Hydration of Nucleobase as Probed by Electron Emission of Uridine-5′-Mono-Phosphate (UMP) in Aqueous Solution Induced by Nitrogen K-Shell Ionization
Abstract
1. Introduction
2. Experimental
3. Calculation
4. Results and Discussion
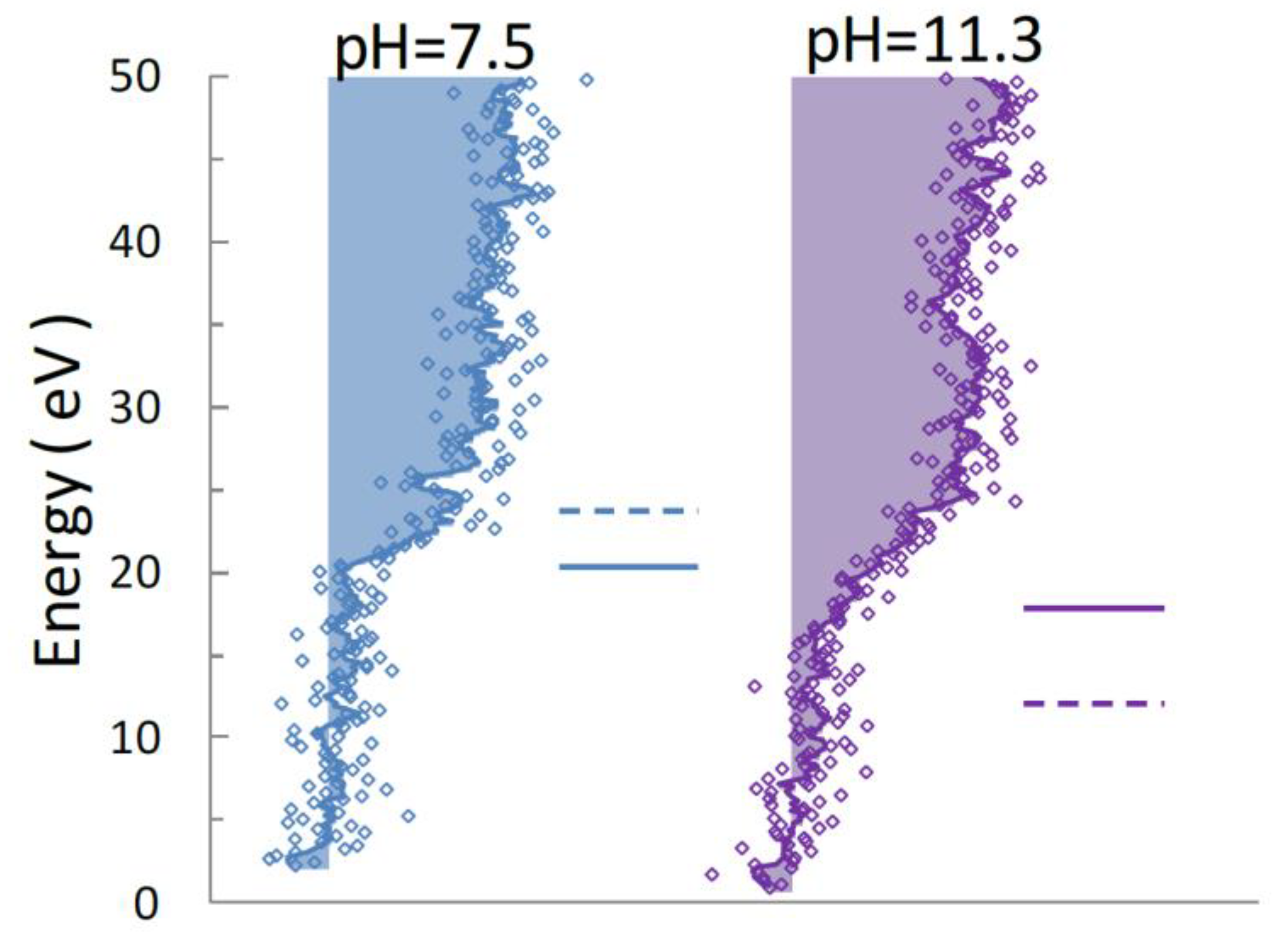
4.1. N 1s Photoelectron Spectra for UMP
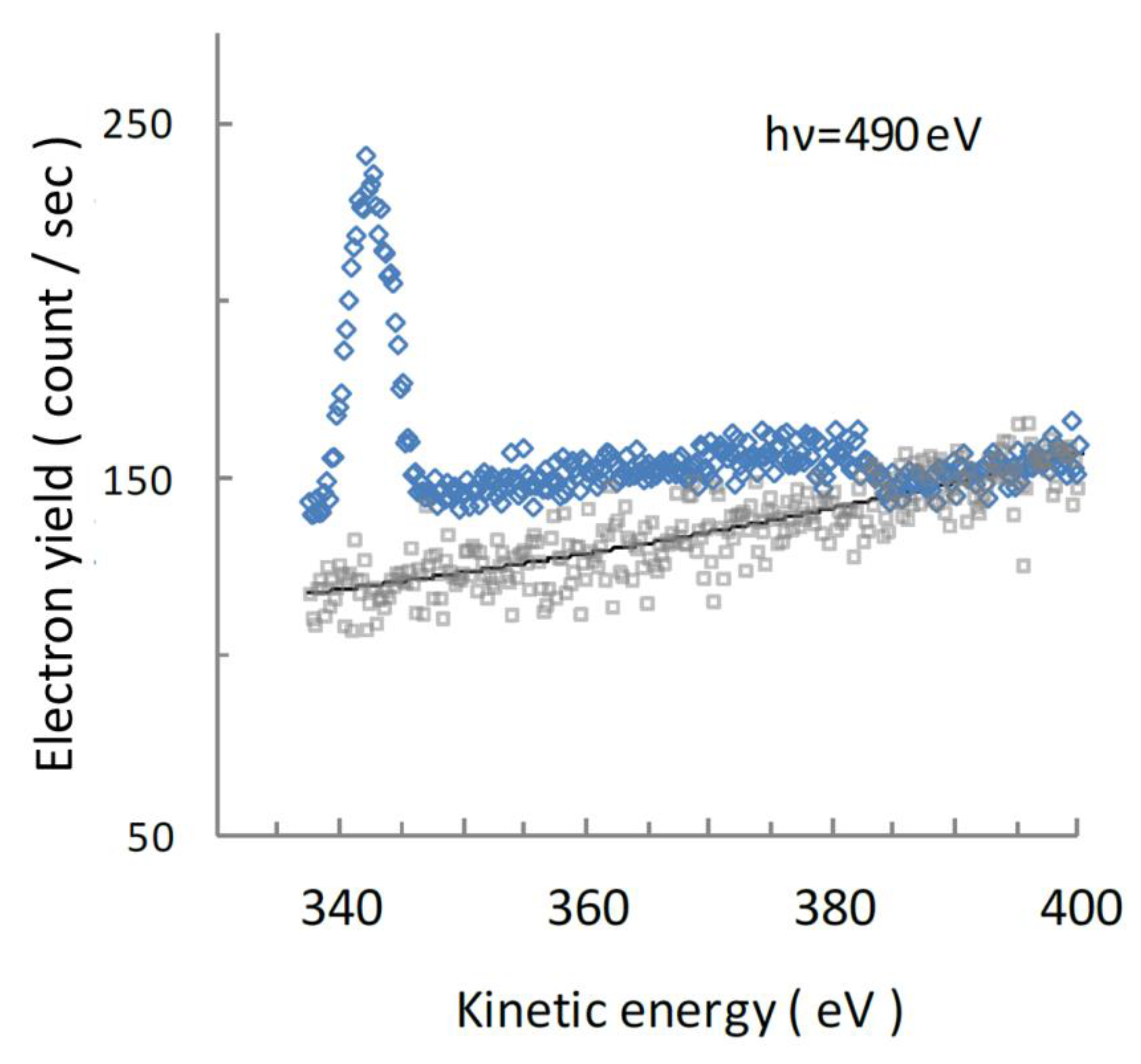
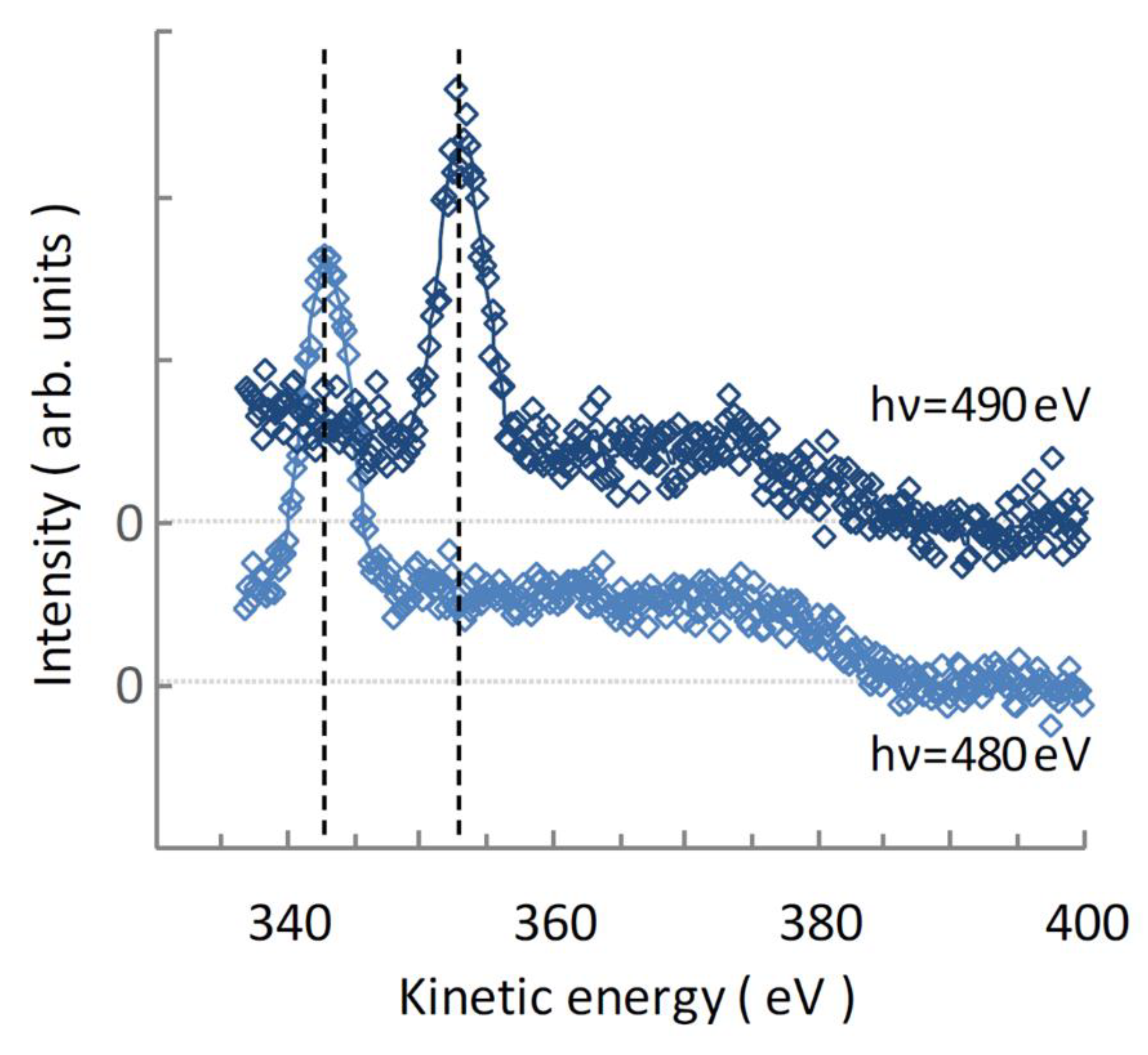
4.2. Auger Electron Spectra of Aqueous UMP Following N 1s Photoionization
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, D.; Sevilla, M.D. The chemical consequences in radiation damage to DNA. Adv. Radiat. Biol. 1993, 17, 121–180. [Google Scholar]
- Bernhard, W.A.; Close, D.M. DNA damage dictates the biological consequences of ionizing irradiation: The chemical pathway. In Charged Particle and Photon Interaction with Matter; Mozunder, A., Hatano, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 431–470. [Google Scholar]
- Yokoya, A.; Fujii, K.; Shikazono, N.; Ukai, M. Spectroscopic study of radiation-induced DNA lesions and their susceptibility to enzymatic repair. In Charged Particle and Photon Interaction with Matter; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 543–574. [Google Scholar]
- O’Neill, P.; Fielden, E.M. Primary free radical processes in DNA. Adv. Radiat. Biol. 1993, 17, 53–120. [Google Scholar]
- Garrett, B.C.; Dixon, D.A.; Camaioni, D.M.; Chipman, D.M.; Johnson, M.A.; Jonah, C.D.; Kimmel, G.A.; Miller, J.H.; Rescigno, T.N.; Rossky, P.J.; et al. Role of water in electron-initiated processes and radical chemistry. Chem. Rev. 2005, 105, 355–390. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Yokoya, A.; Fujii, K.; Fukuda, Y.; Ukai, M. Unpaired electron species in thin films of calf-thymus DNA molecules induced by nitrogen and oxygen K-shell photoabsorption. Phys. Rev. Lett. 2012, 109, 213001. [Google Scholar] [CrossRef]
- Shimada, H.; Fukao, T.; Minami, H.; Ukai, M.; Fujii, K.; Yokoya, A.; Fukuda, Y.; Saitoh, Y. Structural changes of nucleic acid base in aqueous solution as observed in X-ray absorption near edge structure (XANES). Chem. Phys. Lett. 2014, 591, 137. [Google Scholar] [CrossRef]
- Shimada, H.; Fukao, T.; Minami, H.; Ukai, M.; Fujii, K.; Yokoya, A.; Fukuda, Y.; Saitoh, Y. Nitrogen K-edge X-ray absorption near edge structure (XANES) spectra of purine containing nucleotides in aqueous solution. J. Chem. Phys. 2014, 141, 055102. [Google Scholar] [CrossRef]
- Shimada, H.; Minami, H.; Okuizumi, N.; Sakuma, I.; Ukai, M.; Fujii, K.; Yokoya, A.; Fukuda, Y.; Saitoh, Y. Nitrogen K-edge x-ray absorption near edge structure of pyrimidine-containing nucleotides in aqueous solution. J. Chem. Phys. 2015, 142, 05B605_1. [Google Scholar] [CrossRef]
- Lopez-Tarifa, P.; Gaigeot, M.-P.; Vuilleumier, R.; Tavernelli, I.; Alcami, M.; Martin, F.; Herve du Penhoat Politis, M.-F. Ultrafast damage following radiation-induced oxidation of uracil in aqueous solution. Angew. Chem. Int. Ed. 2013, 52, 3160–3163. [Google Scholar] [CrossRef]
- Fujii, K.; Izumi, Y.; Narita, A.; Ghose, K.K.; Lopez-Tarifa, P.; Touati, A.; Spezia, R.; Vuilleumier, R.; Gaigeot, M.-P.; Politis, M.-F.; et al. Roles of hydration for inducing decomposition of 2-deoxy-d-ribose by ionization of oxygen K-shell electrons. Radiat. Res. 2018, 189, 264–272. [Google Scholar] [CrossRef]
- Richter, C.; Hollas, D.; Saak, C.-M.; Förstel, M.; Miteva, T.; Mucke, M.; Björneholm, O.; Sisourat, N.; Slavíček, P.; Hergenhahn, U. Competition between proton transfer and intermolecular Coulombic decay in water. Nat. Commun. 2018, 9, 4988. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Aihara, H.; Kodashima, Y.; Makishima, H.; Nakiri, S.; Takada, S.; Shimada, H.; Ukai, M.; Ozga, C.; Holzapfel, X.; et al. Novel analytical study for reaction intermediates in the primary radiation interaction of DNA using a synchrotron radiation-induced luminescence spectroscopy. Radiat. Protec. Dosim. 2019, 183, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Ukai, M.; Yokoya, A.; Nonaka, Y.; Fujii, K.; Saitoh, Y. Synchrotron radiation photoelectron studies for primary radiation effects using a liquid water jet in vacuum: Total and partial photoelectron yields for liquid water near the oxygen K-edge. Radiat. Phys. Chem. 2009, 78, 1202–1206. [Google Scholar] [CrossRef]
- Ottosson, N.; Faubel, M.; Bradforth, S.E.; Jungwirth, P.; Winter, B. Photoelectron spectroscopy of liquid water and aqueous solution. J. Electron. Spectrosc. Relat. Phenom. 2010, 177, 60–70. [Google Scholar] [CrossRef]
- Mucha, A.; Knobloch, B.; Jeżowska-Bojczuk, M.; Kozłowski, H.; Sigel, R.K.O. Comparison of the acid–base properties of ribose and 2′-deoxyribose nucleotides. Chem. Eur. J. 2008, 14, 6663. [Google Scholar] [CrossRef]
- Ukai, M.; Yokoya, A.; Fujii, K.; Saitoh, Y. X-ray absorption spectrum for guanosine-5′-monophosphate in water solution in the vicinity of the nitrogen K-edge observed in free liquid jet in vacuum. Radiat. Phys. Chem. 2008, 77, 1265–1269. [Google Scholar] [CrossRef]
- Ukai, M.; Yokoya, A.; Fujii, K.; Saitoh, Y. X-ray absorption spectra of nucleotides (AMP, GMP, and CMP) in liquid water solutions near the nitrogen K-edge. Chem. Phys. Lett. 2010, 495, 90–95. [Google Scholar] [CrossRef]
- Chen, C.T.; Ma, Y.; Sette, F. K-shell photoabsorption of the N2 molecule. Phys. Rev. A 1989, 40, 6737. [Google Scholar] [CrossRef]
- Coreno, M.; de Simone, M.; Prince, K.C.; Richter, R.; Vondracek, M.; Avaldi, L.; Camilloni, R. Vibrational-ly resolved oxygen K→ Π* spectra of O and CO. Chem. Phys. Lett. 1999, 306, 269–274. [Google Scholar] [CrossRef]
- Sigel, H. Acid-base properties of purine residues and the effect of metal ions: Quantification of rare nucleobase tautomers. Pure Appl. Chem. 2004, 76, 1869. [Google Scholar] [CrossRef][Green Version]
- Hermann, K.; Pettersson, L.G.M.; Casida, M.E.; Daul, C.; Goursot, A.; Koester, A.; Proynov, E.; St-Amant, A.; Salahub, D.R. StoBe-deMon Version 3.1., Contributing Authors: Carravetta, V.; Duarte, H.; Friedrich, C.; Godbout, N.; Guan, J.; Jamorski, C.; Leboeuf, M.; Leetmaa, M.; Nyberg, M.; Patchkovskii, S.; Pedocchi, L.; Sim, F.; Triguero, L.; and Vela. A. Available online: www.fhi-berlin.mpg.de/KHsoftware/StoBe/ (accessed on 27 September 2019).
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef] [PubMed]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 1998, 1995. [Google Scholar] [CrossRef]
- Takeda, Y.; Shimada, H.; Miura, R.; Ukai, M.; Ozga, C.; Knie, A.; Ehresmann, A.; Yokoya, A.; Fujii, K.; Fukuda, Y.; et al. Effects of hydration on X-ray absorption near the nitrogen K-edge of pyrimidine-containing nucleotides in aqueous solution. (To be published).
- Yeh, J.J.; Lindau, I. Atomics subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. Atom. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Moddeman, W.E.; Carlson, T.A.; Krause, M.O.; Pullen, B.P.; Bull, W.E.; Schweitzer, G.K. Determination of the K-LL Auger Spectra of N2, O2, CO, NO, H2O, and CO2. J. Chem. Phys. 1971, 55, 2317–2336. [Google Scholar] [CrossRef]

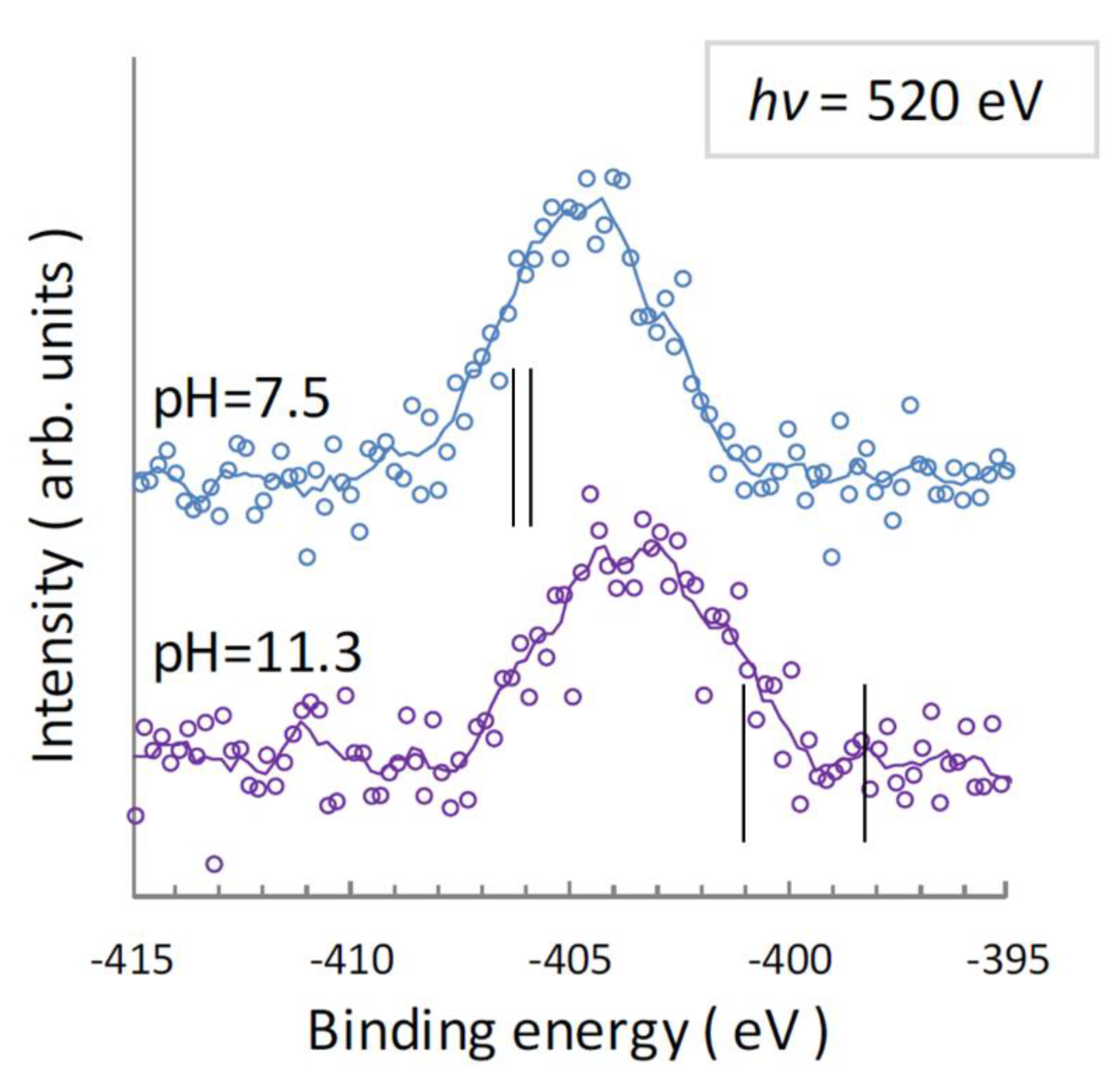
| pH = 7.5 | pH = 11.3 | ||
|---|---|---|---|
| N1, N3 (Mixed) | N1 | N3 | |
| Observed a | 404.7 eV, 404.9 eV | 404.9 eV | 402.5 eV |
| Center of Gravity | 404.7 eV | 403.5 eV | |
| Calculated b | 405.9 eV, 406.3 eV | 401.0 eV | 398.2 eV |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, Y.; Shimada, H.; Miura, R.; Ukai, M.; Fujii, K.; Fukuda, Y.; Saitoh, Y. Hydration of Nucleobase as Probed by Electron Emission of Uridine-5′-Mono-Phosphate (UMP) in Aqueous Solution Induced by Nitrogen K-Shell Ionization. Quantum Beam Sci. 2020, 4, 10. https://doi.org/10.3390/qubs4010010
Takeda Y, Shimada H, Miura R, Ukai M, Fujii K, Fukuda Y, Saitoh Y. Hydration of Nucleobase as Probed by Electron Emission of Uridine-5′-Mono-Phosphate (UMP) in Aqueous Solution Induced by Nitrogen K-Shell Ionization. Quantum Beam Science. 2020; 4(1):10. https://doi.org/10.3390/qubs4010010
Chicago/Turabian StyleTakeda, Yasuaki, Hiroyuki Shimada, Ryosuke Miura, Masatoshi Ukai, Kentaro Fujii, Yoshihiro Fukuda, and Yuji Saitoh. 2020. "Hydration of Nucleobase as Probed by Electron Emission of Uridine-5′-Mono-Phosphate (UMP) in Aqueous Solution Induced by Nitrogen K-Shell Ionization" Quantum Beam Science 4, no. 1: 10. https://doi.org/10.3390/qubs4010010
APA StyleTakeda, Y., Shimada, H., Miura, R., Ukai, M., Fujii, K., Fukuda, Y., & Saitoh, Y. (2020). Hydration of Nucleobase as Probed by Electron Emission of Uridine-5′-Mono-Phosphate (UMP) in Aqueous Solution Induced by Nitrogen K-Shell Ionization. Quantum Beam Science, 4(1), 10. https://doi.org/10.3390/qubs4010010