Experimental Study on the Biological Effect of Cluster Ion Beams in Bacillus subtilis Spores
Abstract
1. Introduction
2. Materials and Methods
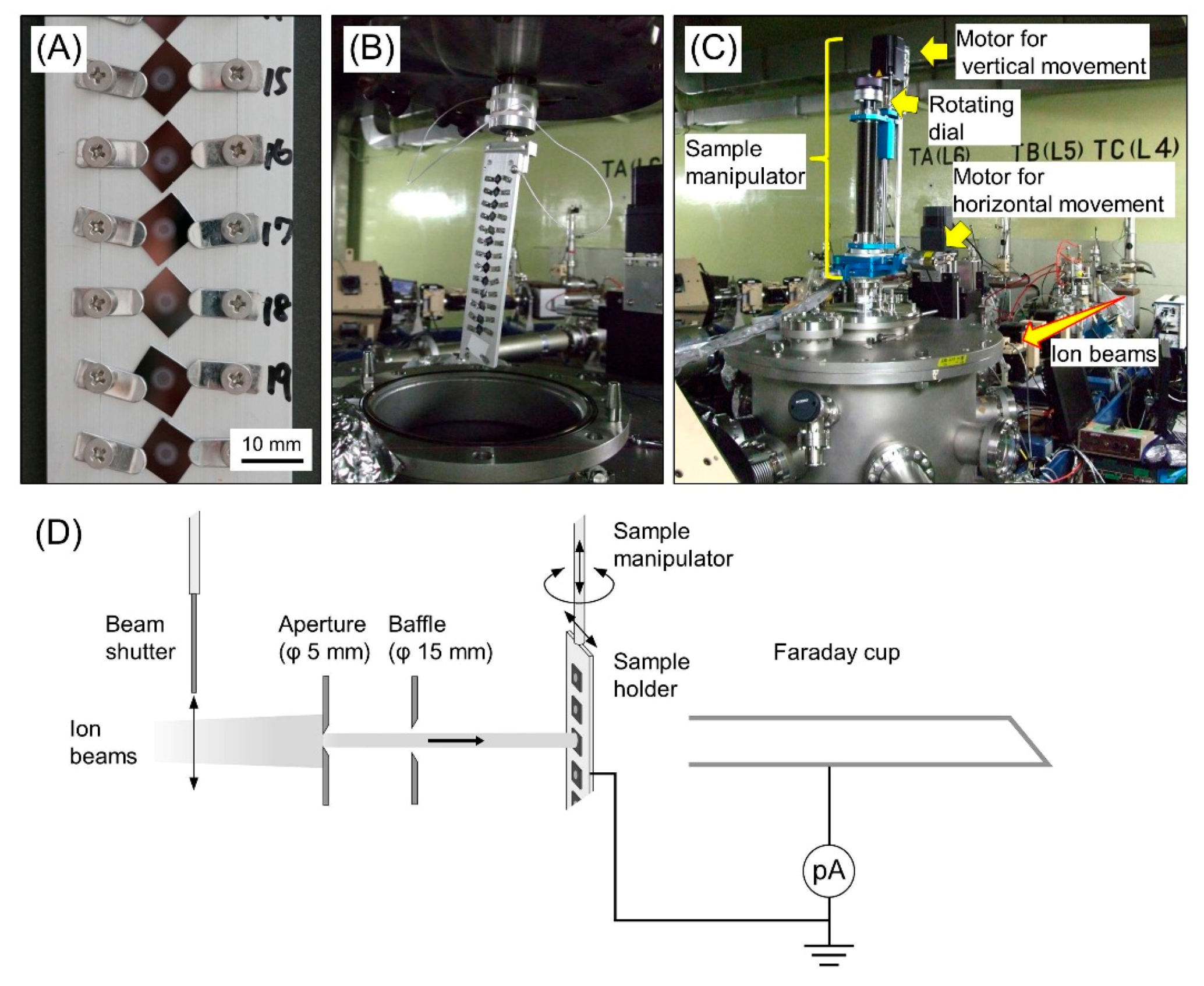
2.1. Sample Preparation for Cluster Ion Irradiation
2.2. Cluster Ion Irradiation
2.3. Survival Assay
2.4. Measurement of Particle Fluence
2.5. Calculation of Physical Parameters
2.6. LET-RBE Relationship on Lethal Effect
3. Results and Discussion
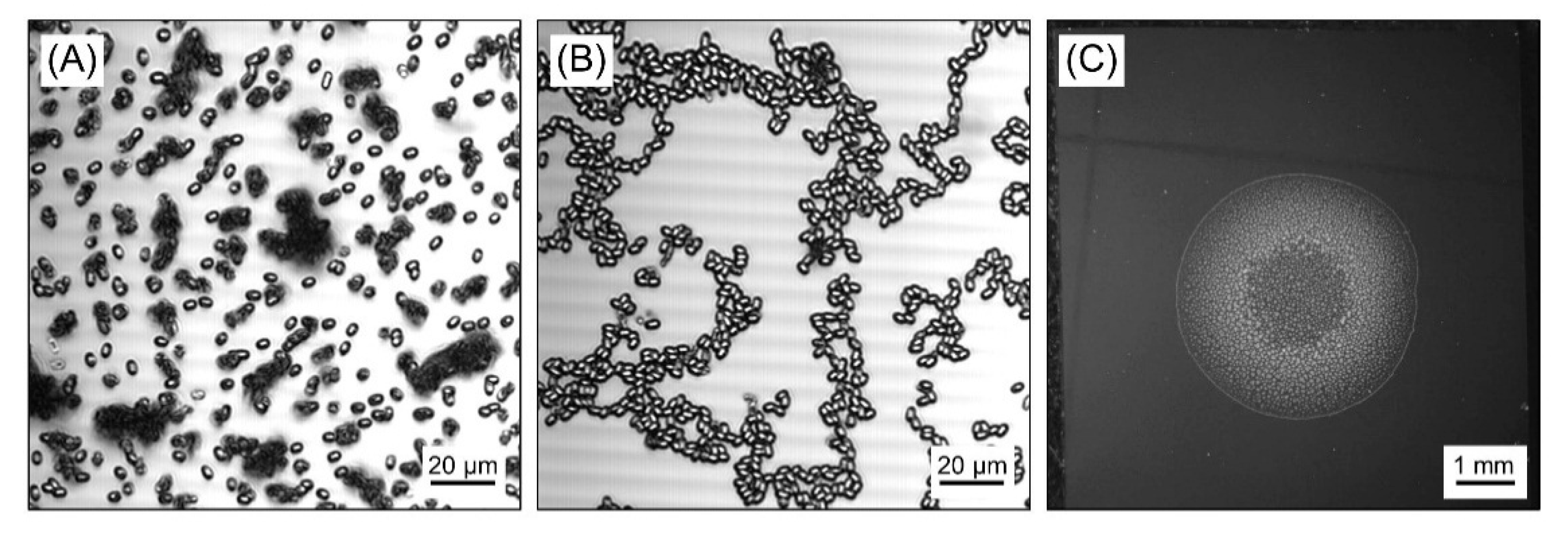
3.1. Sample Preparation for Homogeneous Irradiation of B. Subtilis Spores
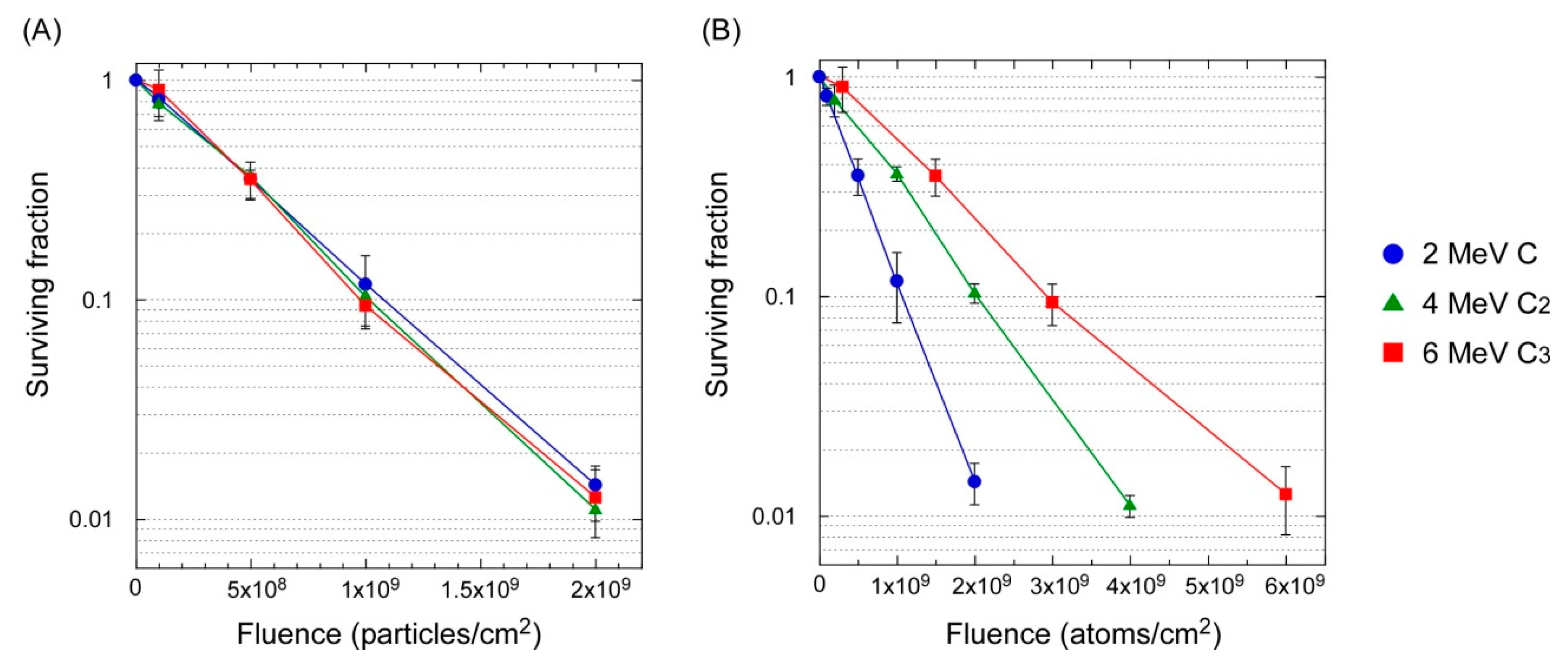
3.2. Lethal Effect: Cluster Ions Versus Monomer Ions
3.3. Accuracy of Irradiation Fluence
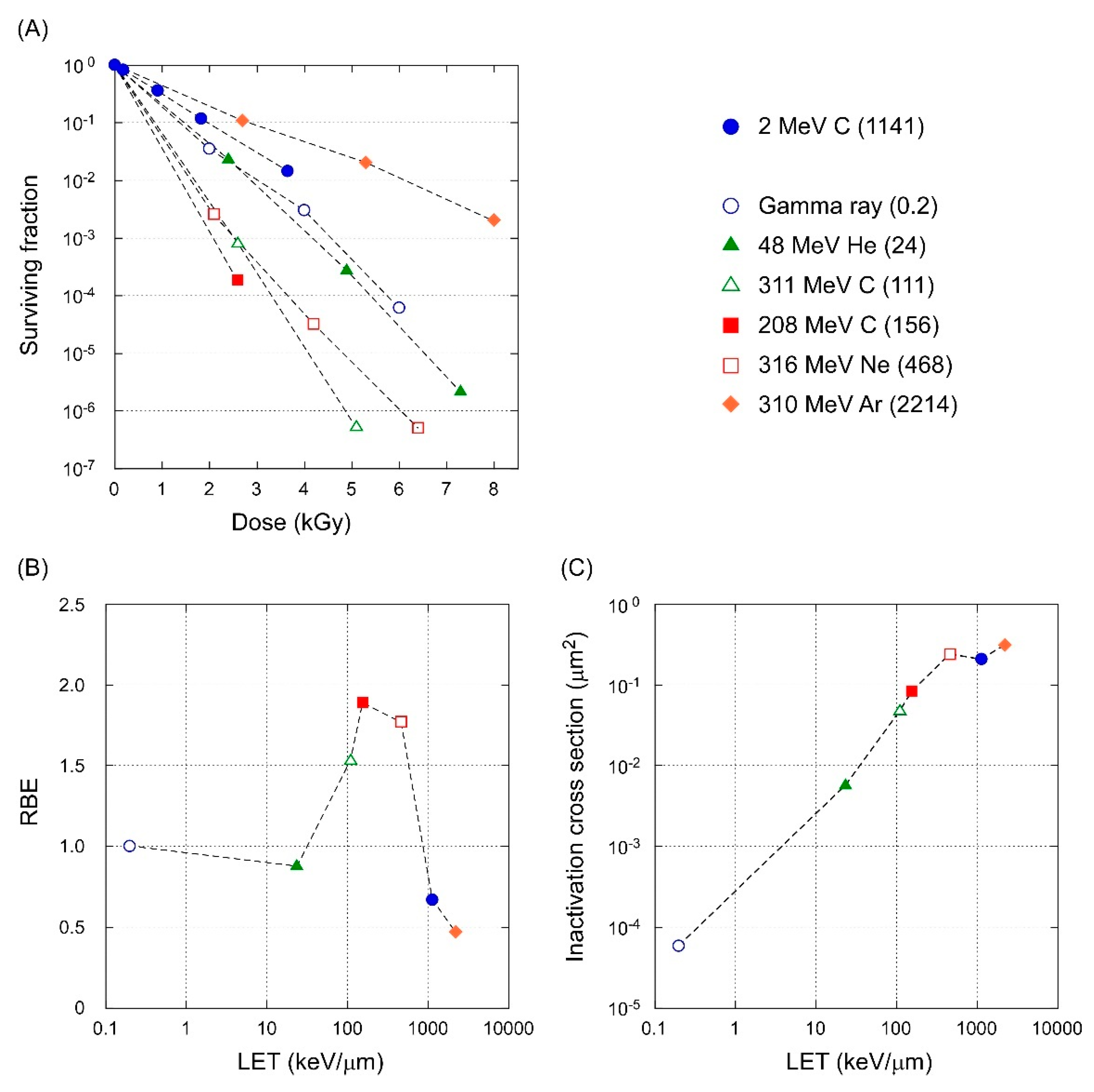
3.4. LET-RBE Relationship
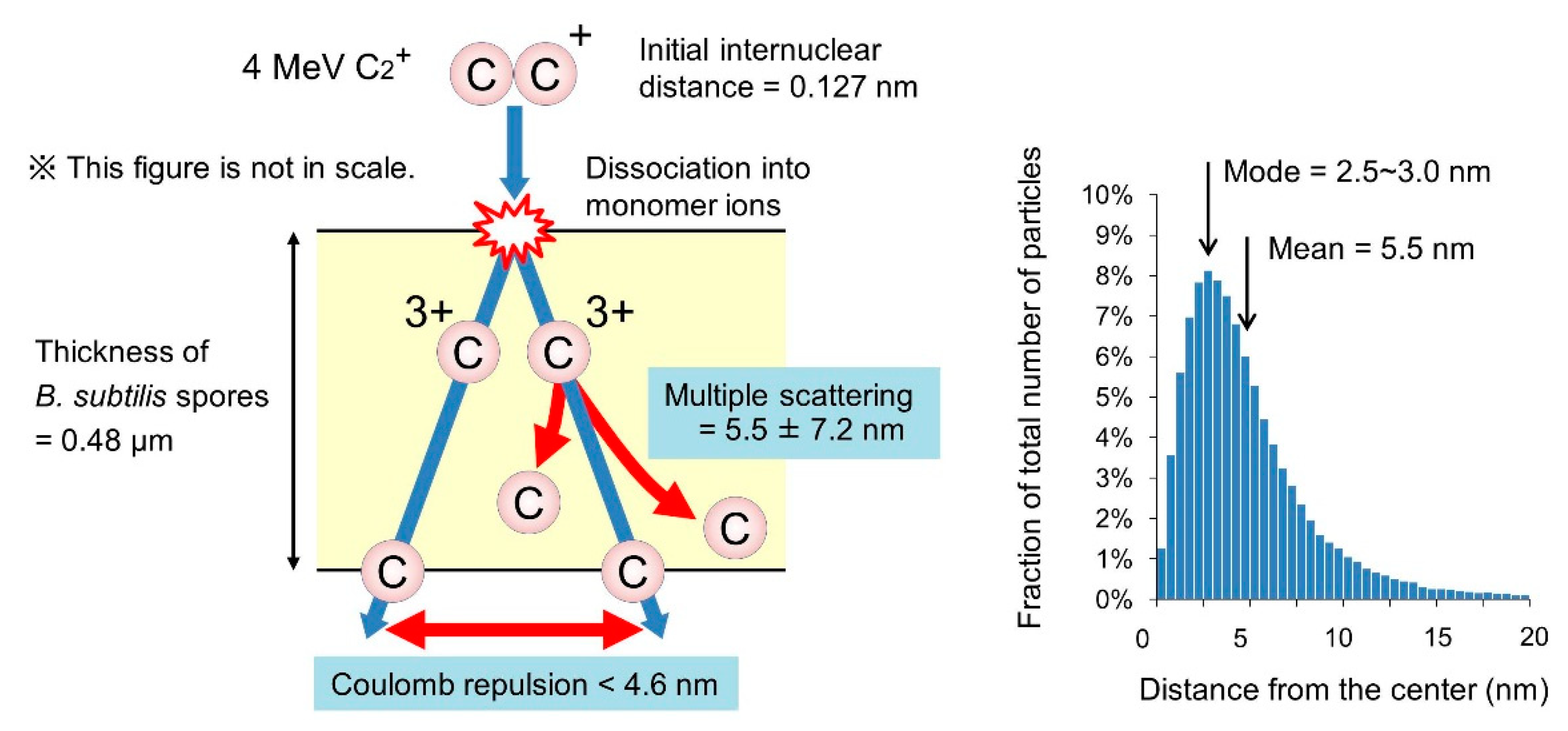
3.5. Behavior of the Atoms of Cluster Ions Bombarded into B. Subtilis Spores
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, H.H.; Brunelle, A.; Della-Negra, S.; Depauw, J.; Jacquet, D.; Le Beyec, Y.; Chaumont, J.; Bernas, H. Giant metal sputtering yields induced by 20-5000 keV/atom gold clusters. Phys. Rev. Lett. 1998, 80, 5433–5436. [Google Scholar] [CrossRef]
- Canut, B.; Bonardi, N.; Ramos, S.M.M.; Della-Negra, S. Latent tracks formation in silicon single crystals irradiated with fullerenes in the electronic regime. Nucl. Instr. Meth. Phys. Res. B 1998, 146, 296–301. [Google Scholar] [CrossRef]
- Saitoh, Y.; Mizuhashi, K.; Tajima, S. Acceleration of cluster and molecular ions by TIARA 3MV tandem accelerator. Nucl. Instr. Meth. Phys. Res. A 2000, 452, 61–66. [Google Scholar] [CrossRef]
- Saitoh, A.; Chiba, A.; Narumi, K. Transmission of cluster ions through a tandem accelerator of several stripper gases. Rev. Sci. Instrum. 2009, 80, 106104. [Google Scholar] [CrossRef]
- Kurashima, S.; Satoh, T.; Saitoh, Y.; Yokota, W. Irradiation facilities of the Takasaki Advanced Radiation Research Institute. Quantum Beam Sci. 2017, 1, 2. [Google Scholar] [CrossRef]
- Koide, T.; Saitoh, Y.; Sakamaki, M.; Amemiya, K.; Iwase, A.; Matsui, T. Change in magnetic and structural properties of FeRh thin films by gold cluster ion beam irradiation with the energy of 1.67 MeV/atom. J. Appl. Phys. 2014, 115, 17B722. [Google Scholar] [CrossRef]
- Narumi, K.; Nakajima, K.; Kimura, K.; Mannami, M.; Saitoh, Y.; Yamamoto, S.; Aoki, Y.; Naramoto, H. Energy losses of B clusters transmitted through carbon foils. Nucl. Instr. Meth. Phys. Res. B 1998, 135, 77–81. [Google Scholar] [CrossRef]
- Chiba, A.; Saitoh, Y.; Narumi, K.; Adachi, M.; Kaneko, T. Average charge and its structure dependence of fragment ions under irradiation of a thin carbon foil with a 1-MeV/atom C3+ cluster ion. Phys. Rev. A 2007, 76, 063201. [Google Scholar] [CrossRef]
- Hirata, K.; Saitoh, Y.; Chiba, A.; Yamada, S.; Matoba, S.; Narumi, K. Time-of-flight secondary ion mass spectrometry with transmission of energetic primary cluster ions through foil targets. Rev. Sci. Instrum. 2014, 85, 033107. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Chiba, A.; Hirano, Y.; Saitoh, Y. Development of an electron-attachment-type negative fullerene ion source. AIP Conf. Proc. 2018, 2011, 050020. [Google Scholar]
- Brandt, W.; Ratkowski, A.; Ritchie, R.H. Energy loss of swift proton clusters in solids. Phys. Rev. Lett. 1974, 33, 1325–1328. [Google Scholar] [CrossRef]
- Takayama, K.; Adachi, T.; Wake, M.; Okamura, K. Racetrack-shape fixed field induction accelerator for giant cluster ions. Phys. Rev. ST-AB 2015, 18, 050101. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Zandomeni, R.O.; Fitzgibbon, J.; Sagripanti, J.-L. Difference between the spore size of Bacillus anthracis and other Bacillus species. J. App. Microbiol. 2007, 102, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Setlow, P. Molecular Biological Methods for Bacillus; Harwood, C.R., Cutting, S.M., Eds.; The Biological Laboratories, Harvard University: Cambridge, MA, USA, 1990; pp. 391–450. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter. Nucl. Instr. Meth. Phys. Res. B 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Tanaka, S.; Fukuda, K.; Nishimura, K.; Watanabe, H.; Yamano, N. IRAC M: A Code System to Calculate Induced Radioactivity Produced by Ions and Neutrons; JAERI-Data/Code 97-019; Japan Atomic Energy Research Institute: Tokyo, Japan, 1997. [Google Scholar]
- Carrera, M.; Zandomeni, R.O.; Sagripanti, J.-L. Wet and dry density of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 2008, 105, 68–77. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, S.; Saga, K.; Minowa, T. Process Simulation of Yeast Cultivation and Ethanol Fermentation in Bio-ethanol Production. Energy Resour. 2010, 31, 335–340. (In Japanese) [Google Scholar]
- Brenner, D.J. Point: The linear-quadratic model is an appropriate methodology for determining iso-effective doses at large doses per fraction. Semin. Radiat. Oncol. 2008, 18, 234–239. [Google Scholar] [CrossRef]
- Yokota, Y.; Hase, Y.; Shikazono, N.; Tanaka, A.; Inoue, M. LET dependence of lethality of carbon ion irradiation to single tobacco cells. Int. J. Radiat. Biol. 2003, 79, 681–685. [Google Scholar] [CrossRef]
- Hirata, K.; Saitoh, Y.; Chiba, A.; Narumi, K.; Kobayashi, Y.; Ohara, Y. Highly sensitive time-of-flight secondary-ion mass spectroscopy for contaminant analysis of semiconductor surface using cluster impact ionization. Appl. Phys. Lettt. 2005, 86, 044105. [Google Scholar] [CrossRef]
- Hirata, K.; Saitoh, Y.; Chiba, K.; Yamada, K.; Takahashi, Y.; Narumi, K. Comparison of secondary ion emission yields for poly-tyrosine between cluster and heavy ion impacts. Nucl. Instr. Meth. Phys. Res. B 2010, 268, 2930–2932. [Google Scholar] [CrossRef]
- Shima, K.; Ishihara, T.; Mikumo, T. Empirical formula for the average equilibrium charge-state of heavy ions behind various foils. Nucl. Instr. Meth. Phys. Res. 1982, 200, 605–608. [Google Scholar] [CrossRef]
- Satoh, K.; Oono, Y. Studies on application of ion beam breeding to industrial microorganisms at TIARA. Quantum Beam Sci. 2019. submitted. [Google Scholar]
- Moribayashi, K. Simulation study of radial dose due to the irradiation of a swift heavy ion aiming to advance the treatment planning system for heavy particle cancer therapy: The effect of emission angles of secondary electrons. Nucl. Instr. Meth. Phys. Res. B 2015, 365, 592–595. [Google Scholar] [CrossRef]
- Moribayashi, K. Effect of the track potential on the motion and energy flow of secondary electrons created from heavy-ion irradiation. Radiat. Phys. Chem. 2018, 146, 68–72. [Google Scholar] [CrossRef]
- Kim, J.; Yoshimura, S.H.; Hizume, K.; Ohniwa, R.L.; Ishihama, K.; Takeyasu, K. Fundamental structural units of the Escherichia coli nucleoid revealed by atomic force microscopy. Nucl. Acids Res. 2004, 32, 1982–1992. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hase, Y.; Satoh, K.; Chiba, A.; Hirano, Y.; Tomita, S.; Saito, Y.; Narumi, K. Experimental Study on the Biological Effect of Cluster Ion Beams in Bacillus subtilis Spores. Quantum Beam Sci. 2019, 3, 8. https://doi.org/10.3390/qubs3020008
Hase Y, Satoh K, Chiba A, Hirano Y, Tomita S, Saito Y, Narumi K. Experimental Study on the Biological Effect of Cluster Ion Beams in Bacillus subtilis Spores. Quantum Beam Science. 2019; 3(2):8. https://doi.org/10.3390/qubs3020008
Chicago/Turabian StyleHase, Yoshihiro, Katsuya Satoh, Atsuya Chiba, Yoshimi Hirano, Shigeo Tomita, Yuichi Saito, and Kazumasa Narumi. 2019. "Experimental Study on the Biological Effect of Cluster Ion Beams in Bacillus subtilis Spores" Quantum Beam Science 3, no. 2: 8. https://doi.org/10.3390/qubs3020008
APA StyleHase, Y., Satoh, K., Chiba, A., Hirano, Y., Tomita, S., Saito, Y., & Narumi, K. (2019). Experimental Study on the Biological Effect of Cluster Ion Beams in Bacillus subtilis Spores. Quantum Beam Science, 3(2), 8. https://doi.org/10.3390/qubs3020008



