Effect of Sintering Temperature on the Properties of CuAlO2 Synthesized from Nanosized Precursors for Application in Smart Infrastructure Systems
Abstract
1. Study Background
2. Research Importance
3. Methodology
4. Characterization
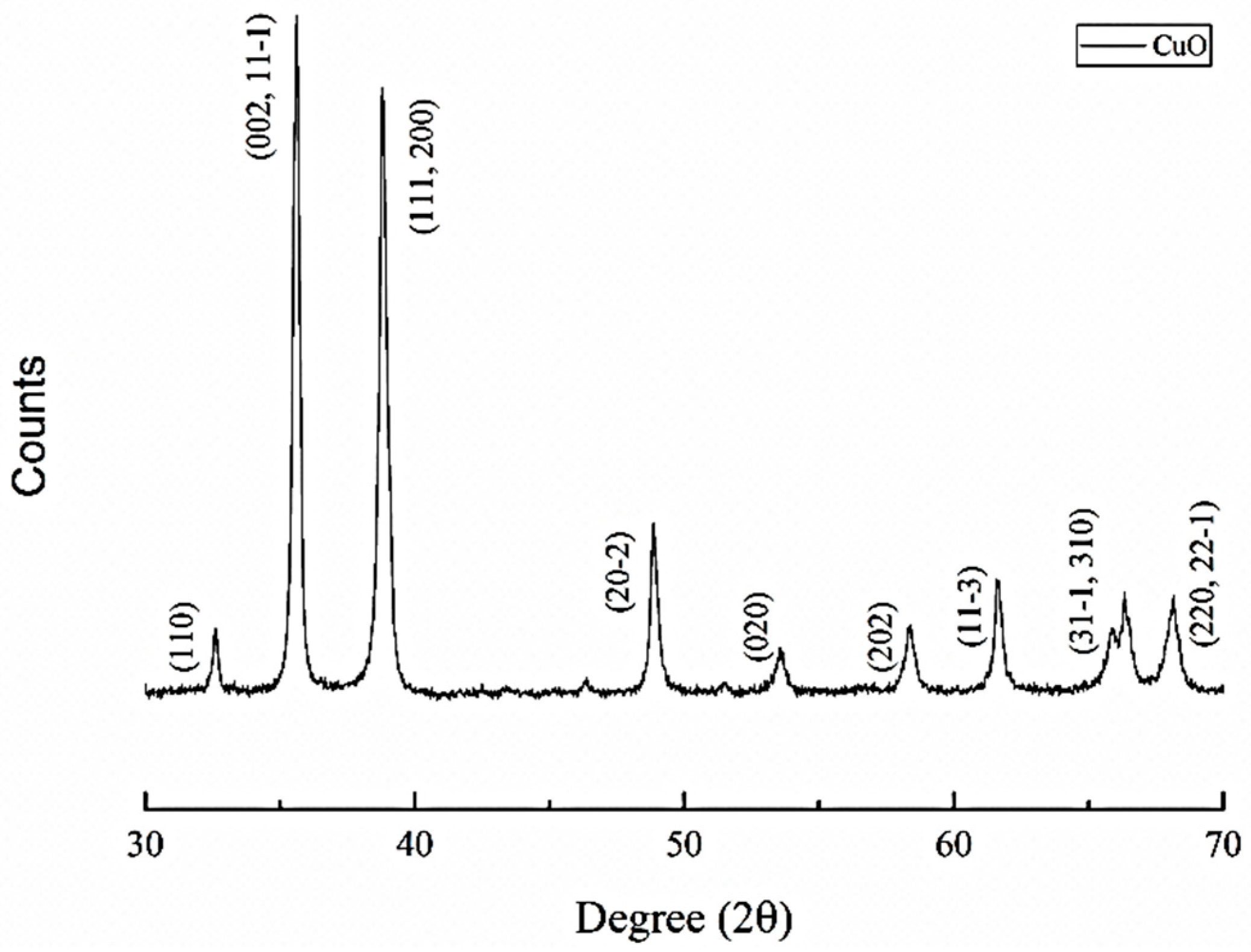
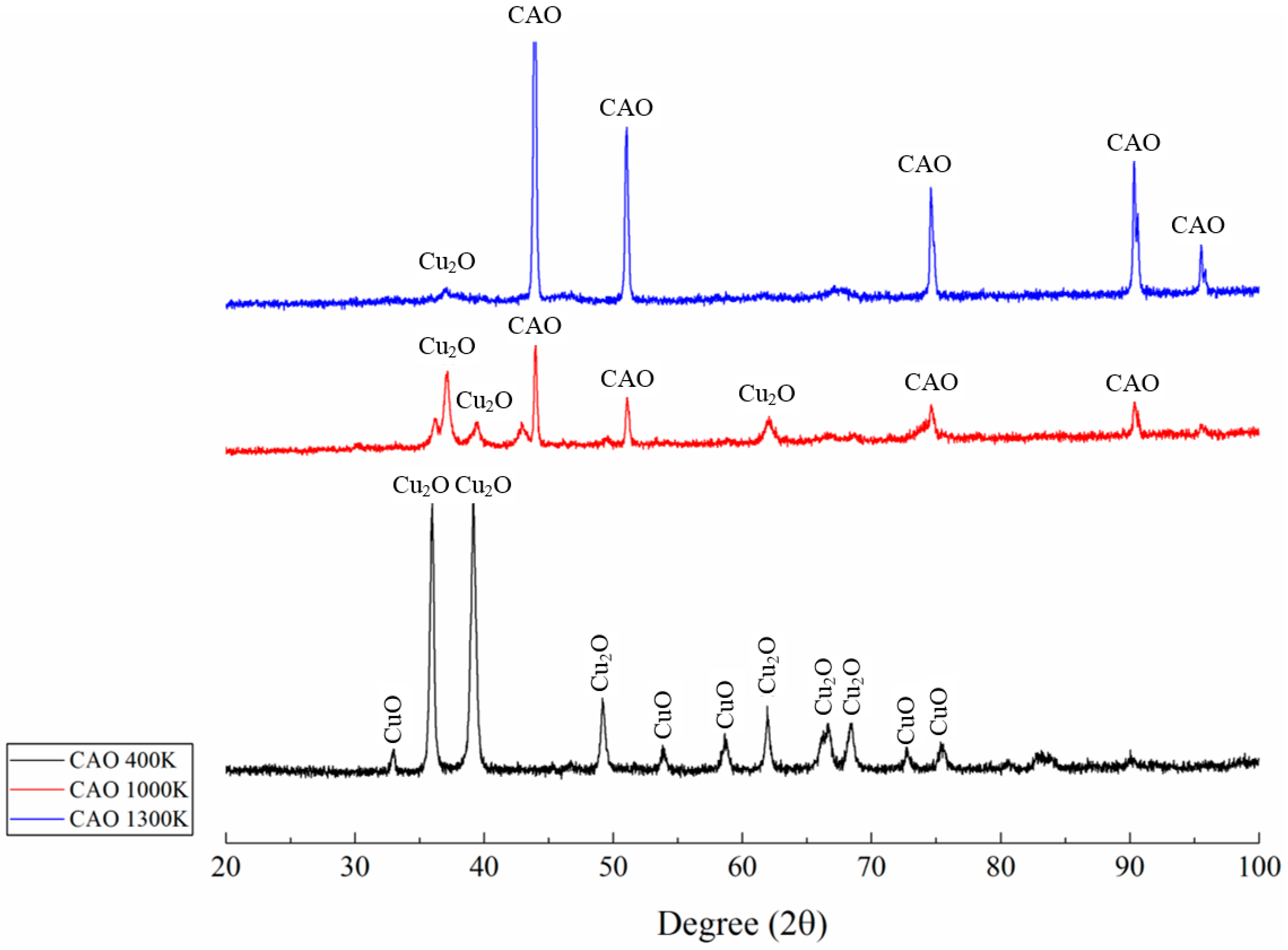
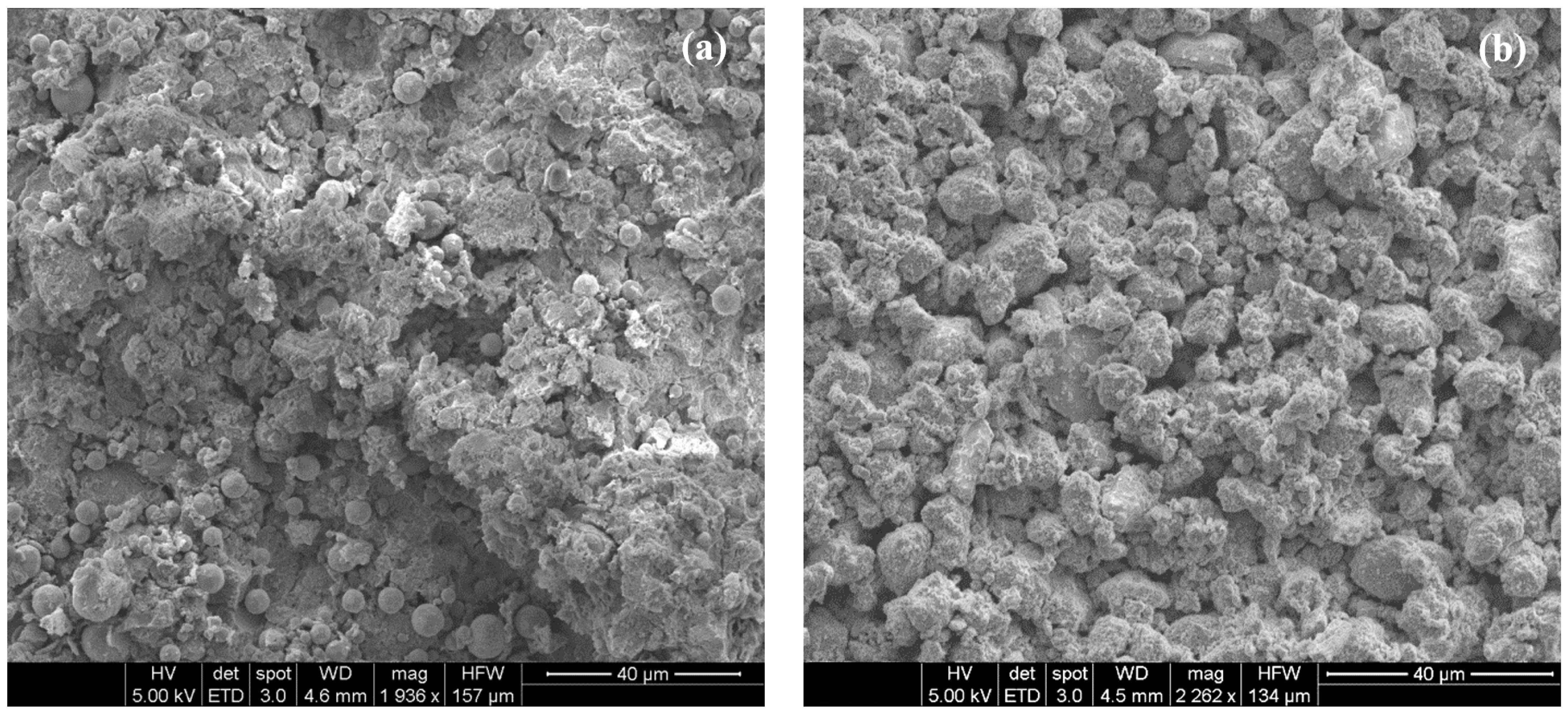
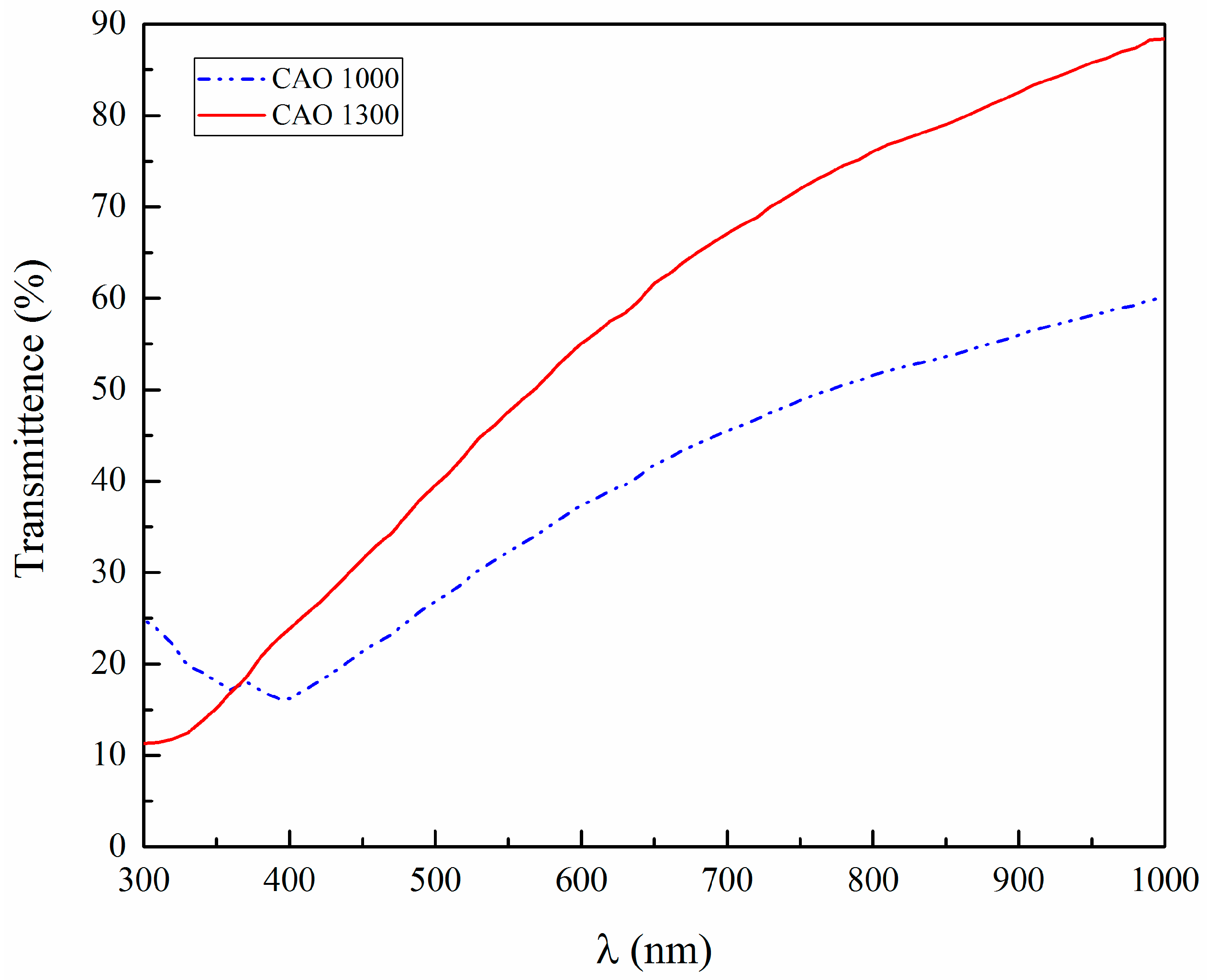
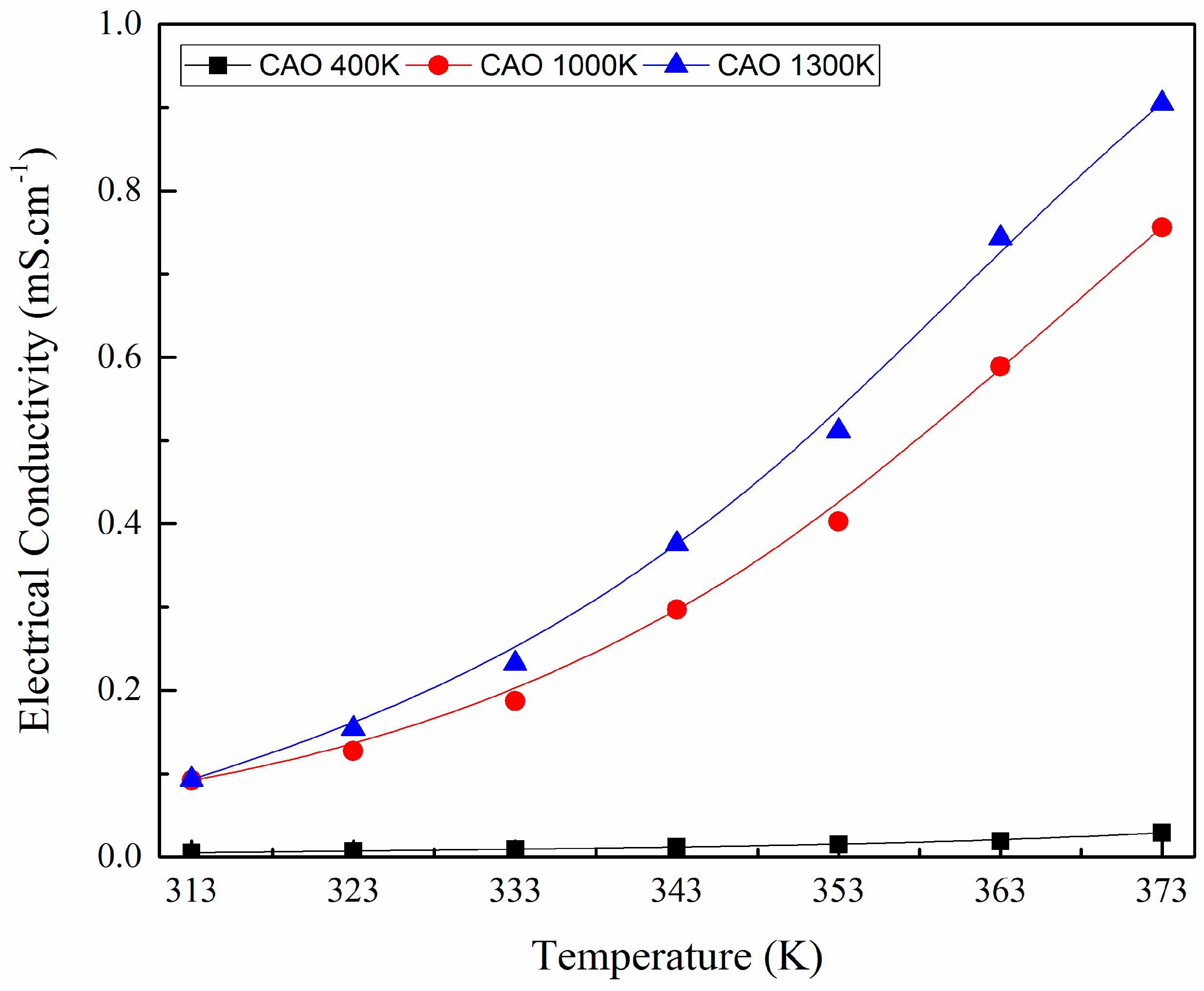
5. Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolas, G.S.; Sharp, J.; Goldsmid, J. Thermoelectrics: Basic Principles and New Materials Developments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 45. [Google Scholar]
- Ghahari, S. The Effect of ZnO nanoparticles on Thermoelectric Behavior and Fresh Properties of Cement Paste; Purdue University: City of West Lafayette, IN, USA, 2016. [Google Scholar]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Yu, R.S.; Lu, C.J.; Tasi, D.C.; Liang, S.C.; Shieu, F.S. Phase transformation and optoelectronic properties of p-type CuAlO2 thin films. J. Electrochem. Soc. 2007, 154, H838–H843. [Google Scholar] [CrossRef]
- Zheng, X.G.; Taniguchi, K.; Takahashi, A.; Liu, Y.; Xu, C.N. Room temperature sensing of ozone by transparent p-type semiconductor CuAlO2. Appl. Phys. Lett. 2004, 85, 1728–1729. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the application of SnO2 in perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Akyildiz, H. Synthesis of CuAlO2 from chemically precipitated nano-sized precursors. Ceram. Int. 2015, 41, 14108–14115. [Google Scholar] [CrossRef]
- Pedapudi, M.C.; Dhar, J.C. Ultrasensitive pn Junction UV-C Photodetector Based on p-Si/β-Ga2O3 Nanowire Arrays. Sens. Actuators A Phys. 2022, 344, 113673. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, Y.; Zhang, J.; Huang, P.; Zhang, X.; Feng, B. Photovoltaic Conversion Enhancement of a Carbon Quantum Dots/p-Type CuAlO2/n-Type ZnO Photoelectric Device. ACS Appl. Mater. Interfaces 2015, 7, 7878. [Google Scholar] [CrossRef] [PubMed]
- Urper, O.; Baydogan, N. Effect of Al concentration on optical parameters of ZnO thin film derived by Sol-Gel dip coating technique. Mater. Lett. 2020, 274, 128000. [Google Scholar] [CrossRef]
- Ingram, B.; Gonzalez, G.B.; Mason, T. Transport and defect mechanisms in cuprous delafossites. 1. Comparison of hydrothermal and standard solid-state synthesis in CuAlO2. Chem. Mater. 2004, 16, 5616–5622. [Google Scholar] [CrossRef]
- Saha, B.; Thapa, R.; Chattopadhyay, K.K. A novel route for the low temperature synthesis of p-type transparent semiconducting CuAlO2. Mater. Lett. 2009, 63, 394–396. [Google Scholar] [CrossRef]
- Tong, B.; Deng, Z.; Meng, G.; Chang, J.; Lu, Y.; Qi, L.; Shan, X.; Shao, J.; Wang, S.; Shen, C. Ultrasensitive and selective CuAlO2 sensor toward H2S based on surface sulfuration-desulfuration reaction. Sens. Actuators B Chem. 2020, 313, 128027. [Google Scholar] [CrossRef]
- Lee, T.-H.; Hwang, S.-M.; Yoo, M.-J. Investigation of CuAlO2 composite dielectric properties and selective metallization by laser direct structure technology. J. Eur. Ceram. Soc. 2020, 40, 1390–1397. [Google Scholar] [CrossRef]
- Neumann-Spallart, M.; Pai, S.P.; Pinto, R. PLD growth of CuAlO2. Thin Solid Film. 2007, 515, 8641–8644. [Google Scholar] [CrossRef]
- Yu, R.-S.; Yin, H.-H. Structural and optoelectronic properties of p-type semiconductor CuAlO2 thin films. Thin Solid Film. 2012, 526, 103–108. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Maity, R.; Ghosh, P.K.; Chattopadhyay, K.K. Thermoelectric properties and electrical characteristics of sputter-deposited p-CuAlO2 thin films. Thin Solid Film. 2005, 474, 261–266. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, G.; Zhang, C.; Chen, Y. Preparation of CuAlO2 thin films with high transparency and low resistivity using sol–gel method. J. Sol-Gel Sci. Technol. 2012, 61, 565–569. [Google Scholar] [CrossRef]
- Gong, H.; Wang, Y.; Luo, Y. Nanocrystalline p-type transparent Cu–Al–O semiconductor prepared by chemical-vapor deposition with Cu(acac)2 and Al(acac)3 precursors. Appl. Phys. Lett. 2000, 76, 3959–3961. [Google Scholar] [CrossRef]
- Sato, T.; Sue, K.; Tsumatori, H.; Suzuki, M.; Tanaka, S.; Kawai-Nakamura, A.; Saitoh, K.; Aida, K.; Hiaki, T. Hydrothermal synthesis of CuAlO2 with the delafossite structure in supercritical water. J. Supercrit. Fluids 2008, 46, 173–177. [Google Scholar] [CrossRef]
- Xiong, D.; Zeng, X.; Zhang, W.; Wang, H.; Zhao, X.; Chen, W.; Cheng, Y.-B. Synthesis and Characterization of CuAlO2 and AgAlO2 Delafossite Oxides through Low-Temperature Hydrothermal Methods. Inorg. Chem. 2014, 53, 4106–4116. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Y.; Kubomura, R.; Ozeki, S.; Liu, S.; Yoshida, H.; Jin, Y.; Lu, Y. Preparation and thermoelectric properties of CuAlO2 compacts by tape casting followed by SPS. J. Alloys Compd. 2021, 853, 157086. [Google Scholar] [CrossRef]
- Ma, L.; Dong, C.; Li, W.; Su, Q.; Zhou, J.; Xie, E.; Lan, W. Room-temperature power factor of CuAlO2 composite tablets enhanced by MWCNTs. Curr. Appl. Phys. 2022, 33, 27–32. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Mastelaro, V.R.; Escanhoela, C.A. In-Depth Understanding of the Relation between CuAlO2 Particle Size and Morphology for Ozone Gas Sensor Detection at a Nanoscale Level. ACS Appl. Mater. Interfaces 2014, 6, 21739–21749. [Google Scholar] [CrossRef]
- Dloczik, L.; Tomm, Y.; Könenkamp, R.; Lux-Steiner, M.C.; Dittrich, T. CuAlO2 prepared by ion exchange from LiAlO2. Thin Solid Film. 2004, 451–452, 116–119. [Google Scholar] [CrossRef]
- Kaya, I.; Cetin, C.; Aydin, H.; Katircioglu, Z.; Buyukbekar, B.; Yavuz, M.S.; Uyaner, M.; Kalem, V.; Akyildiz, H. Production of CuAlO2 in powder, bulk and nanofiber forms. J. Ceram. Process. Res. 2015, 16, 648–655. [Google Scholar]
- Chen, X.-L.; Zhang, J.-X.; Zhao, Z.-Y. First-principles calculations to investigate the polymorph effects of CuAlO2. Comput. Mater. Sci. 2022, 209, 111403. [Google Scholar] [CrossRef]
- Ahmed, J.; Blakely, C.K.; Prakash, J.; Bruno, S.R.; Yu, M.; Wu, Y.; Poltavets, V.V. Scalable synthesis of delafossite CuAlO2 nanoparticles for p-type dye-sensitized solar cells applications. J. Alloys Compd. 2014, 591, 275–279. [Google Scholar] [CrossRef]
- Ruttanapun, C.; Kosalwat, W.; Rudradawong, C.; Jindajitawat, P.; Buranasiri, P.; Naenkieng, D.; Boonyopakorn, N.; Harnwunggmoung, A.; Thowladda, W.; Neeyakorn, W.; et al. Reinvestigation Thermoelectric Properties of CuAlO2. Energy Procedia 2014, 56, 65–71. [Google Scholar] [CrossRef]
- Byrne, D.; Cowley, A.; McNally, P.; McGlynn, E. Dellafossite CuAlO2 film growth and conversion to Cu-Al2O3 metal ceramic composite via control of annealing atmospheres. Crystengcomm 2013, 15, 6144–6150. [Google Scholar] [CrossRef][Green Version]
- Kumekawa, Y.; Hirai, M.; Kobayashi, Y.; Endoh, S.; Oikawa, E.; Hashimoto, T. Evaluation of thermodynamic and kinetic stability of CuAlO2 and CuGaO2. J. Therm. Anal. Calorim. 2010, 99, 57–63. [Google Scholar] [CrossRef]
- Amrute, A.P.; Łodziana, Z.; Mondelli, C.; Krumeich, F.; Pérez-Ramírez, J. Solid-State Chemistry of Cuprous Delafossites: Synthesis and Stability Aspects. Chem. Mater. 2013, 25, 4423–4435. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, M.; Zheng, M.; Hou, Y. Improvement in electrical properties of thermoelectric CuAlO2 ceramics aided by aluminosilicate glass. Int. J. Appl. Ceram. Technol. 2018, 15, 1301–1309. [Google Scholar] [CrossRef]
- Mudenda, S.; Kale, G.M.; Hara, Y.R.S. Rapid synthesis and electrical transition in p-type delafossite CuAlO2. J. Mater. Chem. C 2014, 2, 9233–9239. [Google Scholar] [CrossRef]
- Thu, T.V.; Thanh, P.D.; Suekuni, K.; Hai, N.H.; Mott, D.; Koyano, M.; Maenosono, S. Synthesis of delafossite CuAlO2 p-type semiconductor with a nanoparticle-based Cu(I) acetate-loaded boehmite precursor. Mater. Res. Bull. 2011, 46, 1819–1827. [Google Scholar] [CrossRef]
- Vojisavljević, K.; Malič, B.; Senna, M.; Drnovšek, S.; Kosec, M. Solid state synthesis of nano-boehmite-derived CuAlO2 powder and processing of the ceramics. J. Eur. Ceram. Soc. 2013, 33, 3231–3241. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bertapelle, M.; Bottaro, G.; Sada, C.; Tondello, E. A sol–gel approach to nanophasic copper oxide thin films. Thin Solid Film. 2003, 442, 48–52. [Google Scholar] [CrossRef]
- Das, B.; Renaud, A.; Volosin, A.M.; Yu, L.; Newman, N.; Seo, D.-K. Nanoporous Delafossite CuAlO2 from Inorganic/Polymer Double Gels: A Desirable High-Surface-Area p-Type Transparent Electrode Material. Inorg. Chem. 2015, 54, 1100–1108. [Google Scholar] [CrossRef]
- Ishiguro, T.; Kitazawa, A.; Mizutani, N.; Kato, M. Single-crystal growth and crystal structure refinement of CuAlO2. J. Solid State Chem. 1981, 40, 170–174. [Google Scholar] [CrossRef]
- Shannon, R.D.; Rogers, D.B.; Prewitt, C.T.; Gillson, J.L. Chemistry of noble metal oxides. III. Electrical transport properties and crystal chemistry of ABO2 compounds with the delafossite structure. Inorg. Chem. 1971, 10, 723–727. [Google Scholar] [CrossRef]
- Benko, F.A.; Koffyberg, F.P. Opto-electronic properties of CuAlO2. J. Phys. Chem. Solids 1984, 45, 57–59. [Google Scholar] [CrossRef]
- Ghosh, C.K.; Popuri, S.R.; Mahesh, T.U.; Chattopadhyay, K.K. Preparation of nanocrystalline CuAlO2 through sol–gel route. J. Sol-Gel Sci. Technol. 2009, 52, 75–81. [Google Scholar] [CrossRef]
- Jiang, H.F.; Zhu, X.B.; Lei, H.C.; Li, G.; Yang, Z.R.; Song, W.H.; Dai, J.M.; Sun, Y.P.; Fu, Y.K. Effect of Cr doping on the optical–electrical property of CuAlO2 thin films derived by chemical solution deposition. Thin Solid Film. 2011, 519, 2559–2563. [Google Scholar] [CrossRef]
- Li, G.; Zhu, X.; Lei, H.; Jiang, H.; Song, W.; Yang, Z.; Dai, J.; Sun, Y.; Pan, X.; Dai, S. Preparation and characterization of CuAlO2 transparent thin films prepared by chemical solution deposition method. J. Sol-Gel Sci. Technol. 2010, 53, 641–646. [Google Scholar] [CrossRef]
- Ghahari, S.; Ghafari, E.; Assi, L. Pore structure of cementitious material enhanced by graphitic nanomaterial: A critical review. Front. Struct. Civ. Eng. 2018, 12, 137–147. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, T.Y.; Kim, D. Anisotropic electrical conductivity of delafossite-type CuAlO2 laminar crystal. Appl. Phys. Lett. 2001, 79, 2028–2030. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghotbi, S.; Mousa, M.A.; Assi, L.N.; Ghahari, S. Effect of Sintering Temperature on the Properties of CuAlO2 Synthesized from Nanosized Precursors for Application in Smart Infrastructure Systems. Infrastructures 2022, 7, 97. https://doi.org/10.3390/infrastructures7070097
Ghotbi S, Mousa MA, Assi LN, Ghahari S. Effect of Sintering Temperature on the Properties of CuAlO2 Synthesized from Nanosized Precursors for Application in Smart Infrastructure Systems. Infrastructures. 2022; 7(7):97. https://doi.org/10.3390/infrastructures7070097
Chicago/Turabian StyleGhotbi, Shabnam, Mohammed Abbas Mousa, Lateef Najeh Assi, and SeyedAli Ghahari. 2022. "Effect of Sintering Temperature on the Properties of CuAlO2 Synthesized from Nanosized Precursors for Application in Smart Infrastructure Systems" Infrastructures 7, no. 7: 97. https://doi.org/10.3390/infrastructures7070097
APA StyleGhotbi, S., Mousa, M. A., Assi, L. N., & Ghahari, S. (2022). Effect of Sintering Temperature on the Properties of CuAlO2 Synthesized from Nanosized Precursors for Application in Smart Infrastructure Systems. Infrastructures, 7(7), 97. https://doi.org/10.3390/infrastructures7070097








