Development of a Method to Study Evaporation of a Volatile Solvent in an Isolated Subsurface Structure: A Practical Exercise in Risk Minimization
Abstract
1. Introduction
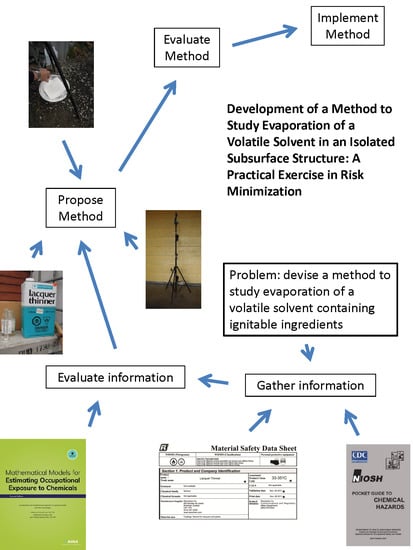
1.1. Considerations in Method Development
2. Materials and Methods
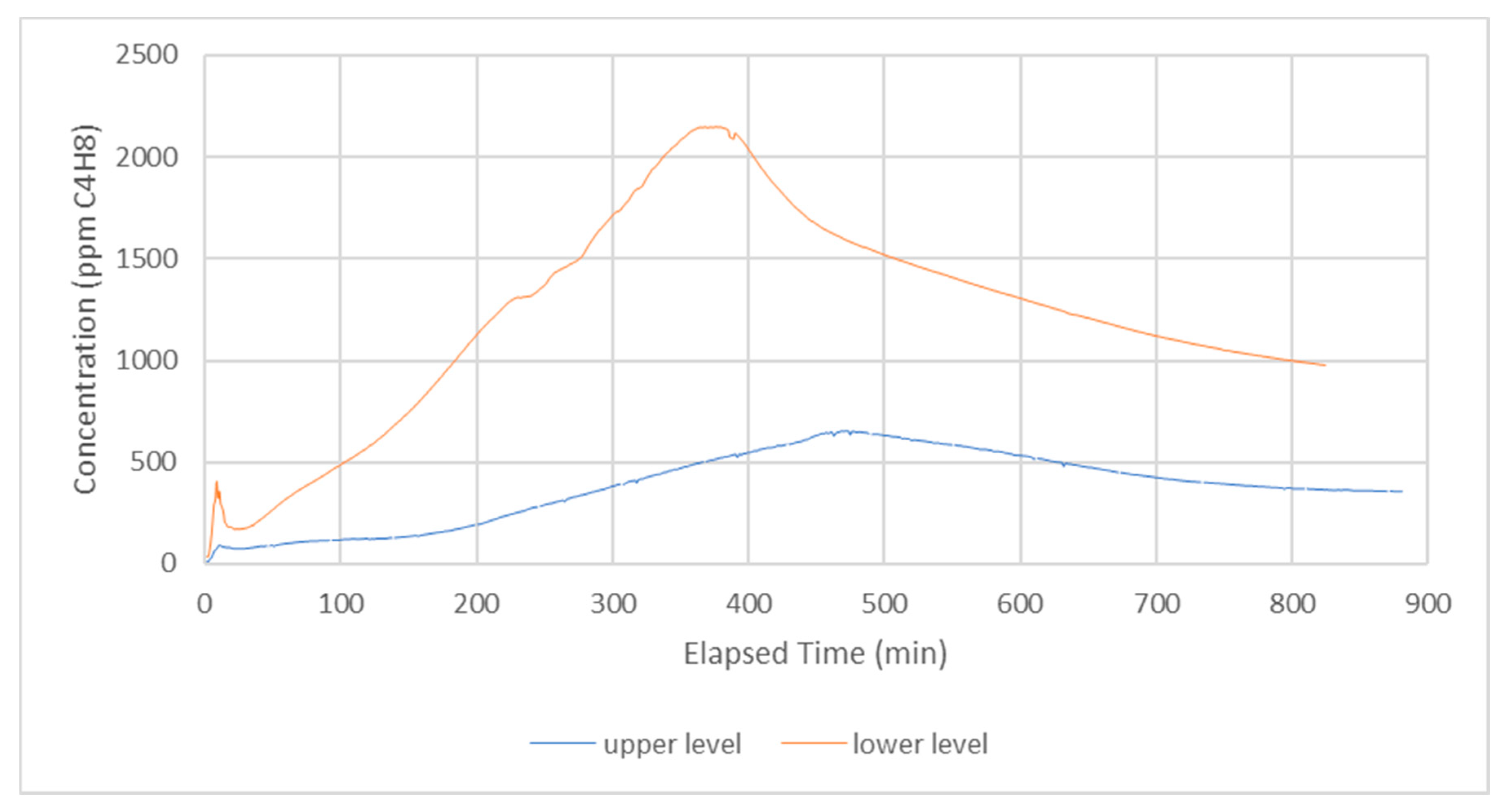
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BARPI (Bureau d’Analyse des Risques et Pollutions Industriels). Explosion of hydrocarbons in an urban sewerage network, April 22 1992, Guadalajara, Mexico. ARIA (Analyse, Recherche et Information sur les Accidents) No. 3543. Lyon, France: Direction Générale de la Prévention des Risques du ministère du Développement durable, Bureau d’Analyse des Risques et Pollutions Industriel. 2018. Available online: http://www.aria.developpement-durable.gouv.fr/wp-content/files_mf/FD_3543_guadalajara_1992_ang.pdf (accessed on 9 December 2018).
- Polonini, J. Bueiros transformam cidade em campo minado: 10 explosões que maracaram o Rio. O Globo, 13/07/2016, Rio de Janeiro. 2016. Available online: http://acervo.oglobo.globo.com/em-destaque/bueiros-transformam-cidade-em-campo-minado-10-explosoes-que-marcaram-rio-19704742 (accessed on 1 December 2018).
- Katz, S.H.; Meiter, E.R.; Bloomfield, J.J. Gas Hazards in Street Manholes (RI 2710); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1925. [Google Scholar]
- Jones, G.W.; Perrott, G.S.J. Gases in Manholes: A Survey of a Utility in Boston, Mass (RI 3109); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1931. [Google Scholar]
- IEEE (Institute of Electrical and Electronic Engineers). Preventing and mitigating manhole events. In White Paper Prepared by IEEE Insulating Conductors Committee–C34D; Institute of Electrical and Electronic Engineers: New York, NY, USA, 2015. [Google Scholar]
- Boeck, W.; Kruger, K. Arc motion and burn through in GIS. IEEE Transact. Power Deliv. 1992, 7, 254–261. [Google Scholar] [CrossRef]
- Jones, G.W.; Miller, W.E.; Campbell, J.; Yant, W.P. Ventilation of Manholes. Effect of Holes in the Covers on Natural Ventilation (RI 3307); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1936; Issue 1. [Google Scholar]
- Jones, G.W.; Miller, W.E.; Campbell, J.; Yant, W.P. Ventilation of Manholes. Effect of the Size of the Manhole on Natural Ventilation (RI 3343); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1937; Issue 2. [Google Scholar]
- Jones, G.W.; Baker, E.S.; Campbell, J. BM Ventilation of MANHOLES. Effect of Wind Velocity on Natural Ventilation (RI 3412); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1938; Issue 3. [Google Scholar]
- Jones, G.W.; Miller, W.E.; Campbell, J. BM Ventilation of Manholes. Effect of Vertical Ducts in Combination with Openings in Manhole Covers on the Natural Ventilation (RI 3496); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1940; Issue 4. [Google Scholar]
- McManus, T.N. Natural Ventilation of Isolated Subsurface Structures in the Infrastructure. D.Sc. Thesis, Universidade Federal Fluminense, Programa de Pós-Graduação em Engenharia Civil, Niteroí, Brasil, 2016. [Google Scholar]
- McManus, N. Safety and Health in Confined Spaces; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). Worker Deaths in Confined Spaces (DHHS/PHS/CDC/NIOSH Pub. No. 94-103); Department of Health and Human Services, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 1994. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). Carbon Monoxide Poisoning and Death after the Use of Explosives in a Sewer Construction Project (DHHS (NIOSH) Publication No. 98-122); Department of Health and Human Services, National Institute for Occupational Health: Cincinnati, OH, USA, 1998. [Google Scholar]
- NIOSH. Suspected Inhalation Fatalities Involving Workers during Manual Tank Gauging, Sampling, and Fluid Transfer Operations on Oil and Gas Well Sites, 2010–2014; Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2015. Available online: http://www.cdc.gov/niosh/topics/fog/data.html (accessed on 19 January 2019).
- NIOSH/OSHA. Health and Safety Risks for Workers Involved in Manual Tank Gauging and Sampling at Oil and Gas Extraction Sites (DHHS (NIOSH) Publication Number 2016-108/ OSHA-HA-3843-2016); Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2016. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). Fatality Assessment and Control Evaluation (FACE) Program; Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2018; Available online: www.cdc.gov/niosh/face/ (accessed on 9 December 2018).
- OSHA (Occupational Safety and Health Administration). Selected Occupational Fatalities Related to Toxic and Asphyxiating Atmospheres in Confined Work Spaces as Found in Reports of OSHA Fatality/Catastrophe Investigations; U.S. Department of Labor, Occupational Safety and Health Administration (U.S. DOL/OSHA): Washington, DC, USA, 1985. [Google Scholar]
- OSHA (Occupational Safety and Health Administration). Fatality and Catastrophe Investigation Summaries; U.S. Department of Labor, Occupational Safety and Health Administration (U.S. DOL/OSHA): Washington, DC, USA, 2018; Available online: www.osha.gov/pls/imis/accidentsearch.html (accessed on 9 December 2018).
- Smith, P.A.; Lockhart, B.; Besser, B.W.; Michalskic, M.A.R. Exposure of unsuspecting workers to deadly atmospheres in below-ground confined spaces and investigation of related whole-air sample composition using adsorption gas chromatography. J. Occup. Environ. Hyg. 2014, 11, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.; Dunne, S.P. Radon in the workplace—A study of underground BT structures. Ann. Occup. Hyg. 1996, 40, 569–581. [Google Scholar] [CrossRef]
- Keil, C.B.; Simmons, C.E.; Anthony, T.R. Mathematical Models for Estimating Occupational Exposure to Chemicals, 2nd ed.; American Industrial Hygiene Association: Fairfax, VA, USA, 2009. [Google Scholar]
- Recochem. Material Safety Data Sheet for Lacquer Thinner; Recochem: Montreal, QC, Canada, 2011; Available online: http://canadiantire.wercsmart.com/SDSviewer/awsClient/document?productid=91002360&productguid=4df2ef6c-7e4f-47b2-9a77-cf3cce024580&DocumentKey=4df2ef6c-7e4f-47b2-9a77-cf3cce024580&HazdocumentKey=&MSDS=3 (accessed on 9 December 2018).
- AIHA (American Industrial Hygiene Association). IH Mod (Industrial Hygiene Models), 2.0, Support File, Generation Rate Estimation; American Industrial Hygiene Association: Falls Church, VA, USA, 2018. Available online: https://www.aiha.org/get-involved/VolunteerGroups/Pages/Exposure-Assessment-Strategies-Committee.aspx (accessed on 9 December 2018).
- Lira, C.T.; Elliot, J.R. ACTCOEFF.xls Spreadsheet. In For Use with Introductory Chemical Engineering Thermodynamics, 2nd ed.; Elliot, J.R., Lira, C.T., Eds.; Prentice Hall: New York, NY, USA, 2012; Available online: https://sourceforge.net/projects/chethermo/ (accessed on 17 December 2018).
- Randhol, P.; Engelien, H.K. Xlunifac, a Program for Calculation of Liquid Activity Coefficients Using the UNIFAC Model, Version 1.0. 2000. Available online: http://www.pvv.org/~randhol/xlunifac/ (accessed on 9 February 2019).
- McManus, N.; Green, G. The WHMIS Training Program; Training by Design, Inc.: North Vancouver, BC, Canada, 2001. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). NIOSH Pocket Guide to Chemical Hazards, DHHS (NIOSH) Publication No. 2005-149; August 2006, with Minor Technical Changes; September 2007, with Minor Technical Changes; Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2007. [Google Scholar]
- Zabetakis, M.G. Flammability Characteristics of Combustible Gases and Vapors (Bull. 627); Department of the Interior, Bureau of Mines: Washington, DC, USA, 1967. [Google Scholar]
- ASTM (American Society for Testing and Materials). Standard Test Methods for Evaporation Rates of Volatile Liquids by Shell Thin-Film Evaporometer, ASTM D3539-11; American Society for Testing and Material: West Conshohocken, PA, USA, 2011; (Withdrawn 2015). [Google Scholar]
- Eastman Chemical Company. Solvent Selector Chart; Eastman Chemical Company: Kingsport, TN, USA, 2018. [Google Scholar]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics; Hodder and Stoughton: London, UK, 1990. [Google Scholar]
- Fingas, M. Evaporation modeling. In Oil Spill Science and Technology; Fingas, M., Ed.; Gulf Publishing: New York, NY, USA, 2011. [Google Scholar]
- Fingas, M. Modeling oil and petroleum evaporation. J. Petrol. Sci. Res. 2013, 2, 104–115. [Google Scholar]
- Fingas, M.F. (Ed.) Oil and petroleum evaporation. In Handbook of Oil Spill Science and Technology; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Chou, J. Hazardous Gas Monitors: A Practical Guide to Selection, Operation, and Applications; McGraw-Hill Professional: New York, NY, USA, 1999. [Google Scholar]
- GfG Instrumentation. Using Photoionization Detectors to Measure Toxic VOCs (AP 1021); GfG Instrumentation: Ann Arbor, MI, USA, 2013; Available online: https://goodforgas.com/wp-content/uploads/2013/12/AP1021_Using_PIDs_to_measure_toxic_VOCs_10_10_13.pdf (accessed on 21 March 2019).
- GfG Instrumentation. Chemical Ionization Potential (eV) and 10.6 eV Correction Factors (CF) (TN2004); GfG Instrumentation: Ann Arbor, MI, USA, 2009; Available online: https://goodforgas.com/wp-content/uploads/2013/12/TN2004_PID_gas_table_01_16_09.pdf (accessed on 7 December 2018).
- McManus, T.N.; Haddad, A. Ventilation of an isolated subsurface structure induced by natural forces. Infrastructures 2019, 4, 33. [Google Scholar] [CrossRef]
- McManus, T.N.; Haddad, A. Solvent evaporation in an isolated subsurface structure: An unrecognized and underappreciated risk. Infrastructures 2019, 4, 47. [Google Scholar] [CrossRef]
- McManus, T.N.; Haddad, A. Short-duration characterization of source emissions for use in predictive software models to assess worker exposure: A note of caution. Environ. Nat. Resour. Res. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- AIHA (American Industrial Hygiene Association). Odor Thresholds for Chemicals with Established Occupational Health Standards, 2nd ed.; American Industrial Hygiene Association: Fairfax, VA, USA, 2013. [Google Scholar]
- Reason, J. Human Error; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Coole, M.; Corkell, J.; Woodward, A. Defence in depth, protection in depth and security in depth: A comparative analysis towards a common usage language. In Proceedings of the 5th Australian Security and Intelligence Conference, Novotel Langley Hotel, Perth, WA, Australia, 5–7 December 2012. [Google Scholar]
- McManus, T.N.; Haddad, A.N. Visualizing air motion involving isolated subsurface structures: A critical tool for understanding ventilation induced by natural forces. Infrastructures 2019, 4, 59. [Google Scholar] [CrossRef]

| Substance | Wt | Basis | MW | Mol | MoleFraction | VPsat | γ | VP Predicted | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (g) | (g) | xi | (mmHg) | (mmHg) | |||||
| 13 °C | 25 °C | 13 °C | 25 °C | |||||||
| Toluene | 60–80 | 70 | 92 | 0.8 | 0.6 | 14.5 | 28 | 1.3 | 10.7 | 20.6 |
| MEK | 10–20 | 15 | 72 | 0.2 | 0.2 | 56.3 | 98.9 | 1.0 | 8.6 | 15.0 |
| Methanol | 5–10 | 7 | 32 | 0.2 | 0.2 | 63.8 | 124 | 2.7 | 28.7 | 55.9 |
| Acetone | 1–9 | 8 | 58 | 0.1 | 0.1 | 143 | 238 | 1.1 | 16.8 | 27.8 |
| Total | 100 | 1.3 | 1.0 * | 64.8 | 119.3 | |||||
| Substance | Flash Point | Lower Flammable Limit (25 °C) | Evaporation Rate Factor n-BuAc = 1 | GfG PID Response Factor | |
|---|---|---|---|---|---|
| °C | % | g/m3 | |||
| Toluene | 4 | 1.3 | 48 | 1.9 | 0.5 |
| MEK | −9 | 1.9 | 56 | 3.8 | 0.9 |
| Methanol | 11 | 6.7 | 88 | 3.5 | NA |
| Acetone | −18 | 2.6 | 62 | 6.3 | 1.2 |
| n-heptane | 3.9 | ||||
| n-decane | 0.1 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McManus, T.N.; Rosa, A.; Haddad, A. Development of a Method to Study Evaporation of a Volatile Solvent in an Isolated Subsurface Structure: A Practical Exercise in Risk Minimization. Infrastructures 2020, 5, 68. https://doi.org/10.3390/infrastructures5080068
McManus TN, Rosa A, Haddad A. Development of a Method to Study Evaporation of a Volatile Solvent in an Isolated Subsurface Structure: A Practical Exercise in Risk Minimization. Infrastructures. 2020; 5(8):68. https://doi.org/10.3390/infrastructures5080068
Chicago/Turabian StyleMcManus, Thomas Neil, Ana Rosa, and Assed Haddad. 2020. "Development of a Method to Study Evaporation of a Volatile Solvent in an Isolated Subsurface Structure: A Practical Exercise in Risk Minimization" Infrastructures 5, no. 8: 68. https://doi.org/10.3390/infrastructures5080068
APA StyleMcManus, T. N., Rosa, A., & Haddad, A. (2020). Development of a Method to Study Evaporation of a Volatile Solvent in an Isolated Subsurface Structure: A Practical Exercise in Risk Minimization. Infrastructures, 5(8), 68. https://doi.org/10.3390/infrastructures5080068