13 | 0.80 (3) | 1.80 (3) | 2.605 (2) | 175 (3) |
| O9-H9⋯O8 | 0.82 (1) | 1.77 (1) | 2.574 (2) | 169(1) |
| N12-H12A⋯O6 | 0.89 (1) | 2.16 (1) | 2.900 (2) | 140 (1) |
| N12-H12B⋯O3 | 0.89 (1) | 2.02 (1) | 2.887 (2) | 166 (1) |
| N12-H12C⋯O8 | 0.89 (1) | 1.91 (1) | 2.776 (2) | 165 (1) |
| O13-H13A⋯O2 | 0.71 (3) | 2.22 (3) | 2.888 (2) | 159 (3) |
| O13-H13B⋯O4 | 0.76 (3) | 2.11 (3) | 2.863 (2) | 179 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, N.; Chaouch, S.; da Silva, I.; Ezahri, M.; Lachkar, M.; Alhasan, R.; Abdin, A.Y.; Jacob, C.; Bali, B.E. Synthesis, Structural Characterization, and Biological Activities of Organically Templated Cobalt Phosphite (H2DAB)[Co(H2PO3)4]·2H2O. Sci 2022, 4, 5. https://doi.org/10.3390/sci4010005
Hamdi N, Chaouch S, da Silva I, Ezahri M, Lachkar M, Alhasan R, Abdin AY, Jacob C, Bali BE. Synthesis, Structural Characterization, and Biological Activities of Organically Templated Cobalt Phosphite (H2DAB)[Co(H2PO3)4]·2H2O. Sci. 2022; 4(1):5. https://doi.org/10.3390/sci4010005
Chicago/Turabian StyleHamdi, Najlaa, Souad Chaouch, Ivan da Silva, Mohamed Ezahri, Mohammed Lachkar, Rama Alhasan, Ahmad Yaman Abdin, Claus Jacob, and Brahim El Bali. 2022. "Synthesis, Structural Characterization, and Biological Activities of Organically Templated Cobalt Phosphite (H2DAB)[Co(H2PO3)4]·2H2O" Sci 4, no. 1: 5. https://doi.org/10.3390/sci4010005
APA StyleHamdi, N., Chaouch, S., da Silva, I., Ezahri, M., Lachkar, M., Alhasan, R., Abdin, A. Y., Jacob, C., & Bali, B. E. (2022). Synthesis, Structural Characterization, and Biological Activities of Organically Templated Cobalt Phosphite (H2DAB)[Co(H2PO3)4]·2H2O. Sci, 4(1), 5. https://doi.org/10.3390/sci4010005








