Microscopic Depictions of Vanishing Shampoo Foam Examined by Time-of-Flight Small-Angle Neutron Scattering
Abstract
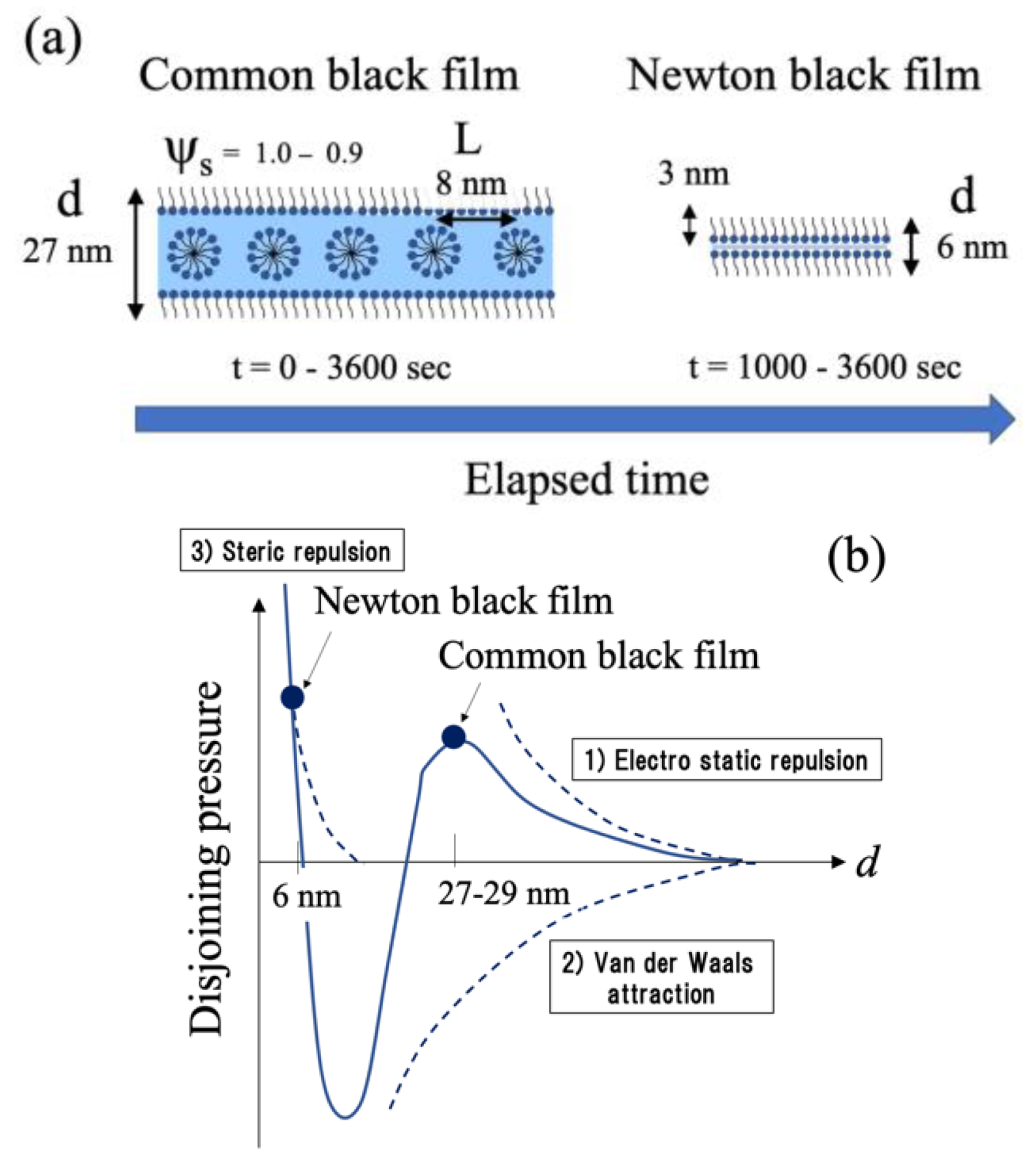
1. Introduction
2. Experimental Section
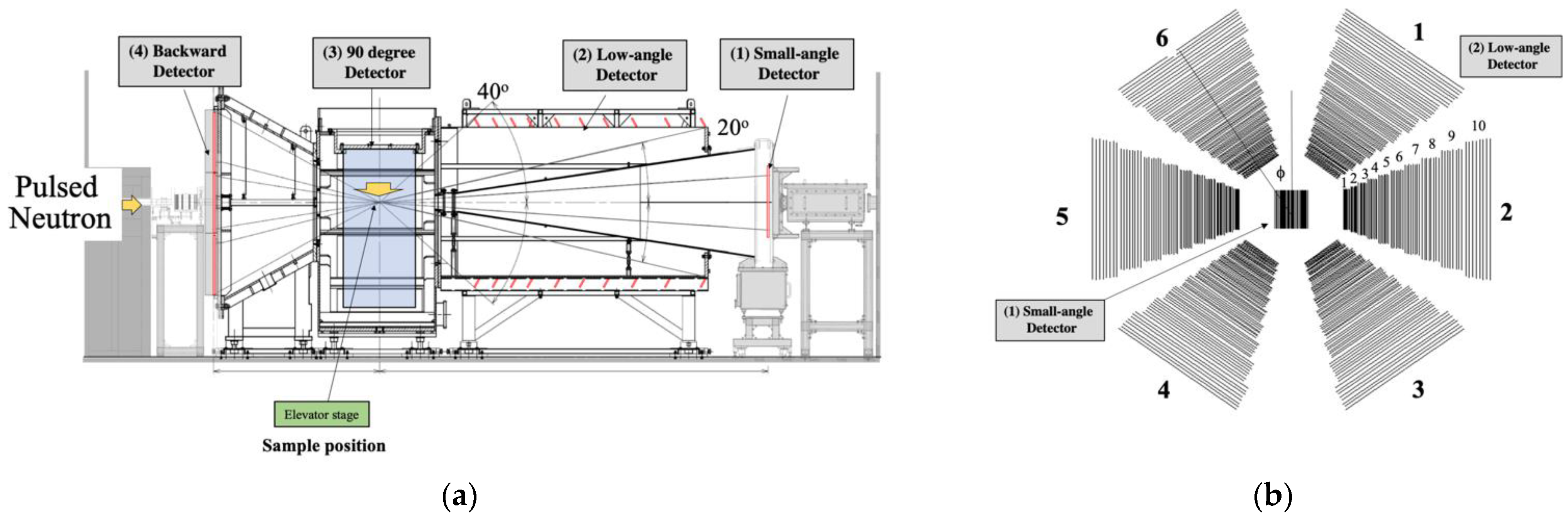
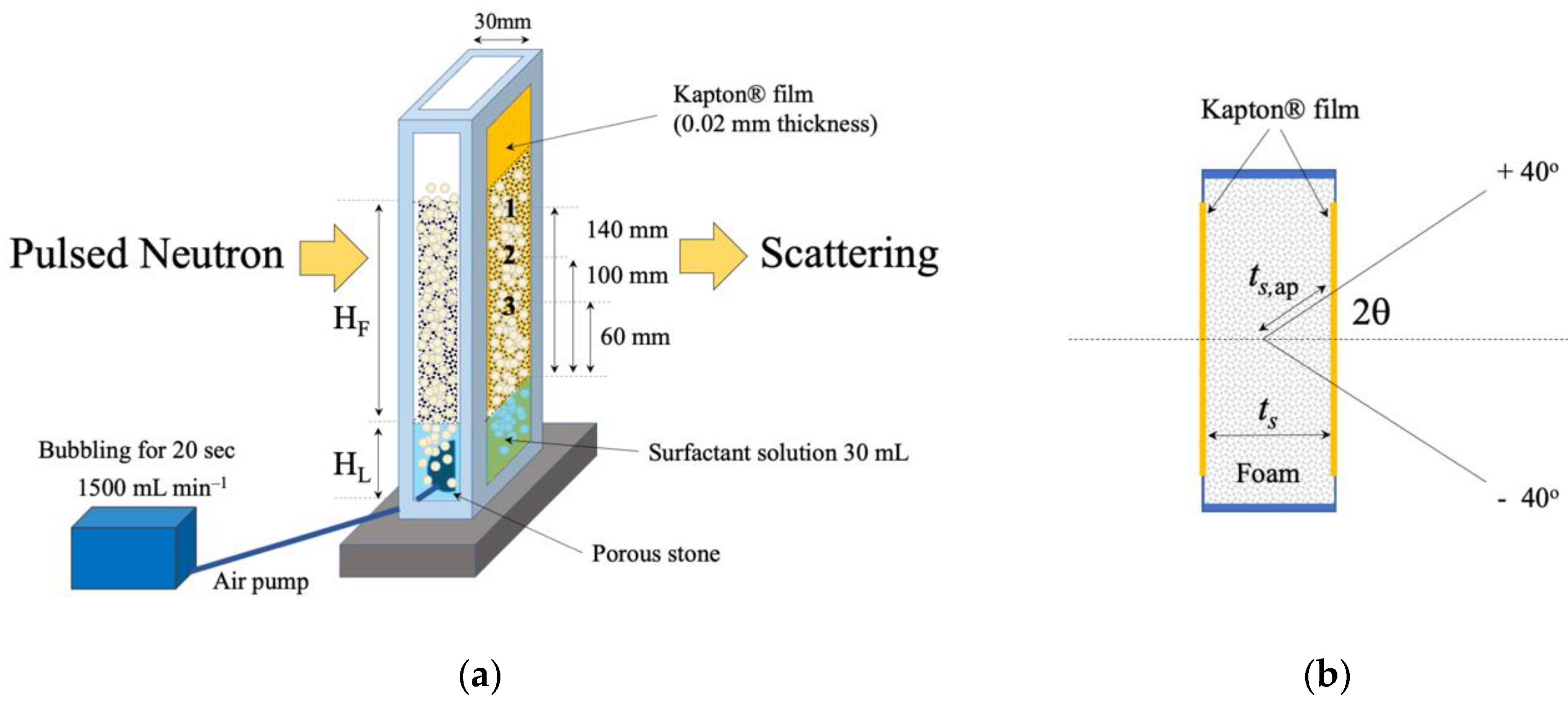
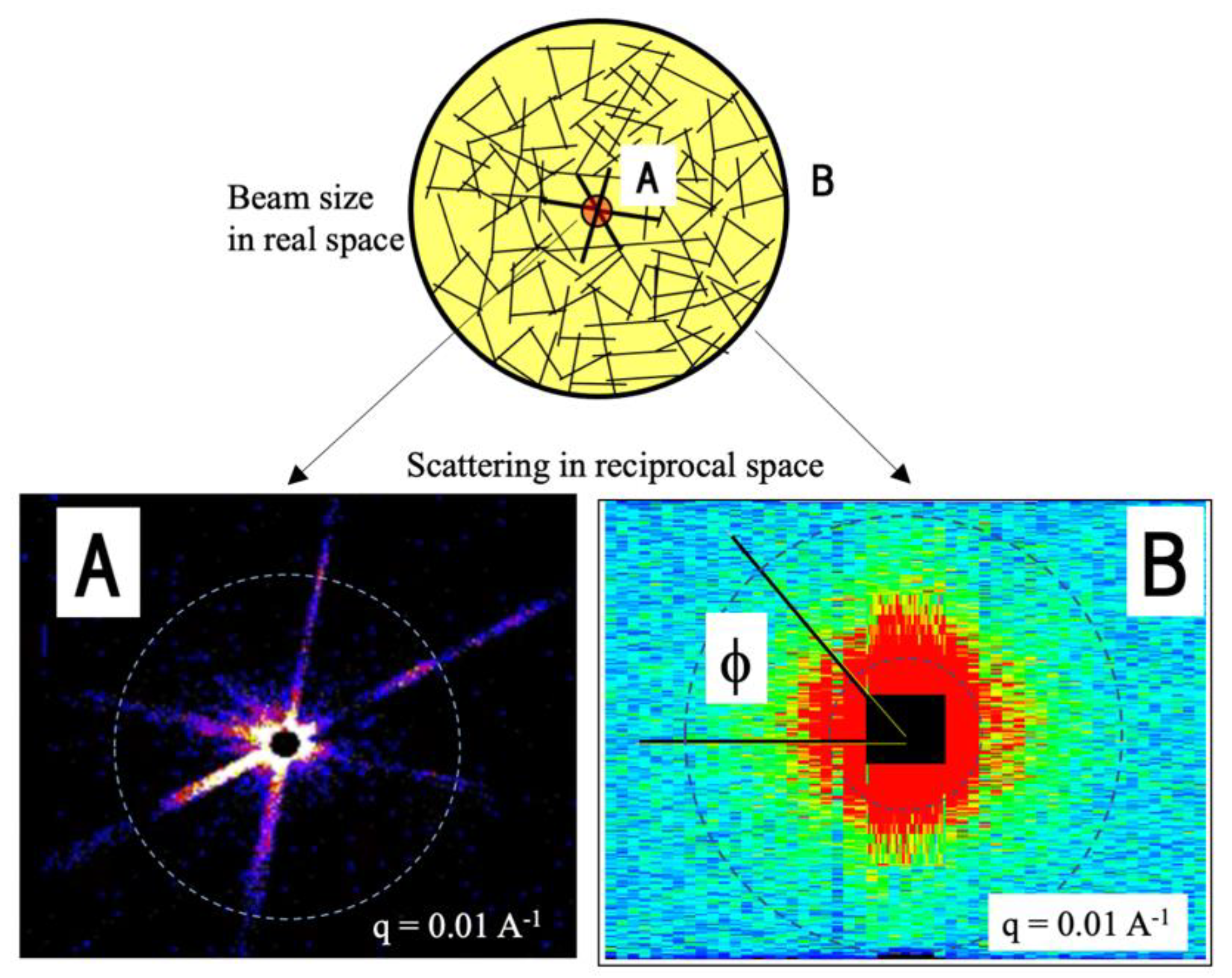
2.1. Time-of-Flight Small-Angle Neutron Scattering Instrument
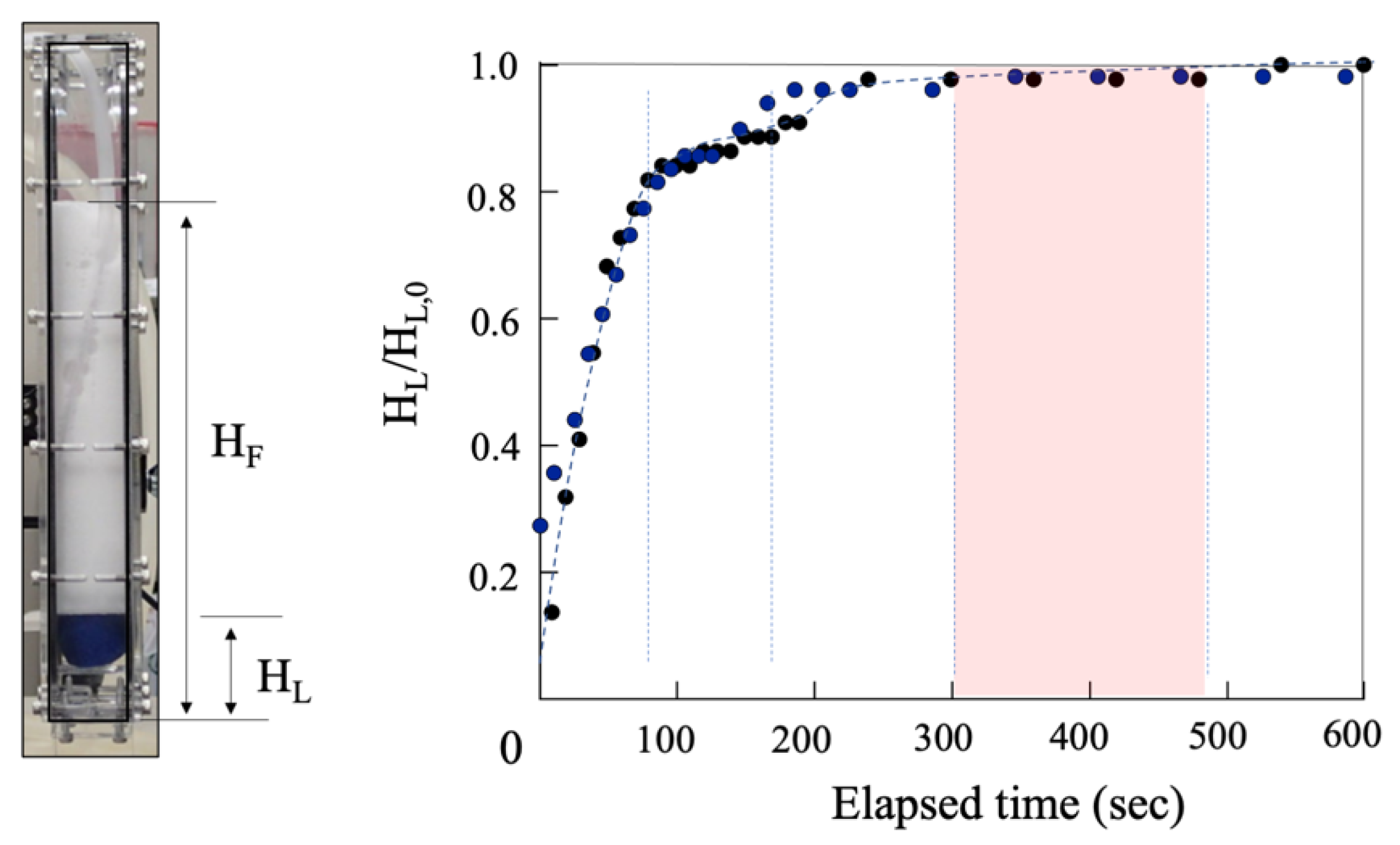
2.2. Height Analysis and Small-Angle X-ray Scattering
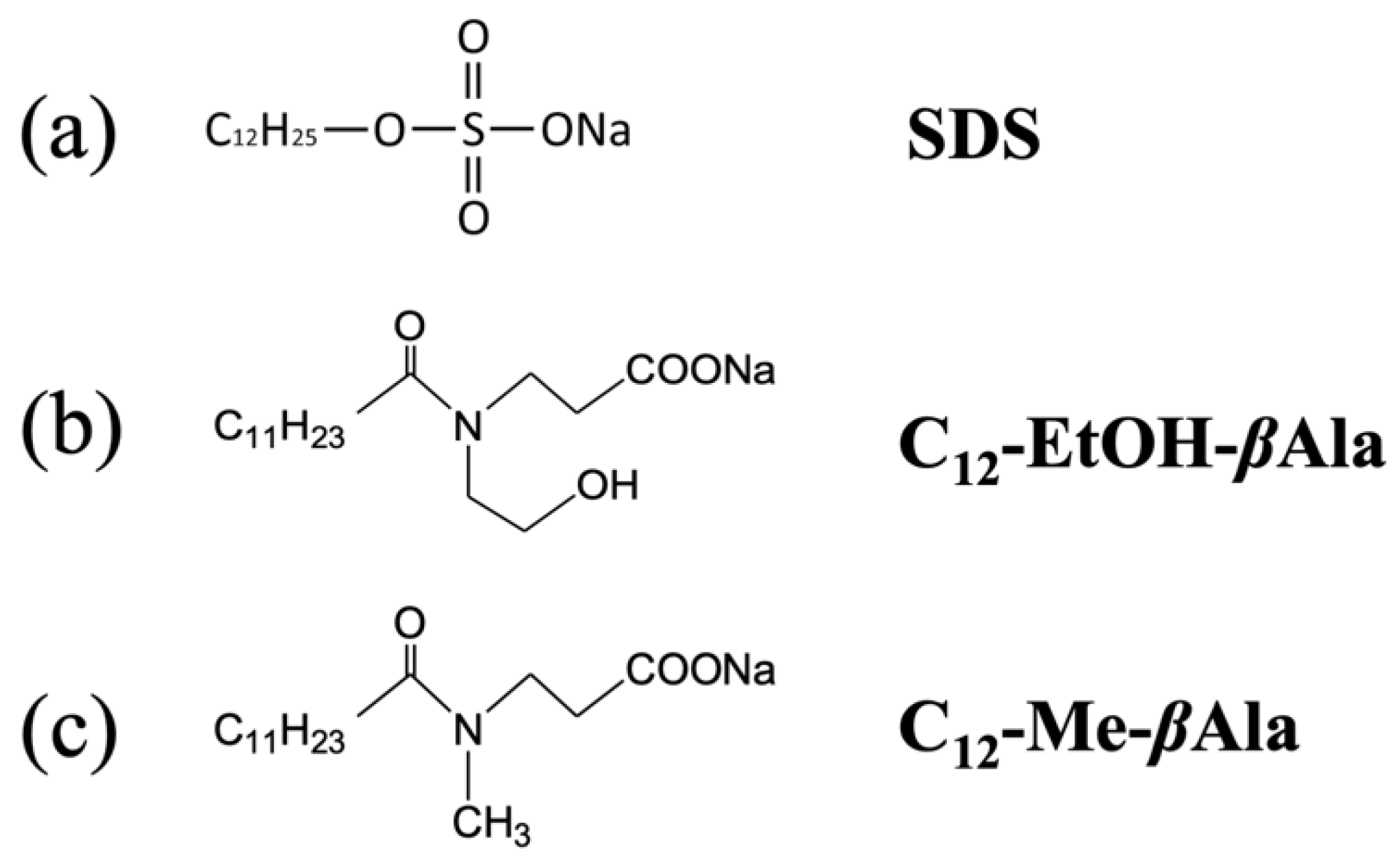
2.3. Materials
3. Time-of-Flight SANS and Scattering Laws
3.1. Data Reduction to Scattering Profile
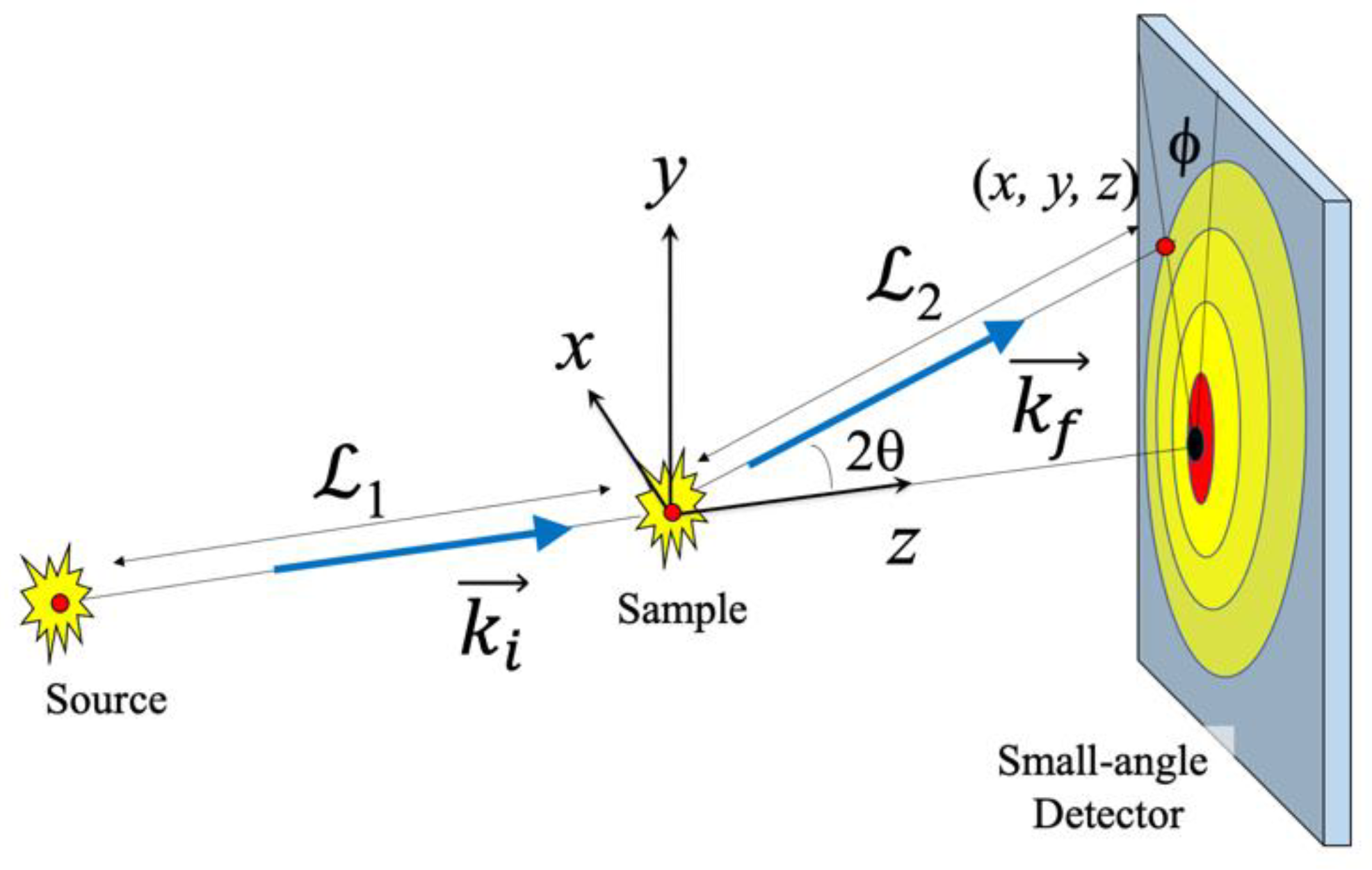
3.2. Scattering Functions for Film Structure
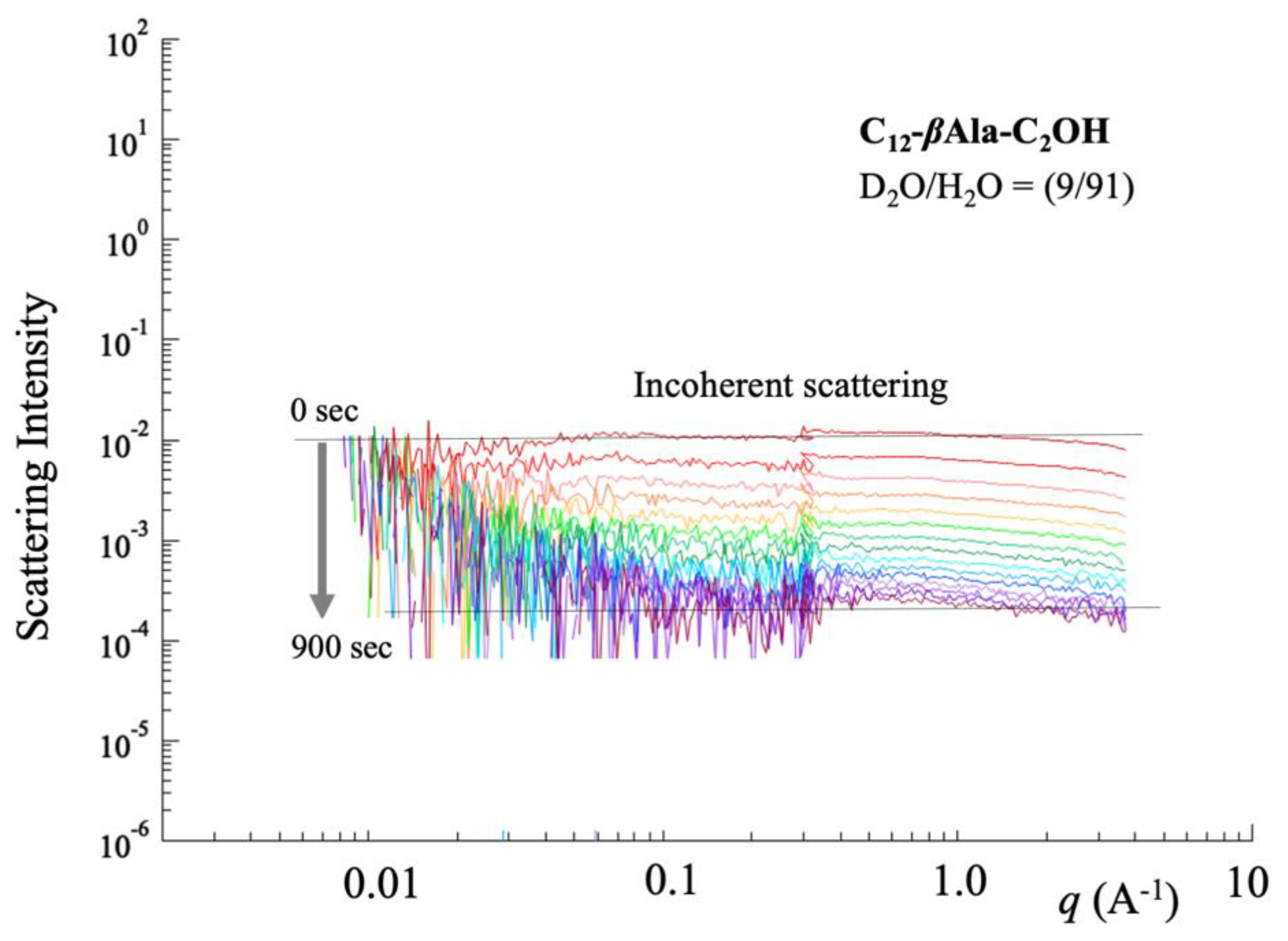
3.3. Deuterium Substitution
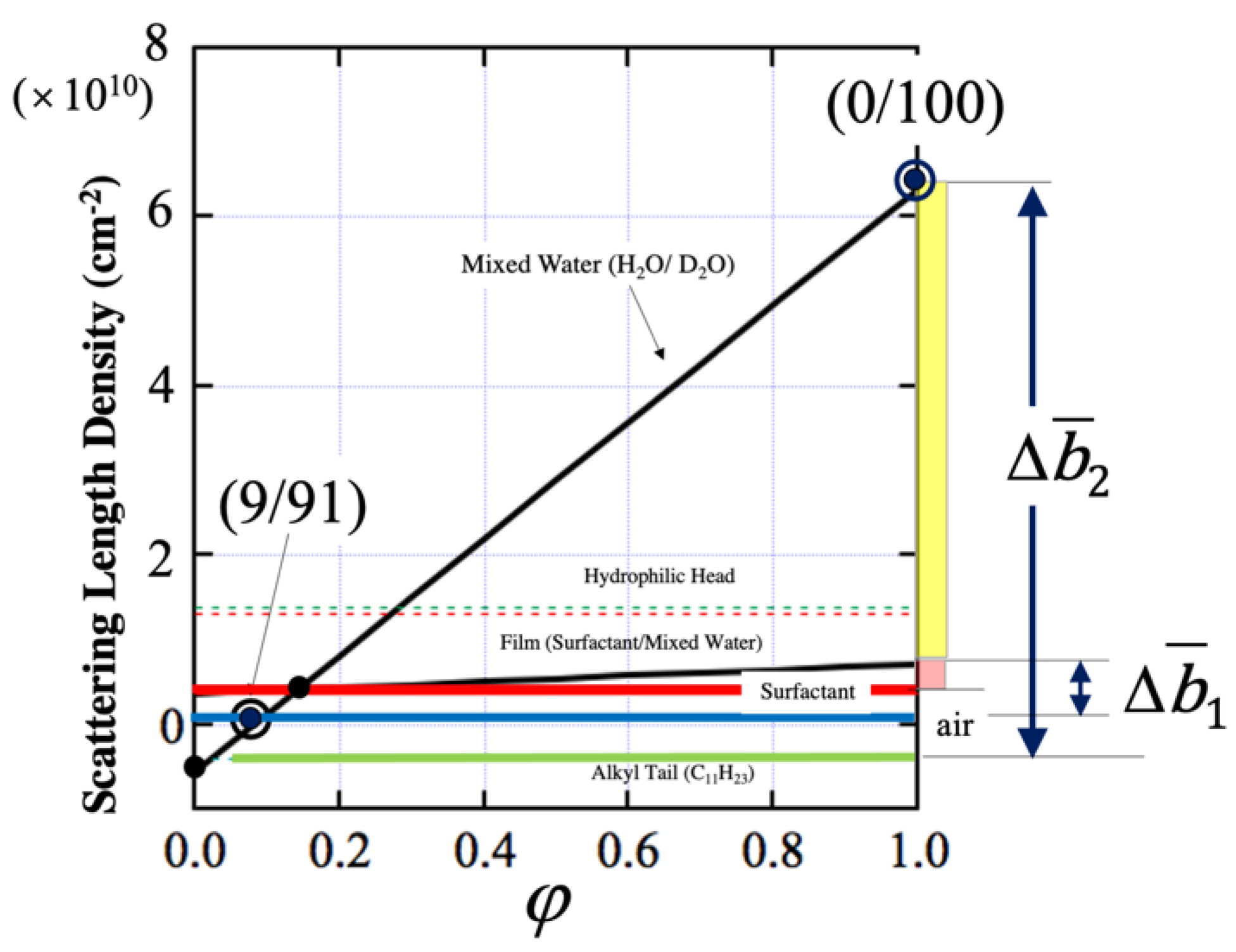
4. Experimental Results
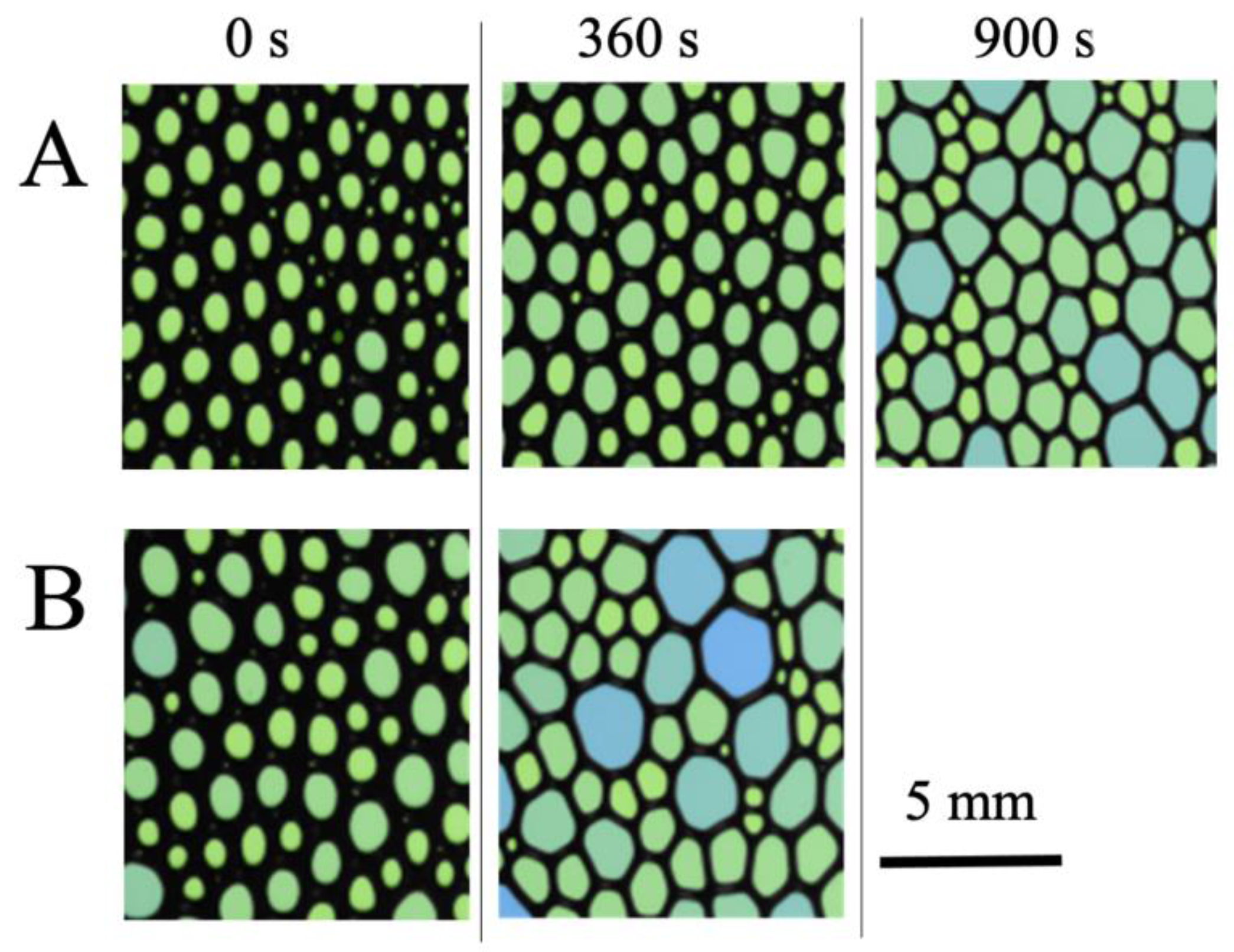
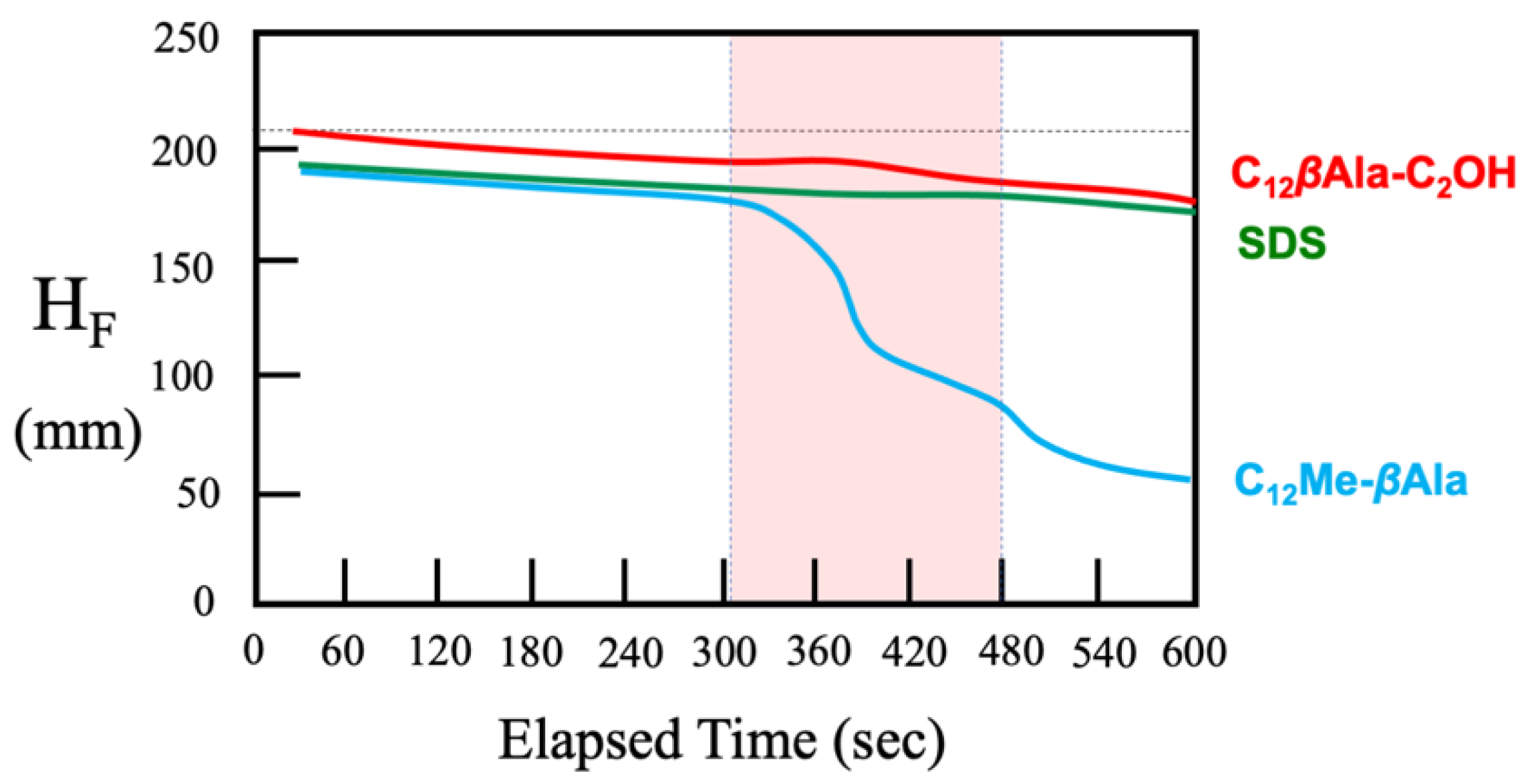
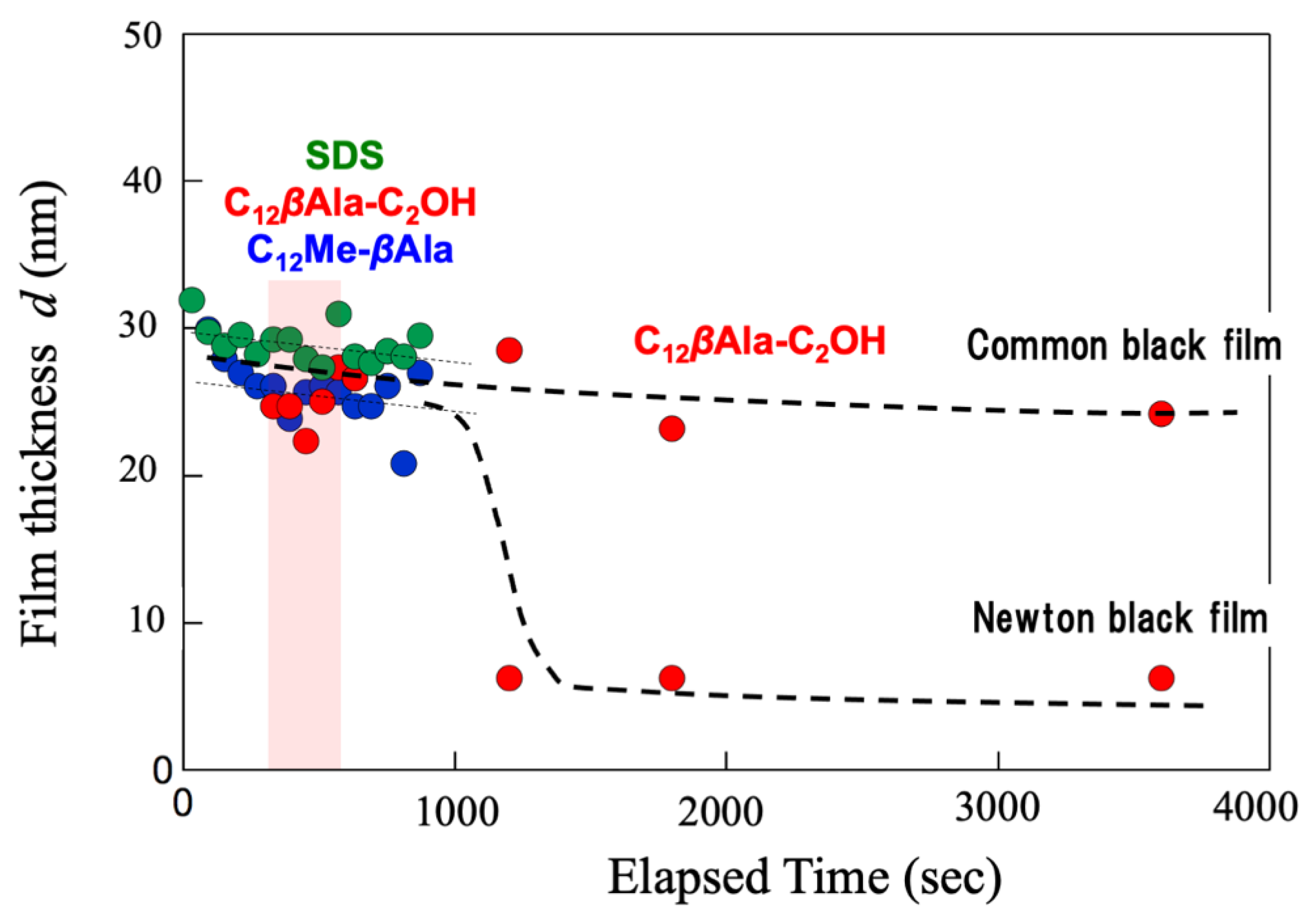
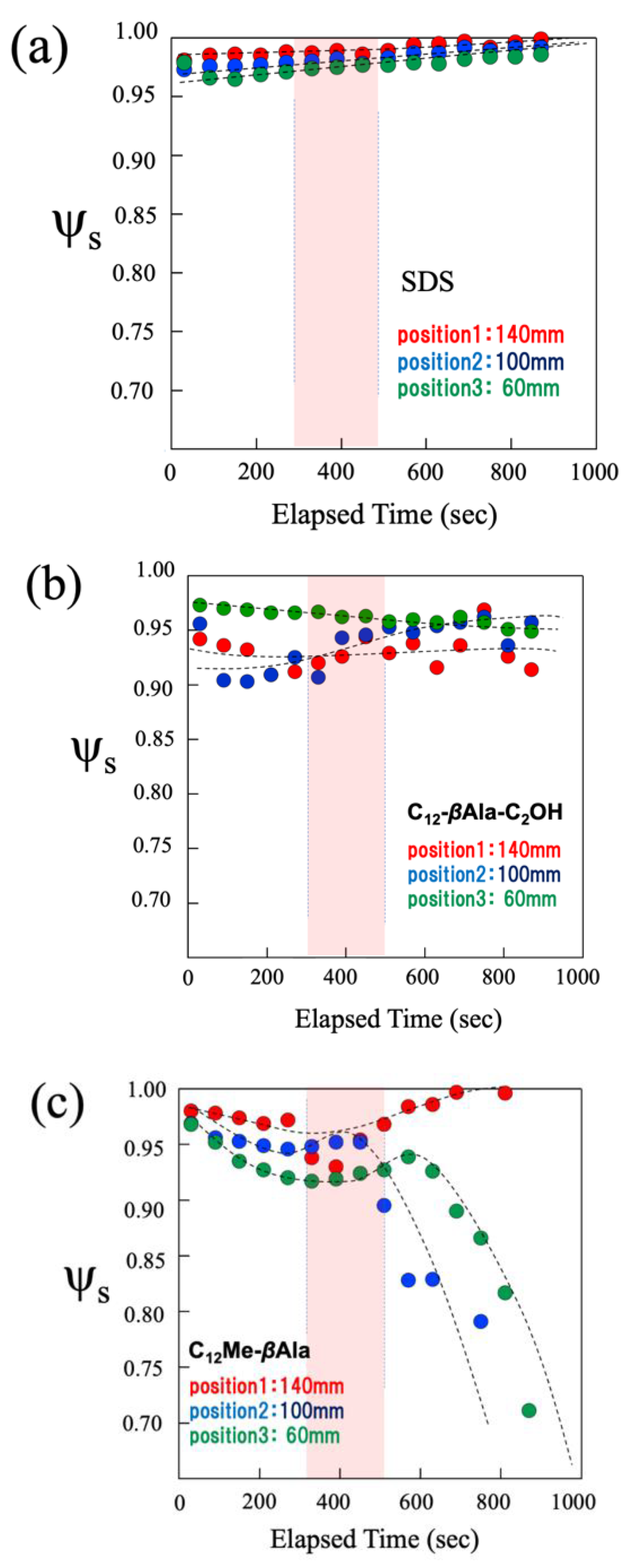
4.1. Macroscopic Behavior (Foam Stability)
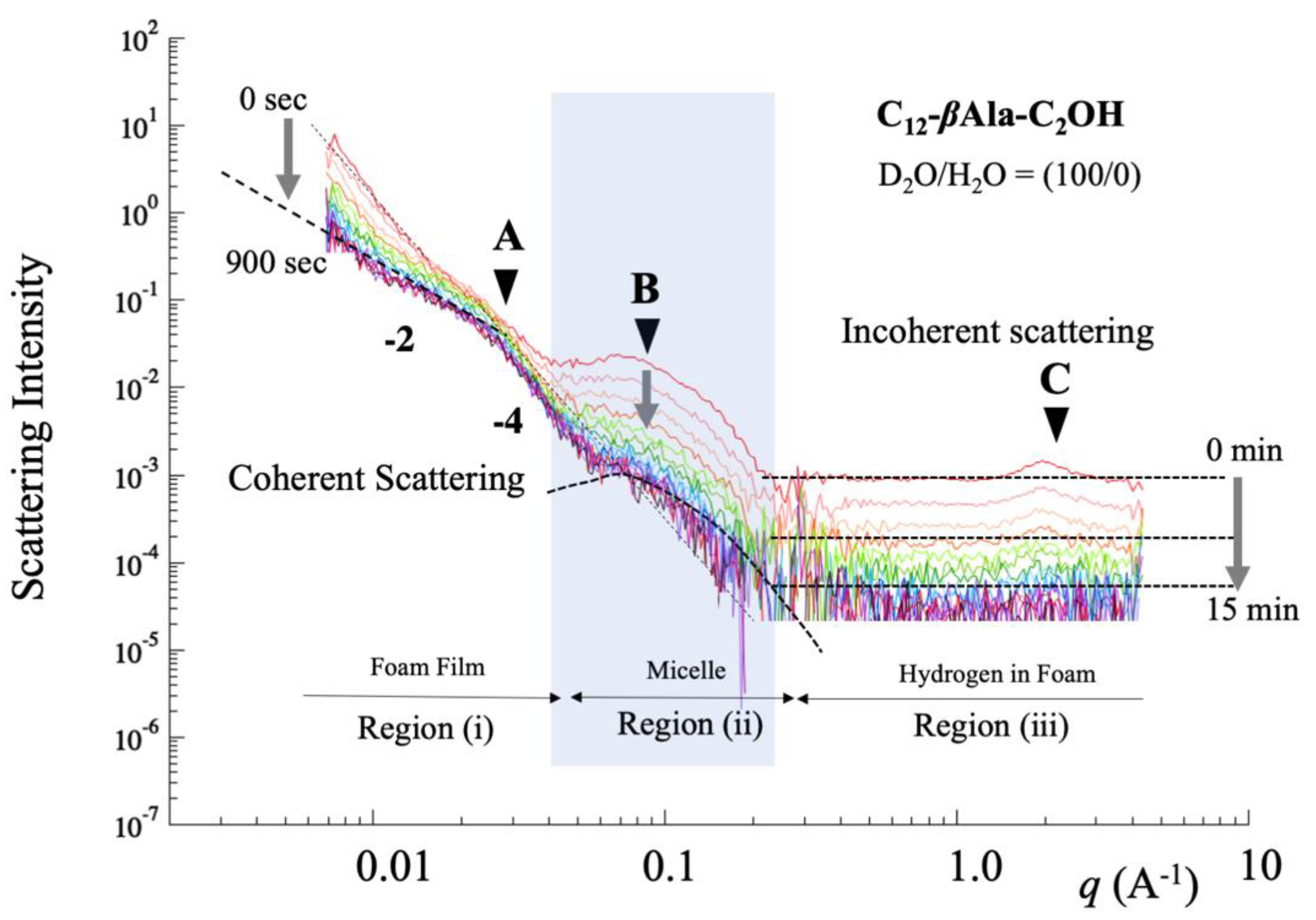
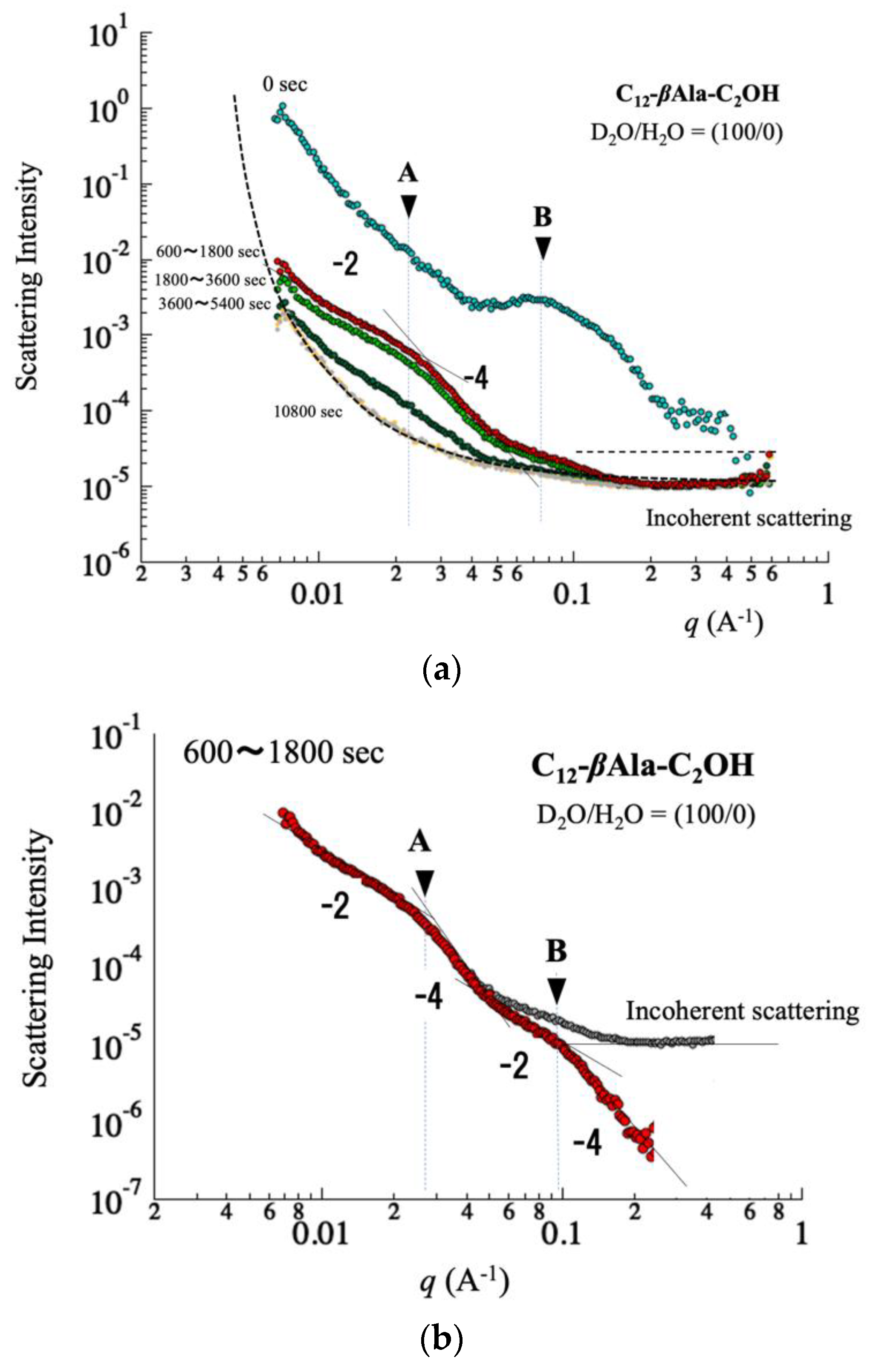
4.2. Microscopic Behavior Determined by Small-Angle Neutron Scattering
5. Discussion
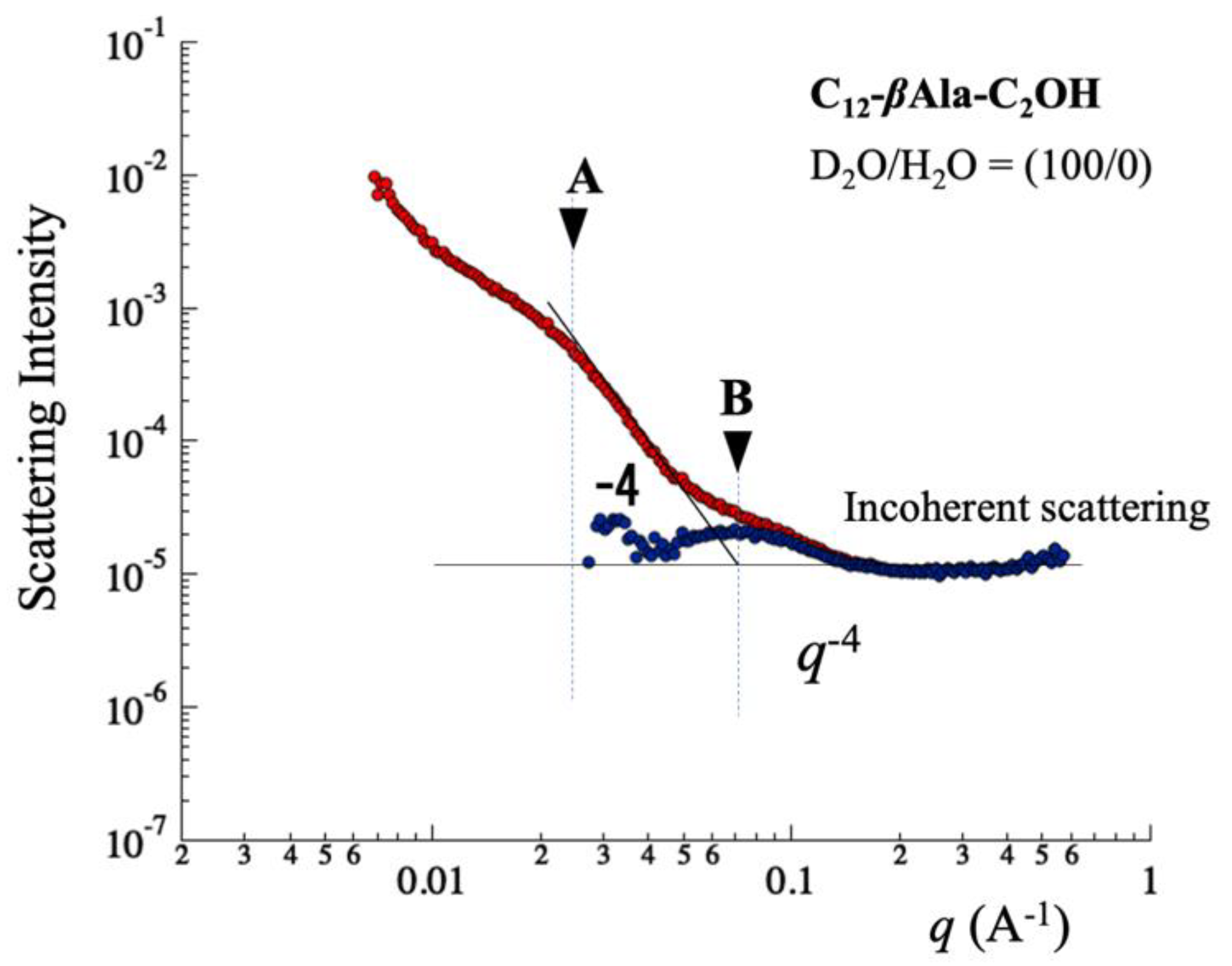
5.1. Foam Film in q-Region (i)
5.2. Micelle in q-Region (ii)
5.3. Water Content in q-Region (iii)
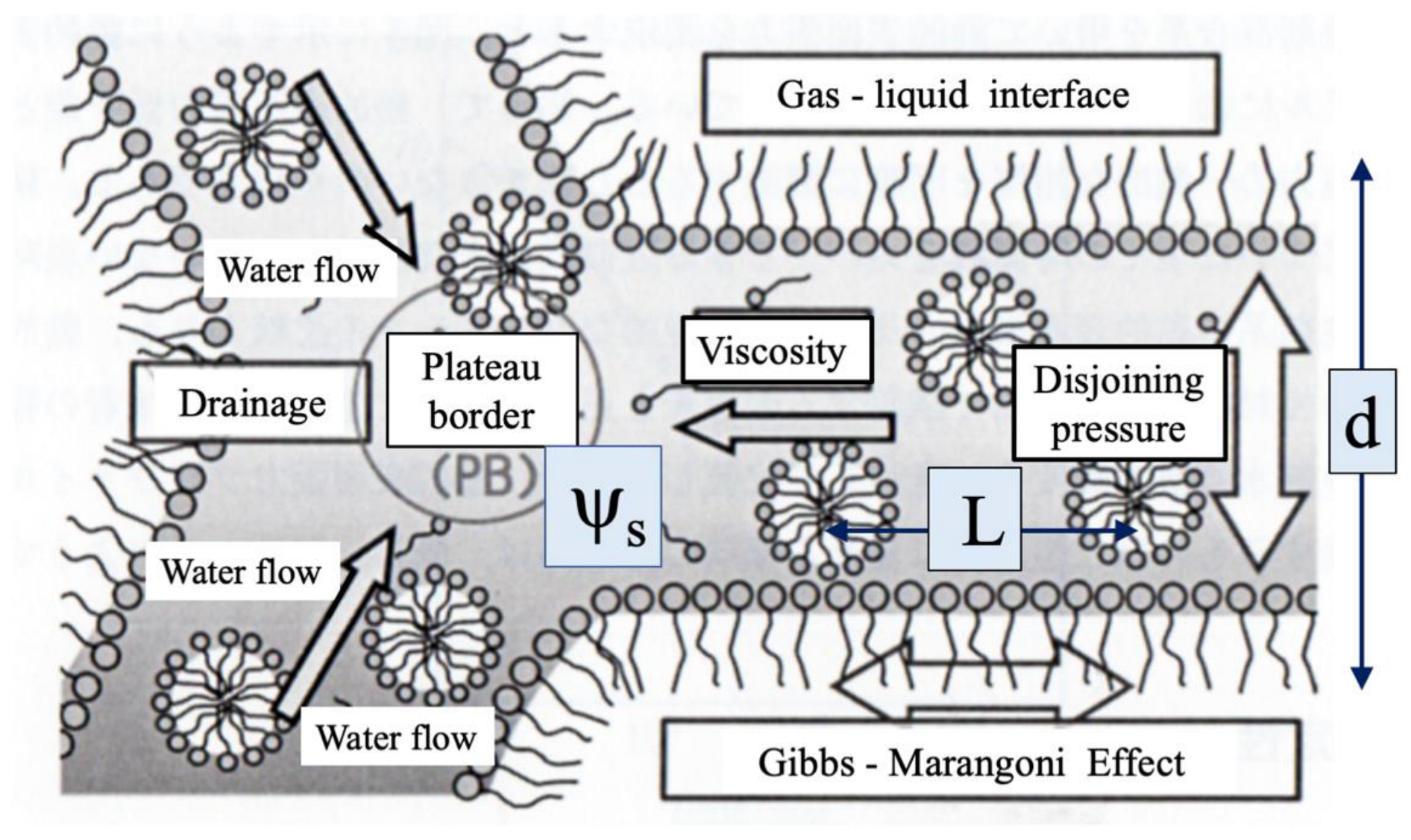
5.4. Microscopic Depictions of Shampoo Foam
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantat, I.; Cohen-Addad, S.; Elias, F.; Graner, F.; Höhler, R.; Pitois, O.; Rouyer, F.; Saint-Jalmes, A. Foams: Structure and Dynamics; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Weaire, D.; Hutzler, S. The Physics of Foams; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Leike, A. Demonstration of the exponential decay law using beer froth. Eur. J. Phys. 2002, 23, 21. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Beaumont, F.; Jeandet, P.; Polidori, G. Flow patterns of bubble nucleation sites (called fliers) freely floating in champagne glasses. Langmuir 2007, 23, 10976–10983. [Google Scholar] [CrossRef] [PubMed]
- Liger-Belair, G.; Voisin, C.; Jeandet, P. Modeling nonclassical heterogeneous bubble nucleation from cellulose fibers: Application to bubbling in carbonated beverages. J. Phys. Chem. B 2005, 109, 14573–14580. [Google Scholar] [CrossRef] [PubMed]
- Liger-Belair, G.; Jeandet, P. Capillary-driven flower-shaped structures around bubbles collapsing in a bubble raft at the surface of a liquid of low viscosity. Langmuir 2003, 19, 5771–5779. [Google Scholar] [CrossRef]
- Ghabache, E.; Liger-Belair, G.; Antkowiak, A.; Séon, T. Evaporation of droplets in a Champagne wine aerosol. Sci. Rep. 2016, 6, 25148. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M. Influence of surfactant and electrolyte concentrations on surfactant Adsorption and foaming characteristics. J. Pet. Sci. Eng. 2017, 149, 612–622. [Google Scholar] [CrossRef]
- Kartono, F.; Maibach, H.I. Irritants in combination with a synergistic or additive effect on the skin response: An overview of tandem irritation studies. Contact Dermat. 2006, 54, 303–312. [Google Scholar] [CrossRef]
- Löffler, H.; Happle, R. Profile of irritant patch testing with detergents: Sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermat. 2003, 48, 26–32. [Google Scholar] [CrossRef]
- Lethcoe, K.; Fox, C.A.; Ryan, R.O. Foam fractionation of a recombinant biosurfactant apolipoprotein. J. Biotechnol. 2022, 343, 25–31. [Google Scholar] [CrossRef]
- Yada, S.; Shimosegawa, H.; Fujita, F.; Yamada, M.; Matsue, Y.; Yoshimura, T. Microstructural Characterization of Foam Formed by a Hydroxy Group-Containing Amino Acid Surfactant Using Small-Angle Neutron Scattering. Langmuir 2020, 36, 7808–7813. [Google Scholar] [CrossRef]
- Sakai, T. Formation Mechanism and Application of Foam; Chapter 1; CMC Publishing: Tokyo, Japan, 2017. [Google Scholar]
- Axelos, M.A.V.; Boué, F. Faoms as viewed by small-angle neutron scattering. Langmuir 2003, 19, 6598–6604. [Google Scholar] [CrossRef]
- Etrillard, J.; Axelos, M.A.V.; Cantat, I.; Artzner, F.; Renault, A.; Weiss, T.; Delannay, R.; Boué, F. In Situ Investigations on Organic Foam Films Using Neutron and Synchrotron Radiation. Langmuir 2005, 21, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Roper, M.H.; Novales, B.; Boue, F.; Axelos, M.A.V. Polysaccharide/Surfactant Complexes at the Air−Water Interface—Effect of the Charge Density on Interfacial and Foaming Behaviors. Langmuir 2008, 24, 12849–12857. [Google Scholar] [CrossRef]
- Schmidt, I.; Novales, B.; Boué, F.; Axelos, M.A.V. Foaming properties of protein/pectin electrostatic complexes and foam structure at nanoscale. J. Colloid Interface Sci. 2010, 345, 316–324. [Google Scholar] [CrossRef]
- Mikhailovskaya, A.; Zhang, L.; Cousin, F.; Boue, F.; Yazhgur, P.; Mullter, F.; Gay, C.; Salonen, A. Probing foam with neutrons. Adv. Colloid Interface Sci. 2017, 247, 444–453. [Google Scholar] [CrossRef]
- Micheau, C.; Bauduin, P.; Diat, O.; Faure, S. Specific salt and pH effects on foam film of a pH sensitive surfactant. Langmuir 2013, 29, 8472–8481. [Google Scholar] [CrossRef] [PubMed]
- Hurcom, J.; Paul, A.; Heenan, R.K.; Davies, A.; Woodman, N.; Schweins, R.; Griffiths, P.C. The interfacial sutrcture of polymeric surfactant stabilized air-in-water foams. Soft Matter 2014, 10, 3003–3008. [Google Scholar] [CrossRef]
- Zhang, L.; Mikhailovskaya, A.; Yazhgur, P.; Muller, F.; Cousin, F.; Langevin, D.; Wang, N.; Salonen, A. Precipitating sodium dodecyl sulfate to create ultrastable and semistable foams. Angew. Chem. Int. Ed. Engl. 2015, 54, 9533–9536. [Google Scholar] [CrossRef] [PubMed]
- Mansour, O.T.; Cattoz, B.; Beaube, M.; Montagnon, M.; Heenan, R.K.; Schweins, R.; Appavou, M.-S.; Griffiths, P.C. Assembly of small molecules surfactants at highly dynamic air-water interfaces. Soft Matter 2017, 13, 8807–8815. [Google Scholar] [CrossRef]
- Imae, T.; Kanaya, T.; Furusawa, M.; Torikai, N. Neutrons in Soft Matter; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Koizumi, S.; Noda, Y.; Maeda, T.; Inada, T.; Ueda, S.; Fujisawa, T.; Izunome, H.; Robinson, R.A.; Frielingshaus, H. Advanced Small-angle scattering Instrument Available in Tokyo area II. Time-of-flight Small-Angle Neutron Scattering Developed on iMATERIA Spectrometer at High Intensity Pulsed Neutron Source J-PARC. Quantum Beam Sci. 2020, 4, 42. [Google Scholar] [CrossRef]
- Roe, R.J. Methods of X-ray and Neutron Scattering in Polymer Science; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Debye, P. Zerstreuung von Röntgenstrahlen. Ann. Phys. 1915, 351, 809–823. [Google Scholar]
- Tanford, C. The Hydrophobic Effect, 2nd ed.; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Onizawa, M.; Koizumi, S.; Noda, Y.; Inada, T. Time-resolved Small-angle Scattering Investigating A Single Shampoo Film. Soft Matter, 2022; manuscript in preparation. [Google Scholar]
- Nikolov, A.D.; Kralchevsky, P.A.; Ivanov, I.B.; Wasan, D.T. Ordered Micelle Structuring in Thin Films Formed from Anionic Surfactant Solutions: Part II—Model Development. J. Colloid Interface Sci. 1989, 133, 13–22. [Google Scholar] [CrossRef]
- Nikolov, A.D.; Wasan, D.T. Ordered Micelle Structuring in Thin Films Formed from Anionic Surfactant Solutions: Part I—Experimental. J. Colloid Interface Sci. 1989, 133, 1–12. [Google Scholar] [CrossRef]
- Danov, K.D.; Basheva, E.S.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P.; Lips, A. The metastable states of foam films containing electrically charged micelles or particles: Experiment and quantitative interpretation. Adv. Colloid Interface Sci. 2011, 168, 50–70. [Google Scholar] [CrossRef]
- Tzocheva, S.S.; Kralchevsky, P.A.; Danov, K.D.; Georgieva, G.S.; Post, A.J.; Ananthapadmanabhan, K.P. Solubility limits and phase diagrams for fatty acids in anionic (SLES) and zwitterionic (CAPB) micellar surfactant solution. J. Colloid Interface Sci. 2012, 369, 274–286. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koizumi, S.; Noda, Y.; Inada, T.; Maeda, T.; Yada, S.; Yoshimura, T.; Shimosegawa, H.; Fujita, H.; Yamada, M.; Matsue, Y. Microscopic Depictions of Vanishing Shampoo Foam Examined by Time-of-Flight Small-Angle Neutron Scattering. Quantum Beam Sci. 2023, 7, 4. https://doi.org/10.3390/qubs7010004
Koizumi S, Noda Y, Inada T, Maeda T, Yada S, Yoshimura T, Shimosegawa H, Fujita H, Yamada M, Matsue Y. Microscopic Depictions of Vanishing Shampoo Foam Examined by Time-of-Flight Small-Angle Neutron Scattering. Quantum Beam Science. 2023; 7(1):4. https://doi.org/10.3390/qubs7010004
Chicago/Turabian StyleKoizumi, Satoshi, Yohei Noda, Takumi Inada, Tomoki Maeda, Shiho Yada, Tomokazu Yoshimura, Hiroshi Shimosegawa, Hiroya Fujita, Munehiro Yamada, and Yukako Matsue. 2023. "Microscopic Depictions of Vanishing Shampoo Foam Examined by Time-of-Flight Small-Angle Neutron Scattering" Quantum Beam Science 7, no. 1: 4. https://doi.org/10.3390/qubs7010004
APA StyleKoizumi, S., Noda, Y., Inada, T., Maeda, T., Yada, S., Yoshimura, T., Shimosegawa, H., Fujita, H., Yamada, M., & Matsue, Y. (2023). Microscopic Depictions of Vanishing Shampoo Foam Examined by Time-of-Flight Small-Angle Neutron Scattering. Quantum Beam Science, 7(1), 4. https://doi.org/10.3390/qubs7010004






