Abstract
Neutron beams, being electrically neutral and highly penetrating, offer unique advantages for the irradiation of biological species such as plants, seeds, and microorganisms. We comprehensively investigated the potential of neutron irradiation for inducing genetic mutations by using simulations of spallation, reactor, and compact neutron sources based on J-PARC BL10, the JRR-3 TNRF, and KUANS. We analyzed neutron flux, energy deposition rates, and Linear Energy Transfer (LET) distributions. The KUANS simulation demonstrated the highest dose rate of 17 Gy/h, significantly surpassing that obtained at BL10, due to the large solid angle achieved with optimal sample placement. The findings highlight KUANS’s suitability for efficiently inducing specific genetic mutations and neutron breeding, particularly for inducing targeted mutations in biological samples, also on account of its LET range of 20–70 keV/μm. Our results emphasize the importance of choosing neutron sources based on LET requirements to maximize mutation induction efficiency. This research study shows the potential of compact neutron sources such as KUANS for effective biological irradiation and neutron breeding, offering a viable alternative to larger facilities. The neutron filters used at BL10 and the TNRF effectively exclude low-energy neutrons while keeping the high-LET component. The neutron capture reaction, 14N(n,p)14C, was found to be the main dose contributor under thermal neutron-dominated conditions.
Keywords:
neutron; irradiation; quantum beam; breeding; mutations; LET; accelerator; compact neutron source; reactor 1. Introduction
Genetic mutations are fundamental to biological evolution and crop improvement and occur naturally or are induced artificially to develop desirable traits in plants, microorganisms, and other biological systems. To achieve targeted genetic modifications, ionizing radiation has emerged as a powerful and chemical-free alternative for mutation induction. Various radiation sources, including ultraviolet (UV), X-rays, gamma rays, heavy ions, and neutron beams, have been utilized for irradiation breeding [1,2,3,4,5,6]. Among these, neutron beams are particularly advantageous due to their deep penetration and high Linear Energy Transfer (LET) characteristics, which result in more efficient DNA damage compared with low-LET sources such as X-rays and gamma rays. High-LET radiation enhances double-strand break (DSB) induction, which plays a key role in mutagenesis efficiency [7].
The primary mechanisms of radiation-induced DNA damage involve single-strand breaks (SSBs) and double-strand breaks (DSBs), with high-LET radiation producing clustered DNA damage that is more biologically consequential. The effectiveness of neutron irradiation in inducing mutations is strongly dependent on LET. For instance, studies have shown that in Arabidopsis thaliana, an LET value of 30 keV/μm is optimal for inducing albino mutants [8], whereas the Minimal Ionization Energy deposition (MIP) due to fast charged particles is just 0.2 keV/μm. Higher LET values (50–100 keV/μm) have been observed to enhance mutations in crops such as rice and chrysanthemum [8,9,10]. This highlights the importance of optimizing LET distributions for targeted genetic modifications.
While low-LET radiation, such as X-rays and gamma rays, induces mutations primarily through base substitutions and single-strand damage, high-LET radiation, including heavy ions and neutron beams, induces complex genetic changes, including large deletions and chromosomal rearrangements [11,12]. Neutron irradiation is particularly effective in inducing these complex mutations with lower doses compared with gamma rays or heavy ions [13]. Fast neutrons (0.1–1 MeV) interact with hydrogen nuclei in biological materials, producing recoil protons that deposit high-LET energy, leading to increased mutation efficiency. Unlike heavy-ion beams, which require precise positioning due to their short penetration range, neutron beams can uniformly irradiate samples, including liquid-phase cultures, making them highly suitable for mutagenesis applications.
Historically, gamma rays were widely used for irradiation treatments from the 1950s to the 1990s [14,15], while heavy-ion beams gained prominence from the 1990s to the 2010s due to their ability to generate higher mutation frequencies through localized ionization effects [15]. However, heavy-ion beams have low material permeability, requiring precise sample placement within a narrow range. In contrast, neutron beams provide significant advantages due to their superior penetration depth and uniform irradiation potential, making them more effective for mutagenesis applications. Studies have shown that neutron irradiation leads to higher mutation efficiency and a broader spectrum of genetic changes compared with traditional radiation sources [13,16].
Neutron irradiation has been performed at large-scale facilities such as spallation neutron sources, e.g., J-PARC (Japan Proton Accelerator Research Complex) [17], or research reactors, e.g., the JRR-3 (Japan Research Reactor-3) TNRF [18], which require extensive planning, have limited access, and incur high operational costs. Since the 1980s, neutron irradiation has primarily been conducted by using nuclear reactors, but recent advancements have led to the development of compact neutron sources, such as Kyoto University Accelerator-driven Neutron Source (KUANS) [19]. These smaller-scale neutron sources offer increased accessibility and irradiation efficiency while maintaining effective neutron flux, making them a promising alternative for future genetic modification studies [20].
By integrating computational analysis with experimental insights, we aim to optimize neutron-based mutagenesis protocols and highlight the potential of compact neutron sources for genetic modifications in plants and microorganisms. In this study, we modeled three neutron sources, i.e., J-PARC BL10, the JRR-3 TNRF, and KUANS, as typical neutron sources and evaluated the radiation parameters by employing Monte Carlo-based PHITS simulations [21]. The results provide quantitative assessments of dose, LET distributions, and depth profiles in biological materials, contributing to the development of efficient neutron irradiation methodologies for genetic research and crop improvement.
2. Method and Results
2.1. Irradiation Setup at J-PARC and Simulation
Neutron irradiation experiments were conducted at the Materials and Life Science Experimental Facility (MLF) within J-PARC BL10 (NOBORU) [17]. This general-purpose beamline uses spallation reactions to generate fast neutron beams for irradiation studies.
We are conducting biological irradiation experiments at this beamline and will perform a quantitative evaluation of its performance. To simulate the experimental setup, we employed PHITS (Particle and Heavy Ion Transport System), which is a general-purpose Monte Carlo particle transport simulation code that calculates neutrons, photons, and electrons [21], incorporating neutron flux and energy spectrum data from published sources [22]. The geometry replicates the experimental arrangement used for irradiation, including the mercury target, moderator, beamline, and irradiation cell dimensions.
When neutrons interact with atomic nuclei, they induce some nuclear reactions, i.e., scattering, capture, or ion production, resulting in a proton or an alpha. The radiation dose generated by neutrons is mainly caused by recoil protons produced by neutron scattering with hydrogen. Thus, high-energy neutrons above 1 keV were used to irradiate biological samples by cutting off slow neutrons, which would have caused the activation of the samples. Figure 1 illustrates the schematic diagram of the BL10 experiment and the associated simulation setup. In the left diagram, the illustration samples (yellow) indicate the irradiation cells, each containing biological materials. Fast neutron beams travel from the left side, retaining a 14 m distance from the irradiation cells. The values of beam flux and divergence at the sample position were obtained from the database in [22]. The neutron beam size was 7 × 7 cm2, and each sample was uniformly packed in a cylindrical aluminum container with a diameter of 5.5 cm [23], a length of 2.3 cm, and a thickness of 0.5 mm. The neutron flux in the irradiation area was n/cm2/s in 1 MW equivalent7. The neutron flux and energy spectrum data were obtained from reference [24], and PHITS simulations were configured to replicate these conditions. The simulation included calculations for recoil nuclei, nuclear spallation reactions, and ion production through the 14N(n,p)14C reaction. The generated secondary particles were tracked over repeated interactions until they reached a cutoff energy of 1 keV.
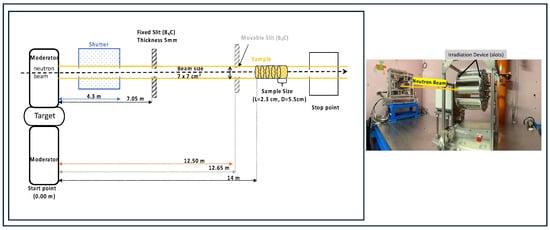
Figure 1.
Schematic side view of the neutron beam irradiation setup at J-PARC BL10 (left) and its photo (right). In the left diagram, the neutrons produced in the target travel to the beamline via the moderator. The sample shown in yellow indicates the irradiation cells containing biological materials. The B4C fixed slit and movable slit serve as an essential filter.
Irradiation targets can be part of a plant, an embryo of a plant, seeds, yeast, microorganisms, or microalgae and are characterized by their chemical composition, basically including carbon (C), hydrogen (H), nitrogen (N), oxygen (O), and other relevant elements. In this study, we employed an irradiation sample with proportions of C, H, N, and O of 42.0%, 7.0%, 4.4%, and 46.7% in weight%, respectively, and a weight density of 1.00 g/cm3 to simulate typical bean (plant) seeds [25].
The aluminum (Al) cylinder was chosen as a sample container, instead of Petri dishes, for its small neutron capture cross-section to avoid the loss of neutrons and the production of gamma rays, which contribute to low-LET radiation on the samples. Notably, aluminum is less likely to be radioactivated. Five Al cells were densely arranged along the beamline to evaluate the depth profile of the dose rate.
Because slow neutrons do not contribute to high LET but cause activation, we utilized a boron carbide filter (B4C) of 5 mm in thickness (Movable Slit in Figure 1) to attenuate them [18]. We simulated the dose of neutrons with and without the B4C filter and calculated their energy spectra with PHITS, as shown in Figure 2a. The neutron spectra are shown in a double-logarithmic graph, with the vertical axis showing per-unit lethargy, which takes the natural logarithm as the unit. The spectrum without B4C shows a peak in cold neutron energy around 10 meV, followed by a gradual increase up to ~1 MeV, i.e., the energy of the generated spallation neutrons. With B4C, neutrons below 10 eV completely disappear, but more than 70% of neutrons survive in the vicinity of 1 MeV, which is useful for irradiation. The total flux was attenuated to n/cm2/s, mainly due to the absence of low-energy neutrons.


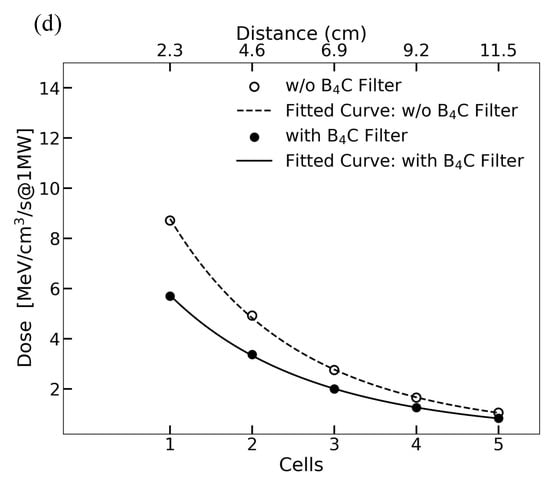
Figure 2.
(a) The neutron flux as a function of energy with and without the B4C filter at J-PARC BL10. The dashed line represents the unfiltered neutron flux, while the solid line illustrates the spectrum after B4C filtering, highlighting the attenuation of low-energy neutrons. The LET distributions for different charged particles calculated with the beam power of 1 MW in the cases without (b) and with (c) the B4C filter. The solid black line shows the total dose from all particles combined, the dotted red line represents the LET distribution of electrons, and the dashed blue line shows that of protons. Additionally, the green dashed line corresponds to 12C ions, the yellow dashed line to 14N ions, the purple dashed line to 16O ions, and the light green dashed line to 14C ions. (d) The exponential decrease in absorbed dose with the increase in depth. The comparison between irradiation with and without the B4C filter highlights the filter’s role in moderating neutron penetration. The fitted curve follows the exponential of a quadratic polynomial fit confirming significant attenuation of absorbed dose with the increase in sample depth.
We also assessed the LET without and with B4C, as shown in Figure 2b,c, and to further quantify neutron dose variation with sample depth, we performed a depth-dependent dose analysis, as shown in Figure 2d. The data confirm an exponential decrease in absorbed dose, consistent with neutron attenuation through the biological samples. The fitted curves, modeled using the exponential of a quadratic polynomial fit, highlight significant neutron energy loss with the increase in depth, emphasizing the impact on biological mutation efficiency. The LET is classified according to electrons, protons, and other nuclei. The scattering of neutrons with materials generates recoil nuclei, which become secondary charged particles with high LET that deposit energy in localized positions, causing significant biological damage.
In the spectrum of the BL10 simulation in Figure 2b, the energy deposited by recoil protons from neutron scattering with hydrogen nuclei is represented in the range of 1–100 keV/μm LET. The low-LET component (~1 keV/μm) is due to the high-energy protons generated by scattering with high-energy neutrons of ~100 MeV, while the high-LET component is due to neutrons of ~1 MeV. The energy deposited by electrons is due to gamma rays from neutron absorption reactions, with a peak in the MIP region of 0.2 keV/μm. The LET corresponding to 100–1000 keV is due to the recoil of nuclei such as C, N, and O in the sample. Additional contributions from heavy ions such as C, N, and O are highlighted in Figure 2c. These heavy ions show significant energy deposition at higher LET values (100–1000 keV/μm). The total dose spectrum (black line) incorporates all particles, with peaks due to electron interactions at lower LET values and broader distributions due to heavier ions and protons at higher LET values.
The total doses are obtained by integrating the LET values in Figure 2b,c. In the case without the B4C filter, the total energy deposited on the sample is 6.7 × 106 MeV/cm3/s with 1 MW beam power. Recoiled protons account for 85% of the total deposit; the contribution of electrons, caused by neutron capture gammas, is 1.5% of the total; the total due to C, N, and O recoil nuclei is 11%. A detailed discussion of the total doses is given in Section 4 based on a comparison with the other facilities.
2.2. Irradiation Setup at JRR-3 (TNRF)
Historically, most neutron irradiation has been carried out by using nuclear reactors. In the JRR-3 experiments conducted at the TNRF, a thermal neutron beam generated by a research reactor with a beam power of 20 MW was used to irradiate the samples, as shown in Figure 3. The neutron flux at the TNRF port is 1.0 × 108 n/cm2/s, with a beam size of W255 × H305 mm [26]. This beamline produces fast neutrons and gamma rays in the thermal neutron beam. For irradiation at this beamline, a cadmium (Cd) plate of 0.5 mm in thickness was used to attenuate the thermal neutrons and reduce the activation of the sample materials.
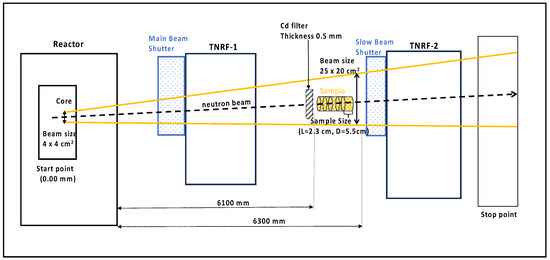
Figure 3.
A schematic of the neutron irradiation setup (side view) at JRR-3 (TNRF), which produces thermal neutrons and fast neutrons. The setup includes a Cd filter covering the irradiation samples to generate fast neutrons in advance. The sample shown in yellow indicates the irradiation cells containing biological materials.
We developed a simulation based on the BL10 case. With the same sample parameters, we used the neutron flux and its energy spectrum in the beam area from reference [18] as the input of the simulation. The neutron beam originated from the reactor’s core in water located 6.1 m away with a beam size of 4 × 4 cm2 and was transported along the beam path with a widening to 25 × 20 cm2 at the sample position.
We calculated the neutron fluxes in the cases with and without the Cd filter, and the calculated neutron energy spectra are shown in Figure 4a. The spectrum without the Cd filter shows a large peak of around 10 meV, indicating a high flux of thermal neutrons. The neutron flux gradually decreases to about 1 MeV, unlike at BL10, covering a broad spectrum from thermal to high-energy neutrons. The use of the Cd filter of 0.5 mm alters the neutron flux spectrum by reducing thermal neutrons while preserving high-energy ones. This is due to the high absorption cross-section of Cd, which effectively eliminates neutrons below 1 eV. The simulation result of the total neutron flux with the Cd filter is 2.1 × 107 n/cm2/s at 20 MW, which is 15% of that without the Cd filter. The reduction is greater than that of BL10 (31%) because the main components at the TNRF are thermal neutrons.

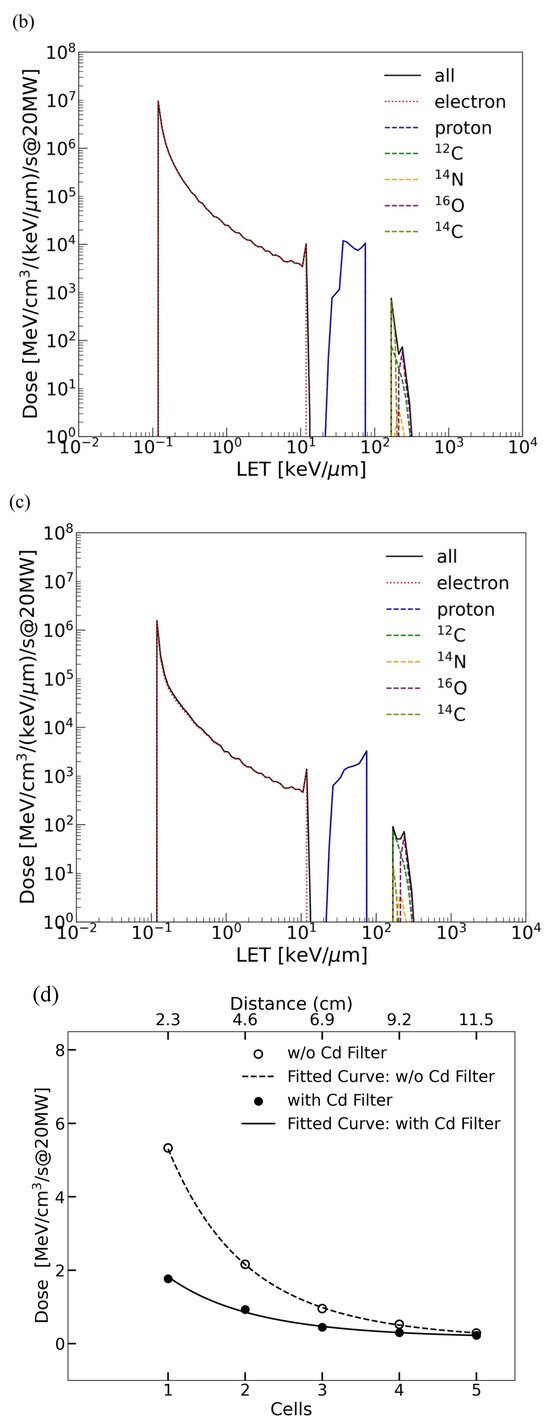
Figure 4.
(a) The neutron flux as a function of energy with and without the Cd filter at JRR-3, TNRF port. The dashed and solid lines represent the neutron flux without and with the Cd filter, respectively. The LET distributions for different charged particles calculated with a reactor power of 20 MW in the cases without (b) and with (c) the Cd filter. The solid black line indicates the total dose from all particles combined, the dotted red line represents the LET distribution of electrons, and the dashed blue line shows that of protons. Additionally, the green dashed line corresponds to 12C ions, the yellow dashed line to 14N ions, the purple dashed line to 16O ions, and the light green dashed line to 14C ions. (d) The depth-dependent neutron dose reduction with and without the Cd filter. The data indicate that the Cd filter effectively suppresses the thermal neutron contribution, significantly reducing the dose compared with the unfiltered case, which is crucial to radiobiological experiments requiring a selective neutron energy spectrum. The fitted curves representing the exponential of a quadratic polynomial fit further highlight the attenuation behavior for both conditions, confirming that the neutron dose decreases exponentially with depth.
The LET values of the JRR-3 simulation without and with the Cd filter are shown in Figure 4b and Figure 4c, respectively. Protons contribute to the dose at higher LET, similar to the BL10 case, but show a sharper peak at 10–100 keV/μm. This is because the maximum energy of neutrons produced by a nuclear reactor is limited to 2 MeV. In the case of the Cd filter, the total dose was 1.7 × 105 MeV/cm3/s, of which 74% was due to protons and 17% to electrons. In the case without the Cd filter, the total dose was 6.6 × 105 MeV/cm3/s. Notably, the contribution of the 14N(n,p)14C reaction accounted for 55% of the total dose. The effect of such ion emission reactions is more significant than that of recoil in thermal neutron irradiation.
The simulation results in Figure 4d demonstrate that the use of a Cd filter significantly reduces the thermal neutron contribution, leading to a sharper decline in absorbed dose with depth. This reduction is particularly relevant for radiobiological experiments where selective neutron energy is required. The fitted curve in the figure illustrates the expected attenuation behavior, emphasizing the importance of neutron filtration in optimizing dose delivery.
By incorporating the Cd filter, the absorbed dose in deeper layers of the biological samples is reduced by approximately 85% compared with unfiltered irradiation. The primary contributor to dose deposition in the filtered setup remains fast neutrons, whereas the unfiltered conditions show a greater influence of thermal neutron interactions and secondary gamma-ray production.
2.3. Simulation of a Compact Neutron Source (KUANS)
In recent years, many compact neutron sources have been constructed by using small accelerators and Be or Li targets [19]. To explore the possibility of the use of neutron irradiation for genetic mutation based on a compact neutron source, we developed a simulation model of Kyoto University Accelerator-driven Neutron Source (KUANS) [20], which is an early-type source and has a relatively low power, as an example to estimate the irradiation dose.
KUANS consists of a proton linear particle accelerator (LINAC), a Be target, a polyethylene moderator, carbon reflectors, and radiation shields made of boron-doped polyethylene and concrete. It has been developed for research and development of neutron devices, as well as for educational purposes. Here, we consider the use of KUANS for biological irradiations. Neutrons in KUANS are generated through the 9Be (p, n) 9B reaction, using a pulsed 3.5 MeV proton beam and a maximum averaged current of 100 μA. The threshold energy of the neutron production reaction is 2057 keV, and the maximum energy for the neutrons is 1.3 MeV. The neutron production rate is expected to be ~1011 n/s [20], which is six orders of magnitude lower than that of J-PARC (~1017 n/s).
At KUANS, the produced fast neutrons are normally moderated with a polyethylene moderator with a volume of 10 × 10 × 10 cm3 to obtain a thermal neutron beam. The moderated neutrons are transported perpendicularly to the proton beam direction and used as a pulsed neutron beam [20]. Because unmoderated fast neutrons are useful for biological irradiation, we set up a computational geometry in our simulation where we removed the polyethylene moderator and set the sample right in front of the target where the generated neutrons could hit it directly. The schematic diagram of KUANS for the simulation is illustrated in Figure 5. Fast neutrons were produced by the Be target. The samples were located 50 mm from the Be target to interact with a higher number of fast neutrons. Because the Be target was covered with a graphite reflector, the transmitted neutrons also had a certain probability of returning to the sample position. The neutron spectrum from the Be target was calculated according to the reaction cross-section [27] and proton energy loss in Be [25]. The neutron distribution was assumed to be isotropic.
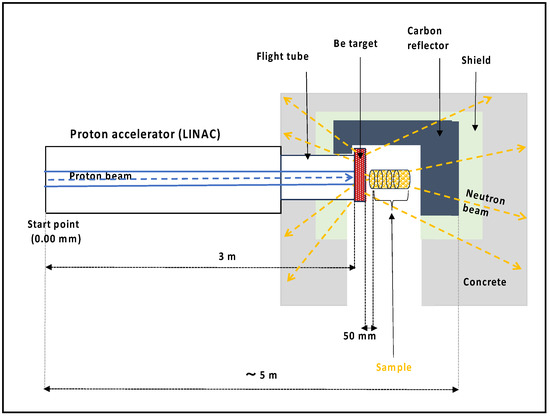
Figure 5.
A schematic diagram (side view) of KUANS. In the diagram, proton beams travel from the left side to the Be target (indicated in red color), producing neutrons traveling around it. The sample shown in yellow indicates the irradiation cells containing biological materials.
The neutron flux in the sample in the KUANS geometry is shown in Figure 6a. It has a high peak of around 1 MeV, and thermal neutrons are vanishingly small due to the absence of moderators. In addition, as the design does not use a moderator, the thermal neutron flux is kept small even without using a thermal neutron filter such as B4C or Cd. This is suitable for higher-LET irradiation without causing activations. The neutron flux at KUANS is 4.6 × 108 n/cm2/s, which is 1.4 times higher than the 3.2 × 108 n/cm2/s at J-PARC. This is because the distance from the target to the sample is very small (50 mm) at KUANS, compared with the 14 m at BL10, and larger solid angles are available. The LET from protons ranges in a relatively narrower region, 20–70 keV/μm, as shown in Figure 6b because the neutrons generated by the Be target are concentrated at 0.5–1.3 MeV.

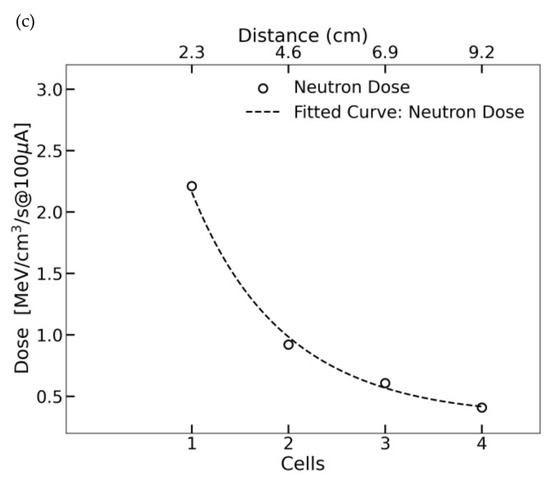
Figure 6.
(a) The simulated neutron flux in the sample at KUANS as a function of energy. (b) LET distributions for different charged particles. The solid black line represents the total dose from all particles, the dashed blue line is that of protons, and the dotted red line is for electrons. The green dashed line corresponds to 12C ions, the yellow dashed line to 14N ions, the purple dashed line to 16O ions, and the light green dashed line to 14C ions. (c) The absorbed dose distribution across a cylindrical sample, showing a depth-dependent attenuation trend. The fitted curve, representing the exponential of a quadratic polynomial fit , closely follows the measured data, confirming a predictable exponential decrease in dose with the increase in depth.
Figure 6c presents the dose profile across a cylindrical biological sample exposed to KUANS neutron irradiation. Unlike reactor-based sources, KUANS produces a dose rate of 17 Gy/h, with absorbed dose attenuation following an exponential decline. The high-LET component in KUANS irradiation ensures efficient mutation induction despite moderate dose rates.
The simulation confirms that neutron energy loss due to scattering is minimized when using compact neutron sources such as KUANS. The results indicate that placing biological samples closer to the neutron source enhances absorbed dose efficiency while maintaining uniform energy deposition across the irradiation target.
At KUANS, we achieved the highest dose rate of 17 Gy/h, 4.5 times higher than that obtained at J-PARC BL10 (3.86 Gy/h without B4C filter) and 42 times higher than that obtained at JRR-3 TNRF (0.38 Gy/h without Cd filter). The LET range at KUANS (20–70 keV/μm) closely aligns with optimal mutation induction, making it a viable alternative to large-scale neutron facilities. While J-PARC BL10 provides broad LET distributions up to 100 keV/μm and high neutron flux, the JRR-3 TNRF is more suited for experiments requiring thermal neutrons with specific activation reactions (14N(n,p)14C). These findings underscore the importance of selecting the appropriate neutron source based on LET requirements and experimental objectives.
3. Discussion
In the previous section, we calculated the irradiation at J-PARC BL10, the JRR-3 TNRF, and KUANS. The results of key parameters, such as neutron flux and energy deposition rates for each particle, are summarized in Table 1. In this section, we discuss the effectiveness of biological irradiation at each site.

Table 1.
The simulation results for J-PARC, JRR-3, and KUANS.
The choice of cylindrical aluminum samples over Petri dishes was motivated by the need to ensure uniform and accurate neutron irradiation. Unlike plastic or glass Petri dishes, which introduce unwanted neutron scattering and low-LET gamma emission, aluminum cells minimize neutron absorption and maintain a high-LET irradiation environment. Given that Petri dishes are primarily suited for monolayer cell cultures, our approach better represents volumetric biological samples, which is essential to neutron irradiation studies targeting genetic mutation induction. By using aluminum cylindrical samples, we achieved a consistent dose profile that aligns with the simulation model, allowing for a more reliable evaluation of neutron-induced biological effects.
The neutron flux directly impacts the dose rate and the irradiation time required to achieve specific biological effects. KUANS produces a neutron flux of 4.6 × 108 n/cm2/s, which is 1.4 and 3.3 times higher than that of BL10 or the TNRF, respectively. The most dominant factor in KUANS inducing the largest flux despite its lower neutron generation rate is the distance from the target. By placing the sample at a distance comparable to the size of the target, a large solid angle of ~1 sr can be obtained, which is a significant advantage compared with using neutrons as a beam, as in the case of large facilities (50 μsr for J-PARC BL10). In addition, the workspace being surrounded by reflectors but without moderators is a major advantage for the use of fast neutrons. Since slow neutrons have low LET, moderators have minimal impact on mutation efficiency.
The dose rate per unit time was the highest for KUANS, 3.0 × 107 MeV/cm3/s, despite the small neutron production rate. This is 4.5 times higher than the BL10 result without the neutron filter. In both cases, KUANS and BL10, the energy deposited is mostly derived from recoil protons. Because the fraction of high-energy neutrons is lower, the total dose rate with the filtered beam at the TNRF was 1/31 that of BL10 with the filter. Based on the density of the sample used of 1.00 g/cm3, the dose rate can be converted to 2.5, 0.08, and 17 Gy/h for BL10 (with B4C), the TNRF (with Cd), and KUANS, respectively. Although a dose rate of 17 Gy/h may be considered moderate for radiobiological experiments, neutron irradiation presents distinct advantages due to its high-LET characteristics. Previous studies indicate that a total dose of 40 Gy with LET values of 30–70 keV/μm is sufficient to induce genetic mutations in seeds and microorganisms. With a dose rate of 17 Gy/h, the required exposure time is approximately 2.4 h, making KUANS an efficient alternative to high-dose gamma or heavy-ion irradiation while ensuring uniform neutron penetration without excessive lethality. The result highlights the potential of compact neutron sources such as KUANS for efficient irradiation. Low-LET doses due to electrons are more likely to cause SSBs rather than DSBs and should be mitigated for efficient mutation. In the present calculation, the dose due to the electrons was suppressed to less than 30% of the total dose under all conditions.
The neutron filters used at BL10 and the TNRF were found to be effective in eliminating neutrons below eV while retaining MeV neutrons. Low-energy neutrons increase the dose by electrons generated by gamma rays; however, these only contribute to low LET values below 10 keV/μm. Therefore, it is expected that the contribution would be comparable to that of gamma irradiation. Thus, neutron filters allow only for irradiation with high LET. With the B4C filter, the dose rate of electrons is reduced to 1/15 that at BL10, while that of protons is kept at 65%. The results obtained at the TNRF without a filter showed that the energy deposited due to the 14N(n,p)14C reaction with thermal neutrons was dominant. This reaction accounted for 54% of the total dose, while the contribution of recoil protons was 11%. The 14N(n,p)14C reaction emits protons at 583 keV and 14C at 42 keV, resulting in LET values of 49–90 keV and 200 keV, respectively, both of which are high enough to induce DSBs in DNAs. This reaction may be responsible for the similar mutations observed with thermal neutron irradiation and fast neutrons [28]. If ion-emitting nuclides with neutron capture, such as 6Li and 10B, can be implemented in the samples, high LET values are obtained even with thermal neutrons only.
Biological effects are determined by genetic mutations induced by DSBs of DNA. Therefore, they are influenced not only by the total dose but also by LET. The optimum LET for mutation induction differs among biological species. For seeds of Arabidopsis, peak lethality occurs at LET values of 290–400 keV/μm [29,30], while the induction of mutation for generating albino mutants appears most effective at around 30 keV/μm [8,9,10]. A LET value of 50 keV/μm of 12C6+ ions was ideal for achieving phenotype mutants in rice seeds [31]. In the case of Chrysanthemum, it was reported that a LET of 76 keV/μm was most effective for inducing flower color mutants [32]. As discussed in the previous sections, neutron irradiation results in a different LET value for heavy ions; typically, the LET of carbon (C) ions ranges from 10 to 200 keV/μm, and that of iron (Fe) ions is 100 to 500 keV/μm [8,30]. The J-PARC and KUANS experiments resulted in different LET distributions. This is because the incident neutron energy at KUANS is 0.5–1.3 MeV, whereas at J-PARC, it includes higher neutrons above 100 MeV. The LET at J-PARC is broadly distributed from 1 to 100 keV/μm for protons and 100–1000 keV/μm for 12C, 14N, and 16O, whereas at KUANS, it is concentrated in the 20–70 keV range. Based on the results of this study, we can use different neutron sources depending on the required LET range to induce more efficient genetic mutations.
The results highlight the benefits of compact neutron sources such as KUANS, which provide high LET and dose rates with minimal sample activation. While J-PARC BL10 and the JRR-3 TNRF offer controlled neutron spectra and established facilities, KUANS achieves comparable mutation efficiency in a more accessible setup. Neutron source selection should be based on the required LET range and irradiation conditions to maximize biological effectiveness.
4. Conclusions
In this study, we developed a simulation that replicated real experimental setups to assess the potential of neutron irradiation for inducing genetic mutation in biological species. We conducted PHITS simulations for J-PARC BL10, the JRR-3 TNRF port, and KUANS. J-PARC BL10 and the JRR-3 TNRF have been previously demonstrated to be facilities that can be effectively employed for mutagenesis, while KUANS was investigated as a compact neutron source alternative. The simulations used a typical bean (plant) seed model for the assessment of irradiation effects.
The dominant contributors to dose deposition were the recoil protons generated by fast neutron scattering (~1 MeV), highlighting the significant role of fast neutrons in radiation-induced mutations. Despite its lower neutron rate, at KUANS, we achieved a dose rate of 17 Gy/h, which is 4.5 times higher than that obtained at J-PARC BL10 (3.86 Gy/h without B4C filter) and 42 times higher than that obtained at the JRR-3 TNRF (0.38 Gy/h without Cd filter). These findings emphasize the efficiency of compact neutron sources in biological irradiation applications. The suppression of gamma-ray contributions ensured optimal LET characteristics for mutation induction. Since the JRR-3 TNRF is customized for thermal neutrons and fast neutrons are suppressed, the effect of recoil protons is small. In the JRR-3 TNRF experiment without the Cd filter, the 14N(n,p)14C reaction was the dominant dose contributor, contributing five times more than the recoil protons. This reaction plays a significant role in neutron-induced mutations, particularly in thermal neutron environments, and doping with 6Li or 10B, which absorb neutrons to produce ions, may enable genetic mutations even under thermal neutron irradiation alone, such as in Boron Neutron Capture Therapy (BNCT).
Recoil protons were the primary contributors to the neutron-induced dose, with LET values ranging from 1 to 100 keV/µm. The KUANS experiment exhibited a narrower LET distribution at 20–70 keV/µm compared with the J-PARC BL10 simulation, as KUANS neutrons were limited to 1.3 MeV, reducing the presence of higher-LET components. We could induce genetic mutations more efficiently by using different neutron sources depending on the required LET range.
The depth-dependent dose analysis confirmed an exponential attenuation trend, with LET distributions significantly influencing DNA damage efficiency. The LET range in KUANS is particularly favorable for genetic mutation studies, aligning with previous findings on optimal LET values for mutagenesis. Our results indicate that compact neutron sources such as KUANS are not only viable but also efficient alternatives to large-scale neutron facilities. With high LET values and effective dose rates, KUANS can facilitate neutron-induced mutagenesis in a more accessible and cost-effective manner, paving the way for advanced radiation breeding techniques. Neutron filtration techniques play a critical role in tailoring neutron spectra to enhance biological effectiveness while minimizing unwanted secondary interactions. LET-dependent DNA damage models indicate that neutron source selection should be tailored to specific mutagenesis goals to optimize genetic modification outcomes. These insights contribute to the future design of radiobiological studies, refining neutron-based breeding techniques and improving neutron irradiation conditions for targeted genetic modifications.
Author Contributions
M.S.: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing—original draft, Writing—review & editing, Visualization. K.M.: Validation, Writing—review & editing, Supervision. M.H., K.K., H.I., S.T.: Methodology, Investigation, Writing—review & editing. N.K.: Conceptualization, Validation, Investigation, Writing—review & editing, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Quantum Flowers & Foods Co., Ltd. through internal research funding. This includes support for project conceptualization and the provision of patented equipment used in the study. The APC was also funded by Quantum Flowers & Foods Co., Ltd. No external grant was involved.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors are deeply grateful to the accelerator operation staff and the experimental groups at J-PARC (BL10), JRR-3 (TNRF), and KUANS for their invaluable support during the experiment. The authors further acknowledge Takashi Ino and Katsuya Hirota for their collaborative research efforts with the High Energy Accelerator Research Organization (KEK).
Conflicts of Interest
Author May Sweet and Norio Kikuchi were employed by the company Quantum Flowers & Foods. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yamada, R.; Kashihara, T.; Ogino, H. Improvement of lipid production by the oleaginous yeast Rhodosporidium toruloides through UV mutagenesis. World J. Microbiol. Biotechnol. 2017, 33, 99. [Google Scholar] [CrossRef]
- Jo, Y.D.; Kim, J.B. Frequency and spectrum of radiation-induced mutations revealed by whole-genome sequencing analyses of plants. Quantum Beam Sci. 2019, 3, 7. [Google Scholar] [CrossRef]
- Yuan, N.; Liang, S.; Zhou, L.; Yuan, X.; Li, C.; Chen, X.; Zhao, H. Comparison of Mutations Induced by Different Doses of Fast-Neutron Irradiation in the M1 Generation of Sorghum (Sorghum bicolor). Genes 2024, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zhang, Y.; Huang, M.; Guo, T.; Yang, G. Genome-Wide Profile of Mutations Induced by Carbon Ion Beam Irradiation of Dehulled Rice Seeds. Int. J. Mol. Sci. 2024, 25, 5195. [Google Scholar] [CrossRef]
- Jankowicz-Cieslak, J.; Hofinger, B.J.; Jarc, L.; Junttila, S.; Galik, B.; Gyenesei, A.; Ingelbrecht, I.L.; Till, B.J. Spectrum and Density of Gamma and X-ray Induced Mutations in a Non-Model Rice Cultivar. Plants 2022, 11, 3232. [Google Scholar] [CrossRef]
- Ogawa, H.; Shilviada, K.; Tomizawa, J.-I. Studies on Radiation-sensitive Mutants of E. coli I. Mutants Defective in the Repair Synthesis. Mol. Gen. Genet. 1968, 101, 227–244. [Google Scholar] [CrossRef]
- Goodhead, D.T. Mechanisms for the Biological Effectiveness of High-LET Radiations. J. Radiat. Res. 1999, 40, S1–S13. [Google Scholar] [CrossRef]
- Kazama, Y.; Saito, H.; Yamamoto, Y.Y.; Hayashi, Y.; Ichida, H.; Ryuto, H.; Fukunishi, N.; Abe, T. LET-dependent effects of heavy-ion beam irradiation in Arabidopsis thaliana. Plant Biotechnol. 2008, 25, 113–117. [Google Scholar] [CrossRef]
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From Classical Radiation to Modern Radiation: Past, Present, and Future of Radiation Mutation Breeding. Front. Public Health 2021, 9, 768071. [Google Scholar] [CrossRef]
- Ishii, K.; Kazama, Y.; Morita, R.; Hirano, T.; Ikeda, T.; Usuda, S.; Hayashi, Y.; Ohbu, S.; Motoyama, R.; Nagamura, Y.; et al. Linear energy transfer-dependent change in rice gene expression profile after heavy-ion beam irradiation. PLoS ONE 2016, 11, e0160061. [Google Scholar] [CrossRef]
- Hwang, W.J.; Kim, M.Y.; Kang, Y.J.; Shim, S.; Stacey, M.G.; Stacey, G.; Lee, S.-H. Genome-wide analysis of mutations in a dwarf soybean mutant induced by fast neutron bombardment. Euphytica 2015, 203, 399–408. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Velalopoulou, A.; Zanni, V.; Tremi, I.; Havaki, S.; Kokkoris, M.; Gorgoulis, V.G.; Koumenis, C.; Georgakilas, A.G. Key biological mechanisms involved in high-LET radiation therapies with a focus on DNA damage and repair. Expert. Rev. Mol. Med. 2022, 24, e15. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, S.; Rana, N.; Bansal, R.; Vishwakarma, G.; Mehetre, S.T.; Das, B.K.; Kumar, M.; Yadav, S.K.; Sonah, H.; Sharma, T.R.; et al. Expanding avenue of fast neutron mediated mutagenesis for crop improvement. Plant 2019, 8, 164. [Google Scholar] [CrossRef]
- Feng, Z.; Du, Y.; Chen, J.; Chen, X.; Ren, W.; Wang, L.; Zhou, L. Comparison and Characterization of Phenotypic and Genomic Mutations Induced by a Carbon-Ion Beam and Gamma-ray Irradiation in Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2023, 24, 8825. [Google Scholar] [CrossRef]
- Marcu, D.; Damian, G.; Cosma, C.; Cristea, V. Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays). J. Biol. Phys. 2013, 39, 625–634. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Century, K.; Straight, S.; Ronald, P.; Dong, X.; Lassner, M.; Zhang, Y. A fast neutron deletion mutagenesis-based reverse genetics system for plants. Plant J. 2001, 27, 235–242. [Google Scholar] [CrossRef]
- Maekawa, F.; Oikawa, K.; Harada, M.; Kai, T.; Meigo, S.; Kasugai, Y.; Ooi, M.; Sakai, K.; Teshigawara, M.; Hasegawa, S.; et al. NOBORU: J-PARC BL10 for facility diagnostics and its possible extension to innovative instruments. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 600, 335–337. [Google Scholar] [CrossRef]
- Matsubayashi, M.; Kobayashi, H.; Hibiki, T.; Mishima, K. Design and characteristics of the JRR-3M thermal neutron radiography facility and its imaging systems. Nucl. Technol. 2000, 132, 309–324. [Google Scholar] [CrossRef]
- IAEA (International Atomic Energy Agency). Compact Accelerator Based Neutron Sources; IAEA Nuclear Energy Series No. NP-T-5.8. International Atomic Energy Agency: Vienna, Austria, 2021. Available online: https://www.iaea.org/publications/14709/compact-accelerator-based-neutron-sources (accessed on 8 March 2025).
- Tasaki, S.; Nagae, T.; Hirose, M.; Yamashita, Y.; Hironaka, K.; Abe, Y.; Yamagata, Y.; Otake, Y.; Hirota, K. Properties and possible applications of Kyoto University accelerator-based neutron source (KUANS). Phys. Procedia 2014, 60, 181–185. [Google Scholar] [CrossRef]
- Sato, T.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.-I.; Kai, T.; Matsuya, Y.; Matsuda, N.; Hirata, Y.; et al. Recent improvements of the particle and heavy ion transport code system—PHITS version 3.33. J. Nucl. Sci. Technol. 2024, 61, 127–135. [Google Scholar] [CrossRef]
- Harada, M.; Teshigawara, M.; Ohi, M.; Klinkby, E.; Zanini, L.; Batkov, K.; Oikawa, K.; Toh, Y.; Kimura, A.; Ikeda, Y. Experimental validation of the brightness distribution on the surfaces of coupled and decoupled moderators composed of 99.8% parahydrogen at the J-PARC pulsed spallation neutron source. Nucl. Instrum. Methods Phys. Res. Sect. A 2018, 903, 38–45. [Google Scholar] [CrossRef]
- Quantum Flowers & Foods Co., Ltd. Neutron Ray Irradiation Target Apparatus, Mutation Induction Method, and Irradiation Target Manufacturing Method. U.S. Patent No. 11980148 B2, 14 May 2024. Available online: https://qff.jp/en/news/notice-of-obtaining-u-s-patent (accessed on 8 March 2025).
- Harada, M.; Teshigawara, M.; Ooi, M.; Oikawa, K.; Takada, H.; Ikeda, Y. Experimental characterization of high-energy component in extracted pulsed neutrons at the J-PARC spallation neutron source. Nucl. Instrum. Methods Phys. Res. Sect. A 2021, 1000, 165252. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Manoyan, J.M. The Stopping of Ions in Compounds. Nucl. Instrum. Methods Phys. Res. Sect. B 1988, 35, 215–228. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Yao, Z.; Yin, Y.; Feng, H.; Wei, Z. Study on the Relationship between Seed Absorbed Dose and Seed Composition of 252Cf Neutron Source Irradiated Bean Seed. Sci. Rep. 2019, 9, 9635. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.H.; Macklin, R.L. Total Neutron Yields from Light Elements under Proton and Alpha Bombardment. Phys. Rev. 1959, 114, 571. [Google Scholar] [CrossRef]
- Kadam, S.T.; Vishwakarma, G.; Kashyap, Y.; Shukla, M.; Roy, T.; Sahu, P.K.; Sharma, D.; Shitre, A.S.; Kumar, V.; Das, B.K. Thermal neutron as a potential mutagen for induced plant mutation breeding: Radiosensitivity response on wheat and rice. Genet. Resour. Crop Evol. 2022, 70, 789–798. [Google Scholar] [CrossRef]
- Ma, L.; Kazama, Y.; Hirano, T.; Morita, R.; Tanaka, S.; Abe, T.; Hatakeyama, S. LET dependence on killing effect and mutagenicity in the model filamentous fungus Neurospora crassa. Int. J. Radiat. Biol. 2018, 94, 1125–1133. [Google Scholar] [CrossRef]
- Kazama, Y.; Hirano, T.; Saito, H.; Liu, Y.; Ohbu, S.; Hayashi, Y.; Abe, T. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 161. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Gao, Y.; Cao, G.; Yang, Y.; Lu, D.; Li, W. A genome-wide view of mutations in respiration-deficient mutants of Saccharomyces cerevisiae selected following carbon ion beam irradiation. Appl. Microbiol. Biotechnol. 2019, 103, 1851–1864. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimizu, A.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Morishita, T. Effects of ion beam irradiation on mutation induction and nuclear DNA content in Chrysanthemum. Breed. Sci. 2010, 60, 398–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).