Analysis of Cr(VI) Bioremediation by Citrobacter freundii Using Synchrotron Soft X-ray Scanning Transmission X-ray Microscopy
Abstract
:1. Introduction
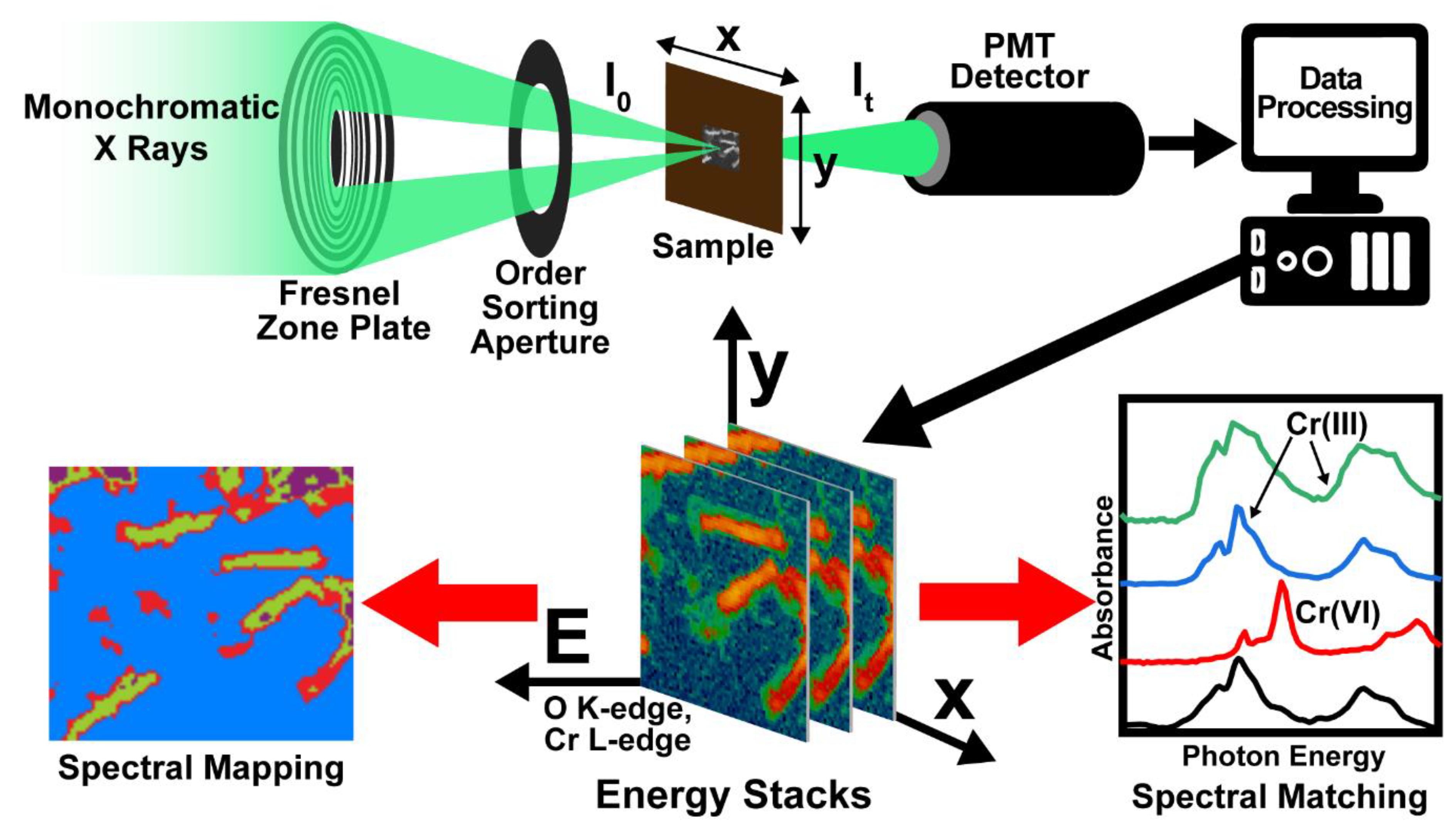
2. Material and Methods
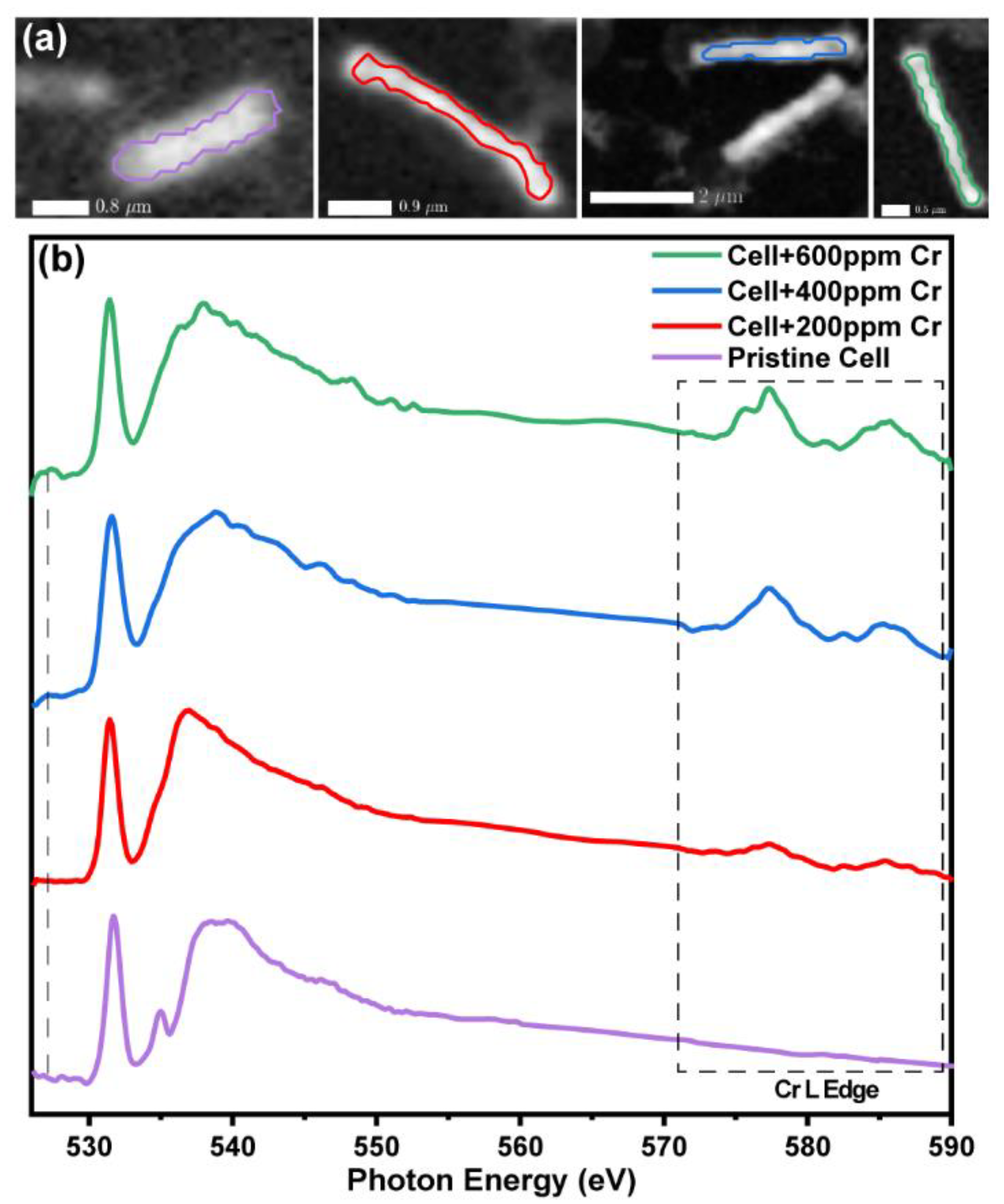
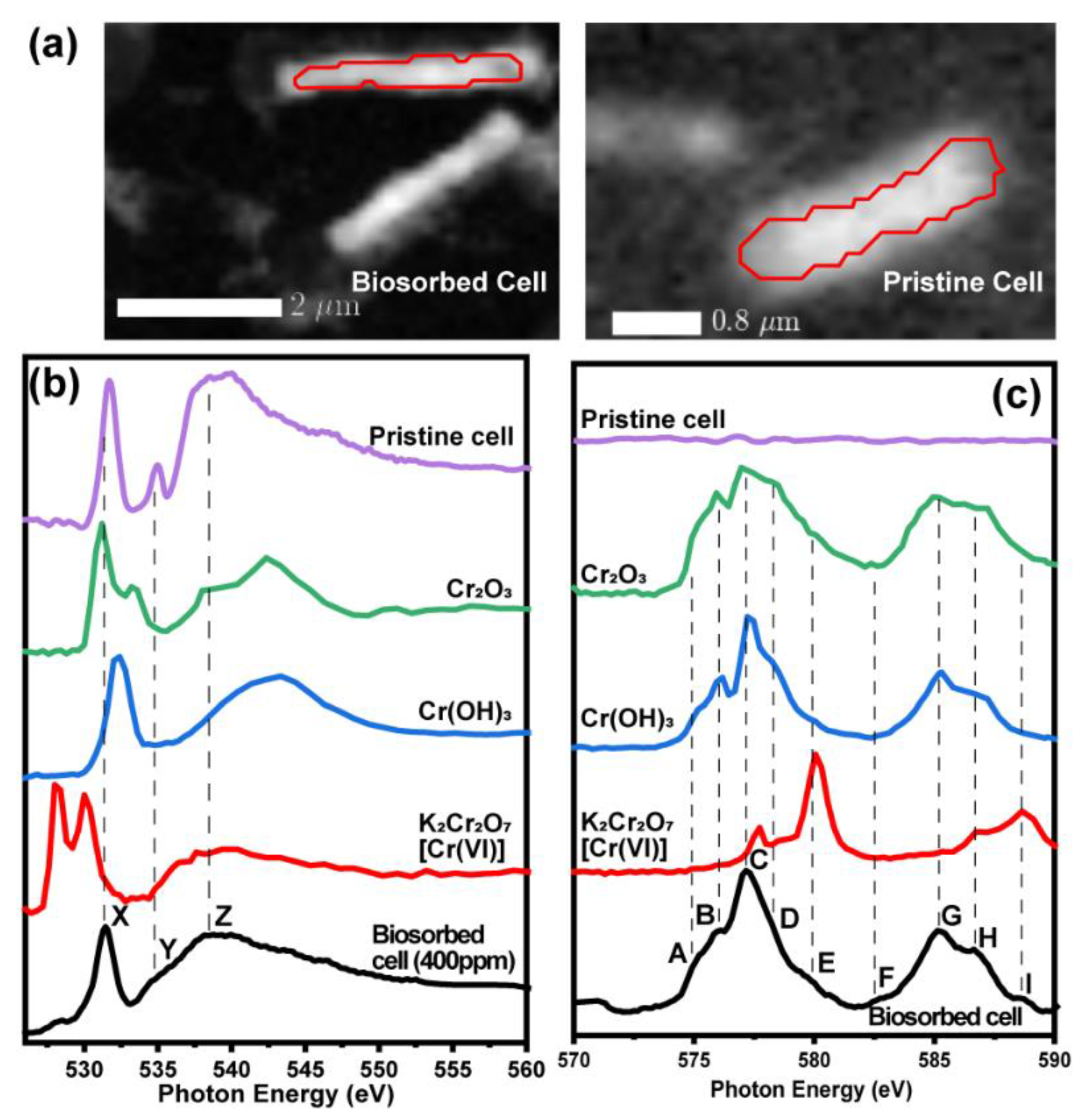
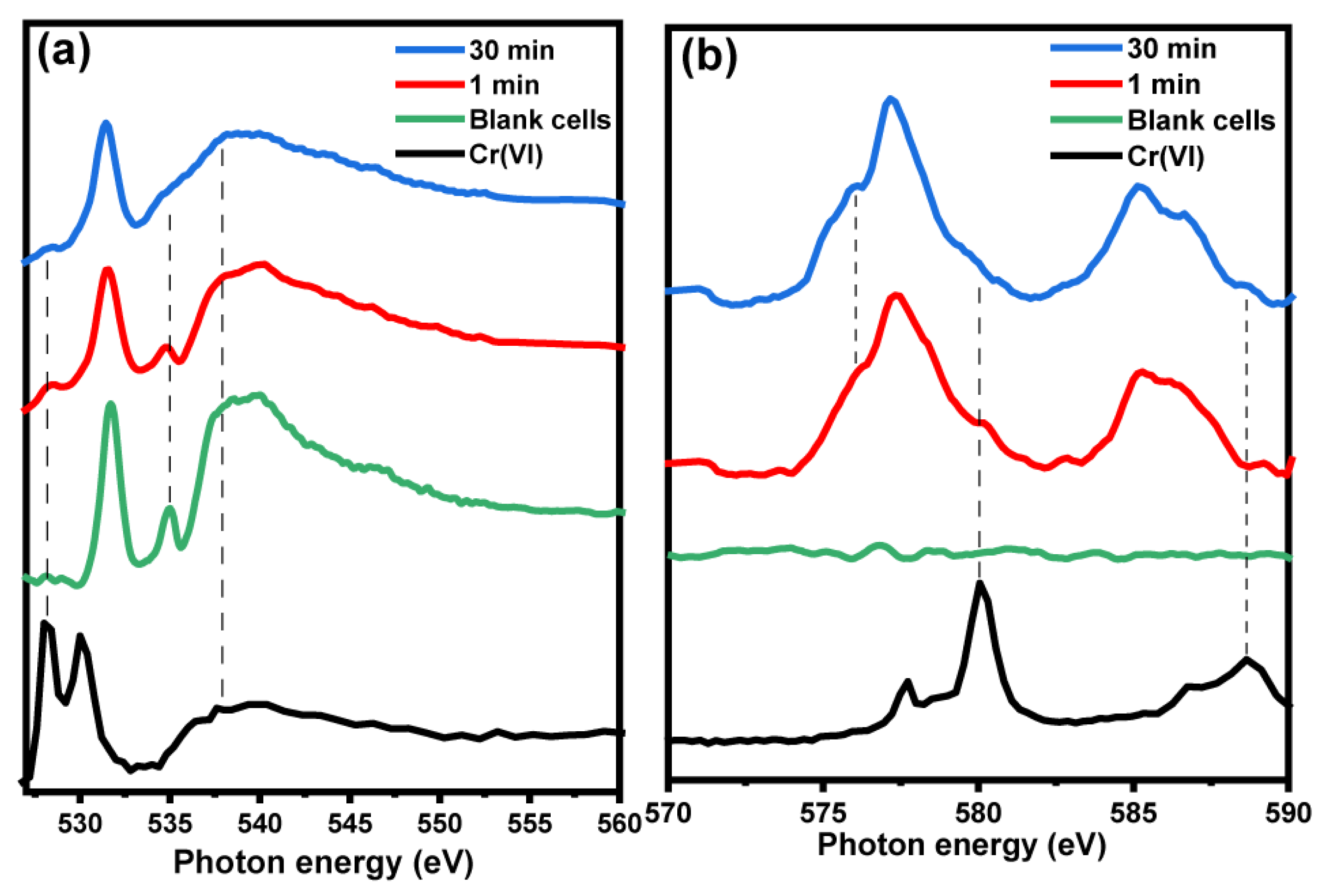
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium(VI) Toxicity in Legume Plants: Modulation Effects of Rhizobial Symbiosis. BioMed Res. Int. 2018, 2018, 8031213. [Google Scholar] [CrossRef]
- Rowbotham, A.L.; Levy, L.S.; Shuker, L.K. Chromium in the environment: An evaluation of exposure of the UK general population and possible adverse health effects. J. Toxicol. Environ. Health Part B 2000, 3, 145–178. [Google Scholar] [CrossRef]
- Pandey, N.; Shukla, S.K.; Singh, N.B. Water purification by polymer nanocomposites: An overview. Nanocomposites 2017, 3, 47–66. [Google Scholar] [CrossRef]
- Sharma, P.; Bihari, V.; Agarwal, S.K.; Verma, V.; Kesavachandran, C.N.; Pangtey, B.S.; Mathur, N.; Singh, K.P.; Srivastava, M.; Goel, S.K. Groundwater contaminated with hexavalent chromium [Cr (VI)]: A health survey and clinical examination of community inhabitants (Kanpur, India). PLoS ONE 2012, 7, e47877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamyrbaev, A.A.; Dzharkenov, T.A.; Imangazina, Z.A.; Satybaldieva, U.A. Mutagenic and carcinogenic actions of chromium and its compounds. Environ. Health Prev. Med. 2015, 20, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Sarkar, A.; Sen, S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Cheng, Y.; Yan, F.; Huang, F.; Chu, W.; Pan, D.; Chen, Z.; Zheng, J.; Yu, M.; Lin, Z.; Wu, Z. Bioremediation of Cr(VI) and immobilization as Cr(III) by Ochrobactrum Anthropi. Environ. Sci. Technol. 2010, 44, 6357–6363. [Google Scholar] [CrossRef]
- Tarekegn, M.M.; Salilih, F.Z.; Ishetu, A.I. Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric. 2020, 6, 1783174. [Google Scholar] [CrossRef]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Zeng, G.; Luo, Y.; Li, H.; Xu, H. Bioreduction and biosorption of Cr(VI) by a Novel Bacillus Sp. CRB-B1 strain. J. Hazard. Mater. 2020, 386, 121628. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [Green Version]
- Tekerlekopoulou, A.G.; Tsiflikiotou, M.; Akritidou, L.; Viennas, A.; Tsiamis, G.; Pavlou, S.; Bourtzis, K.; Vayenas, D.V. Modelling of biological Cr(VI) removal in draw-fill reactors using microorganisms in suspended and attached growth systems. Water Res. 2013, 47, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Braun, J.-J.; Subramanian, S. Comparative studies on the bioremediation of hexavalent and trivalent chromium using Citrobacter Freundii: Part I-effect of parameters controlling biosorption. Int. J. Environ. Res. 2014, 8, 1127–1134. [Google Scholar]
- Prabhakaran, D.C.; Riotte, J.; Subramanian, S. Bioremediation of hexavalent and trivalent chromium using Citrobacter Freundii: A mechanistic study. Nat. Resour. Eng. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Liss, K.D.; Chen, K. Frontiers of synchrotron research in materials Science. MRS Bull. 2016, 41, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.J.; Foster, G.F.; Burge, R.E.; Ali, S.Y.; Scotchford, C.A. Elemental Mapping of Biological Tissue by X-ray Absorption Difference Imaging in the STXM BT—X-ray Microscopy III; Michette, A.G., Morrison, G.R., Buckley, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 423–426. [Google Scholar]
- Burge, R.E.; Beswetherick, J.T.; Browne, M.T.; Charalambous, P.S.; Duke, P.J.; Foster, G.F.; Hare, A.R.; Michette, A.G.; Morris, D.; Morrison, G.R.; et al. Scanning X-ray Microscopy. In X-ray Instrumentation in Medicine and Biology, Plasma Physics, Astrophysics, and Synchrotron Radiation; Benattar, R., Ed.; SPIE: Bellingham, WA, USA, 1989; Volume 1140, pp. 528–529. [Google Scholar]
- Benzerara, K.; Menguy, N.; Guyot, F.; Skouri, F.; de Luca, G.; Barakat, M.; Heulin, T. Biologically controlled precipitation of calcium phosphate by Ramlibacter Tataouinensis. Earth Planet. Sci. Lett. 2004, 228, 439–449. [Google Scholar] [CrossRef]
- Obst, M.; Wang, J.; Hitchcock, A.P. Soft X-Ray spectro-tomography study of cyanobacterial biomineral nucleation. Geobiology 2009, 7, 577–591. [Google Scholar] [CrossRef]
- Obst, M.; Schmid, G. 3D Chemical mapping: Application of scanning transmission (Soft) X-ray microscopy (STXM) in combination with angle-scan tomography in bio-, geo-, and environmental sciences. Methods Mol. Biol. 2014, 1117, 757–781. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Dynes, J.J.; Lawrence, J.R.; Obst, M.; Swerhone, G.D.W.; Korber, D.R.; Leppard, G.G. Soft X-ray spectromicroscopy of nickel sorption in a natural river biofilm. Geobiology 2009, 7, 432–453. [Google Scholar] [CrossRef]
- Belkhou, R.; Stanescu, S.; Swaraj, S.; Besson, A.; Ledoux, M.; Hajlaoui, M.; Dalle, D. HERMES: A soft X-Ray beamline dedicated to X-Ray microscopy. J. Synchrotron Radiat. 2015, 22, 968–979. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, C.T.; Meigs, G.; Randall, K.; Sette, F. High-resolution K-shell photoabsorption measurements of simple molecules. Phys. Rev. A 1991, 44, 1848–1858. [Google Scholar] [CrossRef]
- Lerotic, M.; Mak, R.; Wirick, S.; Meirer, F.; Jacobsen, C. MANTiS: A Program for the analysis of X-Ray spectromicroscopy data. J. Synchrotron Radiat. 2014, 21, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Alrehaily, L.; Joseph, J.; Wren, J.C.; Wang, J.; Sham, T.K. Scanning Transmission X-Ray Microscopy studies of chromium hydroxide hollow spheres and nanoparticles formed by gamma radiation. Can. J. Chem. 2017, 95, 1146–1150. [Google Scholar] [CrossRef]
- Bae, S.; Hikaru, F.; Kanematsu, M.; Yoshizawa, C.; Noguchi, T.; Yu, Y.; Ha, J. Removal of hexavalent chromium in Portland cement using ground granulated blast-furnace slag powder. Materials 2017, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Varshney, M.; Nanda, S.S.; Shin, H.J.; Kim, N.; Yi, D.K.; Chae, K.-H.; Ok Won, S. Structural, electronic structure and antibacterial properties of graphene-oxide nano-sheets. Chem. Phys. Lett. 2018, 698, 85–92. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Sadigh, B.; Baumann, T.F.; Wang, Y.M.; Felter, T.E.; Van Buuren, T.; Gash, A.E.; Satcher, J.H.; Hamza, A.V. Electronic structure of chromia aerogels from soft X-ray absorption spectroscopy. J. Appl. Phys. 2007, 101, 124315. [Google Scholar] [CrossRef]
- de Groot, F.M.F.; Grioni, M.; Fuggle, J.C.; Ghijsen, J.; Sawatzky, G.A.; Petersen, H. Oxygen 1s X-Ray-absorption edges of transition-metal oxides. Phys. Rev. B 1989, 40, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anil, A.G.; Swaraj, S.; Subramanian, S.; Ramamurthy, P.C. Analysis of Cr(VI) Bioremediation by Citrobacter freundii Using Synchrotron Soft X-ray Scanning Transmission X-ray Microscopy. Quantum Beam Sci. 2021, 5, 28. https://doi.org/10.3390/qubs5040028
Anil AG, Swaraj S, Subramanian S, Ramamurthy PC. Analysis of Cr(VI) Bioremediation by Citrobacter freundii Using Synchrotron Soft X-ray Scanning Transmission X-ray Microscopy. Quantum Beam Science. 2021; 5(4):28. https://doi.org/10.3390/qubs5040028
Chicago/Turabian StyleAnil, Amith G., Sufal Swaraj, Sankaran Subramanian, and Praveen C. Ramamurthy. 2021. "Analysis of Cr(VI) Bioremediation by Citrobacter freundii Using Synchrotron Soft X-ray Scanning Transmission X-ray Microscopy" Quantum Beam Science 5, no. 4: 28. https://doi.org/10.3390/qubs5040028
APA StyleAnil, A. G., Swaraj, S., Subramanian, S., & Ramamurthy, P. C. (2021). Analysis of Cr(VI) Bioremediation by Citrobacter freundii Using Synchrotron Soft X-ray Scanning Transmission X-ray Microscopy. Quantum Beam Science, 5(4), 28. https://doi.org/10.3390/qubs5040028






