On the Theoretical CO2 Sequestration Potential of Pervious Concrete
Abstract
:1. Introduction
1.1. CO2 Emissions and CO2 Sequestration
1.2. Scope of Work
2. Computational Methods
2.1. Theoretical Formulation
2.1.1. Chemical and Mineral Composition of OPC
2.1.2. Cement Hydration Reactions
2.1.3. Carbonation Reaction
2.1.4. CO2 Sequestration Potential of Cement Paste
2.1.5. Carbonation Depth
2.1.6. Total Carbonated Volume
2.1.7. Total Mass of Sequestered CO2
2.2. Carbonation Model Implementation
2.3. Lifecycle Assessment (LCA) Methodology
2.3.1. Goal and Scope Definition
2.3.2. Lifecycle Inventory (LCI) Data
2.3.3. Limitations of the Study
- As a screening LCA, emissions associated with construction and transportation (A4 and A5) were not considered. Only lifecycle stages A1–A3 and B1 were included in the scope of this study. To perform a complete LCA specific to a building project using pervious concrete, these stages should be included in the system boundary.
- Only CH (i.e., portlandite) was considered to carbonate in the model used by this study. While other calcium silicate phases also have the potential to carbonate (as discussed), thus it is conservative to not consider their CO2 uptake.
- This study assumed that all cement paste carbonates fully to report a conservative theoretical maximum. It has been shown that the actual degree of carbonation is less than 1.0 and may likely vary from 0.2 to 0.7 as previously discussed.
- Due to limitations in IE4B, aggregate size was not differentiated in the formulation of the mix designs in the User Defined Concrete Mix Design Library. While different aggregate sizes require different manufacturing processes, for this study, “Coarse Aggregate Natural” was used as the input for the IE4B software. It is expected that smaller aggregate sizes will require a marginal increase in manufacturing emissions, but are ignored in this study.
3. Results and Discussion
3.1. Initial CO2 e Emissions
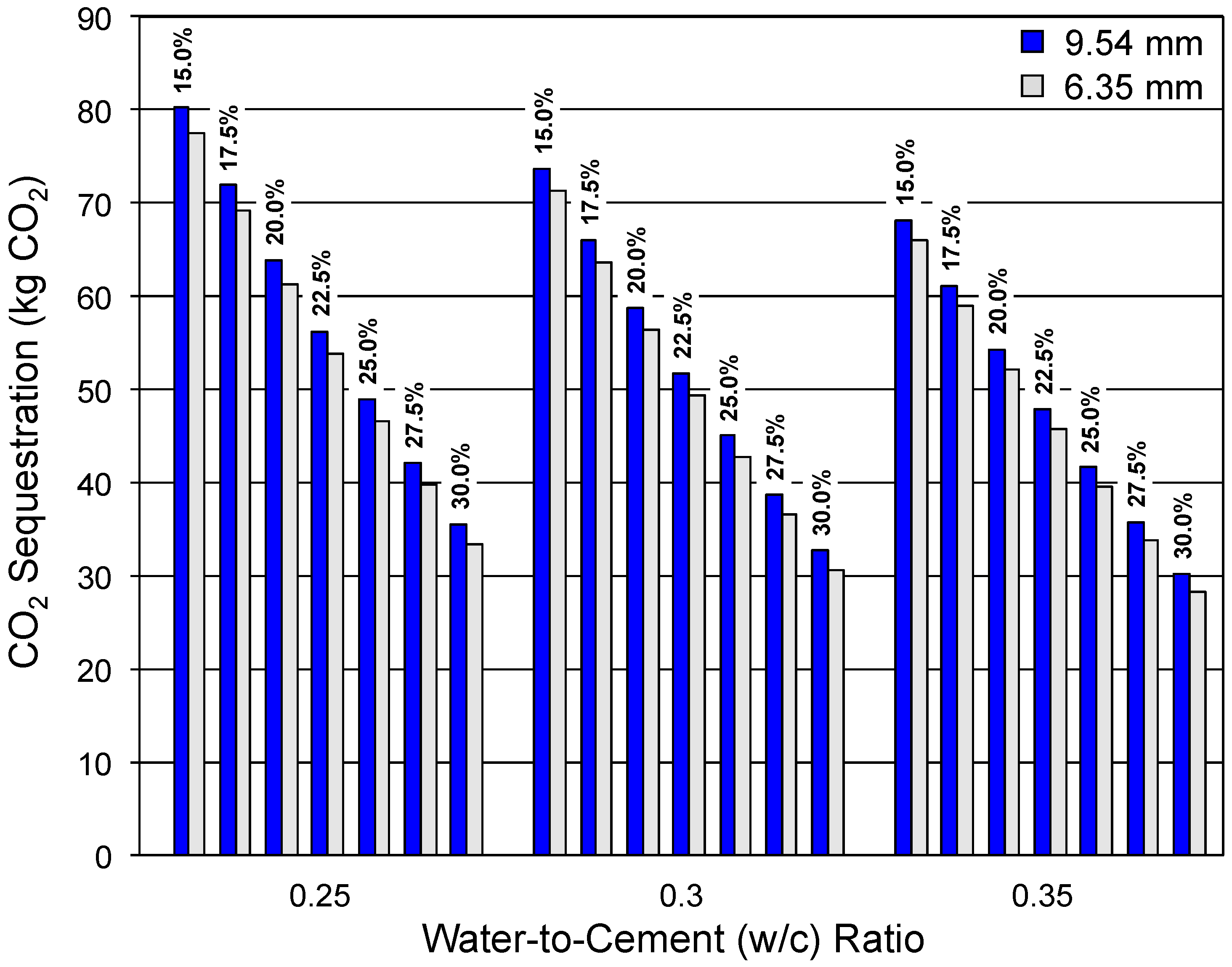
3.2. CO 2 Sequestration Potential
3.2.1. Effect of w/c Ratio
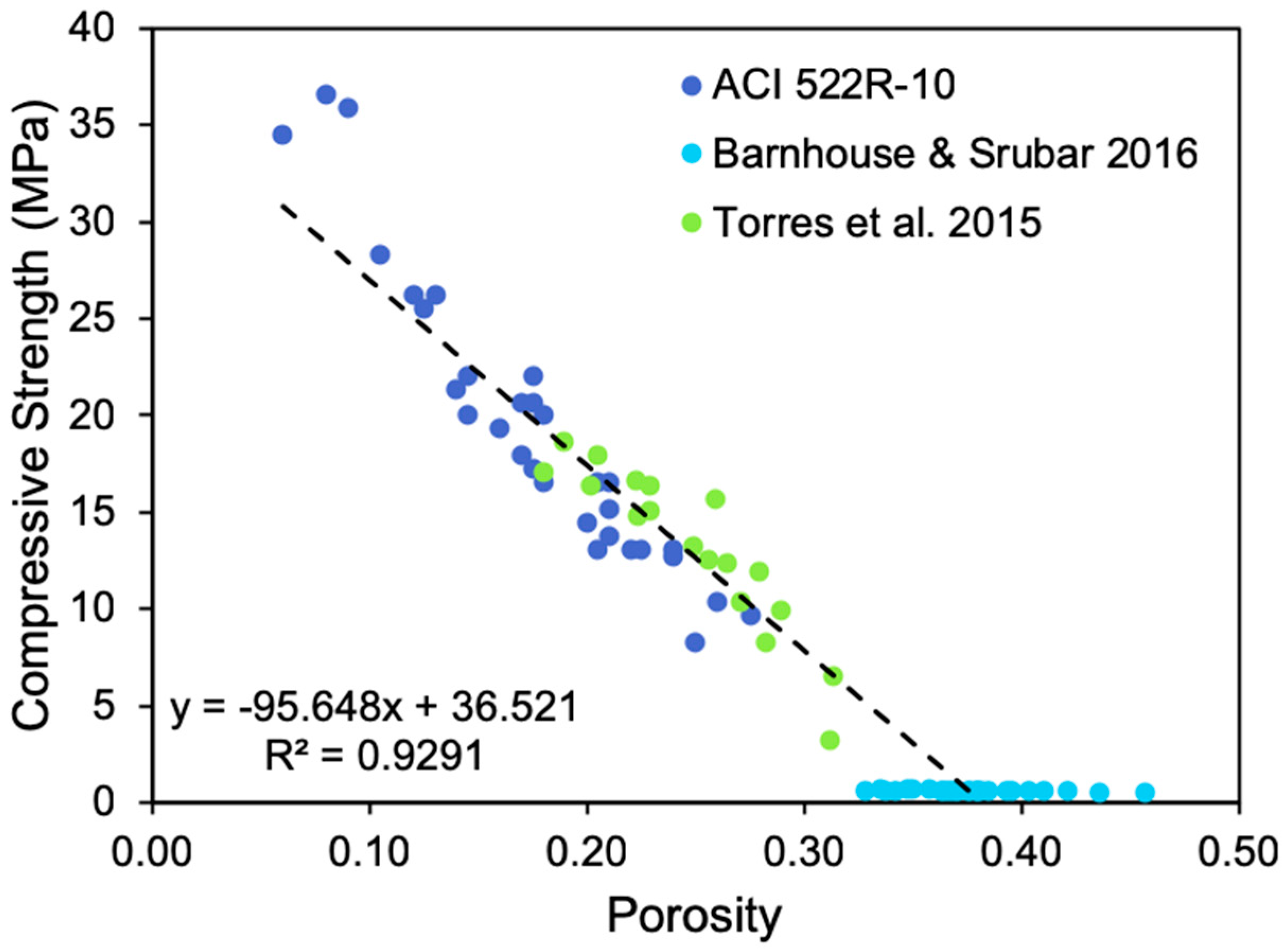
3.2.2. Effect of Compressive Strength
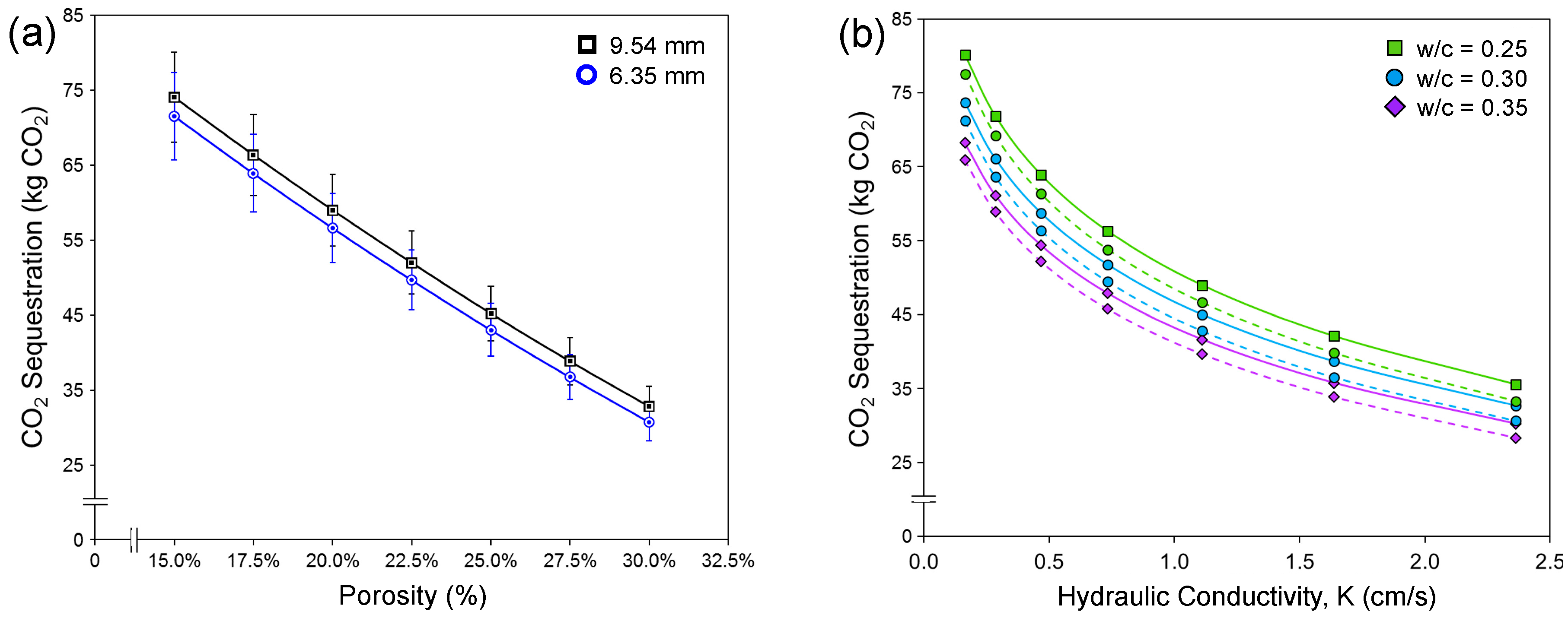
3.2.3. Effect of Design Porosity and Hydraulic Conductivity
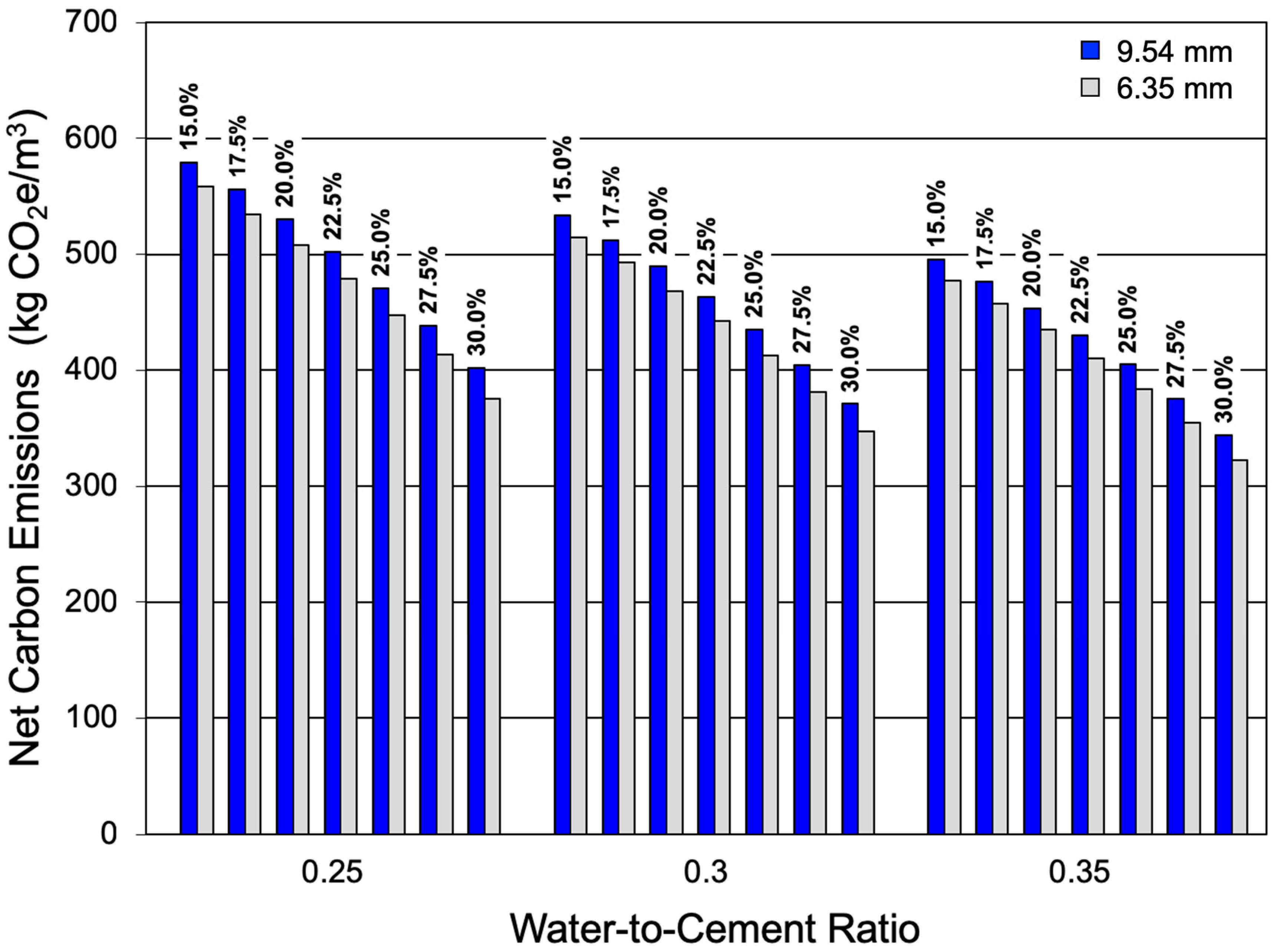
3.3. Net Lifecycle (and Recoverable) CO2 Emissions
4. Conclusions
- The maximum amount of initial CO2 emissions of pervious concrete that can be recovered through CO2 sequestration is estimated to be approximately 12%.
- Higher w/c ratios, design porosities, and hydraulic conductivities correspond to decreased CO2 sequestration potential, while higher compressive strength corresponds to higher CO2 sequestration potential. Aggregate size imparts a negligible effect.
- LCA results indicate that net CO2e emissions decline with increases in w/c ratio, design porosity, and hydraulic conductivity, while increased cement content increases both CO2 sequestration potential and, to a greater extent, initial CO2e emissions.
- Mixtures with higher cement contents always exhibit higher net CO2e emissions despite their increased CO2 sequestration potential.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poh, S.; Zhao, P.; Chen, C.; Goh, Y.; Adebayo, H.; Hung, K.; Wah, C. Characterization of pervious concrete with blended natural aggregate and recycled concrete aggregates. J. Clean. Prod. 2018, 181, 155–165. [Google Scholar]
- Carsana, M.; Tittarelli, F.; Bertolini, L. Use of no-fines concrete as a building material: Strength, durability properties and corrosion protection of embedded steel. Cem. Concr. Res. 2013, 48, 64–73. [Google Scholar] [CrossRef]
- Tamai, M.; Mizuguchi, H.; Hatanaka, S.; Katahira, H.; Nakazawa, T.; Yanagibashi, K.; Kunieda, M. Design, construction and recent applications of porous concrete in Japan. In Proceedings of the JCI Symposium on Design, Construction, and Recent Applications of Porous Concrete; Japan Concrete Institute: Tokyo, Japan, 2004. [Google Scholar]
- American Concrete Institute (ACI). ACI 522R-10: Report on Pervious Concrete; American Concrete Institute (ACI): Farmington Hills, MI, USA, 2010; Volume 10. [Google Scholar]
- Welker, A.L.; Barbis, J.D.; Jeffers, P.A. A side-by-side comparison of pervious concrete and porous asphalt. J. Am. Water Resour. Assoc. 2012, 48, 809–819. [Google Scholar] [CrossRef]
- Haselbach, L.; Boyer, M.; Kevern, J.; Schaefer, V. Cyclic heat island impacts on traditional versus pervious concrete pavement systems. Transp. Res. Rec. J. Transp. Res. Board 2011, 2240, 107–115. [Google Scholar] [CrossRef]
- Horst, M.; Welker, A.L.; Traver, R. Multiyear performance of a pervious concrete infiltration basin BMP. J. Irrig. Drain. Eng. 2011, 137, 352–358. [Google Scholar] [CrossRef]
- Nemirovsky, E.M.; Welker, A.L.; Lee, R. Quantifying evaporation from pervious concrete systems: Methodology and hydrologic perspective. J. Irrig. Drain. Eng. 2012, 139, 271–277. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Welker, A.L.; Traver, R.G.; Vanacore, M.; Ladd, T. Evaluation of an infiltration best management practice utilizing pervious concrete. J. Am. Water Resour. Assoc. 2008, 43, 1208–1222. [Google Scholar] [CrossRef]
- Torres, A.; Hu, J.; Ramos, A. The effect of the cementitious paste thickness on the performance of pervious concrete. Constr. Build. Mater. 2015, 95, 850–859. [Google Scholar] [CrossRef]
- Neithalath, N.; Sumanasooriya, M.S.; Deo, O. Characterizing pore volume, sizes, and connectivity in pervious concretes for permeability prediction. Mater. Charact. 2010, 61, 802–813. [Google Scholar] [CrossRef]
- Huang, B.; Wu, H.; Shu, X.; Burdette, E.G. Laboratory evaluation of permeability and strength of polymer-modified pervious concrete. Constr. Build. Mater. 2010, 24, 818–823. [Google Scholar] [CrossRef]
- Zhong, R.; Wille, K. Compression response of normal and high strength pervious concrete. Constr. Build. Mater. 2016, 109, 177–187. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Carbon 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Res. 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Gajda, J.; Miller, F.M. Concrete as a Sink for Atmospheric Carbon Dioxide: A Literature Review and Estimation of CO2 Absorption by Portland Cement Concrete; Portland Cement Association: Skokie, IL, USA, 2000. [Google Scholar]
- Yang, K.H.; Seo, E.A.; Tae, S.H. Carbonation and CO2 uptake of concrete. Environ. Impact Assess. Rev. 2014, 46, 43–52. [Google Scholar] [CrossRef]
- García-Segura, T.; Yepes, V.; Alcalá, J. Life cycle greenhouse gas emissions of blended cement concrete including carbonation and durability. Int. J. Life Cycle Assess. 2014, 19, 3–12. [Google Scholar] [CrossRef]
- Souto-Martinez, A.; Delesky, E.A.; Foster, K.E.O.; Srubar, W.V. Accounting for the carbon sequestration potential of reinforced concrete in a whole-building life-cycle assessment. In AEI 2017; American Society of Civil Engineers: Reston, VA, USA, 2017; pp. 285–298. [Google Scholar]
- Souto-Martinez, A.; Arehart, J.H.; Srubar, W.V. Cradle-to-gate CO2e emissions vs. in situ CO2 sequestration of structural concrete elements. Energy Build. 2018, 167, 301–311. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar]
- Collins, F. Inclusion of carbonation during the life cycle of built and recycled concrete: Influence on their carbon footprint. Int. J. Life Cycle Assess. 2010, 15, 549–556. [Google Scholar] [CrossRef]
- Lagerblad, B. Carbon Dioxide Uptake during Concrete Life Cycle—State of the Art; Nordic Innovation Centre: Oslo, Norway, 2006; Volume CBI 2005:2, ISBN 9197607002. [Google Scholar]
- ASTM C150/C150M-18 Standard Specification for Portland Cement. ASTM Int. 2018, 1–9.
- Mehta, P.K. Concrete Structure, Properties and Materials. 1986. Available online: https://trid.trb.org/view/273357 (accessed on 18 November 2017).
- Souto-Martinez, A.; Delesky, E.A.; Foster, K.E.O.; Srubar, W.V. A mathematical model for predicting the carbon sequestration potential of ordinary Portland cement (OPC) concrete. Constr. Build. Mater. 2017, 147, 417–427. [Google Scholar] [CrossRef]
- Haselbach, L.M.; Liu, L. Calcium hydroxide formation in thin cement paste exposed to air. ACI Mater. J. 2010, 107, 365–371. [Google Scholar]
- Golroo, A.; Tighe, S.L. Pervious concrete pavement performance modeling using the bayesian statistical technique. J. Transp. Eng. 2012, 138, 603–609. [Google Scholar] [CrossRef]
- Engelsen, C.J.; Justnes, H.; Rønning, A.R. The quantity of CO2 bound by concrete carbonation in Norway. In Proceedings of the Fourth International Conference on Sustainable Construction Materials and Technologies, Las Vegas, NV, USA, 7–11 August 2016. [Google Scholar]
- Fridh, K.; Lagerblad, B. Carbonation of indoor concrete: Measurements of depths and degrees of carbonation. Lund 2013. Available online: https://lup.lub.lu.se/search/publication/4390005 (accessed on 18 November 2017).
- Thiery, M.; Dangla, P.; Belin, P.; Habert, G.; Roussel, N. Carbonation kinetics of a bed of recycled concrete aggregates: A laboratory study on model materials. Cem. Concr. Res. 2013, 46, 50–65. [Google Scholar] [CrossRef]
- Van Balen, K. Carbonation reaction of lime, kinetics at ambient temperature. Cem. Concr. Res. 2005, 35, 647–657. [Google Scholar] [CrossRef]
- National Ready Mixed Concrete Association (NRMCA) Pervious Concrete: Guideline to Mixture Proportioning. Available online: https://my.nrmca.org/Main/ItemDetail?iProductCode=2PE001 (accessed on 18 November 2017).
- Barnhouse, P.W.; Srubar, W.V. Material characterization and hydraulic conductivity modeling of macroporous recycled-aggregate pervious concrete. Constr. Build. Mater. 2016, 110, 89–97. [Google Scholar] [CrossRef]
- Torres, A.; Gaedicke, C.; Hu, J.; Bejugam, R.; Mcmasters, S. Comparing Design Void Content with Actual Void Content of Laboratory Prepared Pervious Concrete. Mater. Sci. Appl. 2018, 9, 596–613. [Google Scholar]
- Joshaghani, A.; Ramezanianpour, A.A.; Ataei, O.; Golroo, A. Optimizing pervious concrete pavement mixture design by using the Taguchi method. Constr. Build. Mater. 2015, 101, 317–325. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Yan, C.; Liu, Y. Influence of crushing index on properties of recycled aggregates pervious concrete. Constr. Build. Mater. 2017, 135, 112–118. [Google Scholar] [CrossRef]
- Tennis, P.D.; Leming, M.L.; Akers, D.J. Pervious Concrete Pavements; EB302.02; Portland Cement Association: Skokie, IL, USA, 2004; ISBN 0893122424. [Google Scholar]


| Average Oxide Composition (%) | Average Mineral (Bogue) Composition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (SiO2) | A (Al2O3) | F (Fe2O3) | C (CaO) | M (MgO) | Š (SO3) | N (Na2O) | Other | C3S | C2S | C3A | C4AF | Other |
| 20.5 | 5.4 | 2.6 | 63.9 | 2.1 | 3.0 | 0.61 | 1.9 | 54 | 18 | 10 | 8 | 10 |
| w/c | Aggregate Size [mm] | Component | Design Porosity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 15.0% | 17.5% | 20.0% | 22.5% | 25.0% | 27.5% | 30.0% | |||
| 0.25 | 9.54 | Cement | 572 | 528 | 484 | 440 | 396 | 352 | 308 |
| Water | 143 | 132 | 121 | 110 | 99 | 88 | 77 | ||
| CA1 | 1349 | 1349 | 1349 | 1349 | 1349 | 1349 | 1349 | ||
| 6.35 | Cement | 553 | 508 | 465 | 421 | 377 | 333 | 288 | |
| Water | 138 | 127 | 116 | 105 | 94 | 83 | 72 | ||
| CA1 | 1383 | 1383 | 1383 | 1383 | 1383 | 1383 | 1383 | ||
| 0.3 | 9.54 | Cement | 526 | 485 | 445 | 405 | 364 | 323 | 283 |
| Water | 158 | 145 | 133 | 121 | 109 | 97 | 85 | ||
| CA1 | 1349 | 1349 | 1349 | 1349 | 1349 | 1349 | 1349 | ||
| 6.35 | Cement | 508 | 468 | 427 | 387 | 346 | 306 | 265 | |
| Water | 152 | 140 | 128 | 116 | 104 | 92 | 79 | ||
| CA1 | 1383 | 1383 | 1383 | 1383 | 1383 | 1383 | 1383 | ||
| 0.35 | 9.54 | Cement | 486 | 449 | 412 | 374 | 336 | 299 | 262 |
| Water | 170 | 157 | 144 | 131 | 118 | 105 | 91 | ||
| CA1 | 1349 | 1349 | 1349 | 1349 | 1349 | 1349 | 1349 | ||
| 6.35 | Cement | 470 | 432 | 395 | 358 | 320 | 283 | 245 | |
| Water | 164 | 151 | 138 | 125 | 112 | 99 | 86 | ||
| CA1 | 1383 | 1383 | 1383 | 1383 | 1383 | 1383 | 1383 | ||
| Aggregate Size (mm) | w/c = 0.25 | w/c = 0.30 | w/c = 0.35 | ||||
|---|---|---|---|---|---|---|---|
| Design Porosity | 9.54 | 6.35 | 9.54 | 6.35 | 9.54 | 6.35 | |
| 15.0 | 12.17% | 12.19% | 12.14% | 12.16% | 12.11% | 12.12% | |
| 17.5 | 11.45% | 11.47% | 11.42% | 11.44% | 11.37% | 11.40% | |
| 20.0 | 10.75% | 10.77% | 10.71% | 10.74% | 10.71% | 10.71% | |
| 22.5 | 10.07% | 10.09% | 10.05% | 10.06% | 10.01% | 10.03% | |
| 25.0 | 9.42% | 9.43% | 9.37% | 9.40% | 9.32% | 9.36% | |
| 27.5 | 8.76% | 8.79% | 8.73% | 8.75% | 8.71% | 8.71% | |
| 30.0 | 8.13% | 8.15% | 8.10% | 8.11% | 8.07% | 8.07% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellingboe, E.; Arehart, J.H.; Srubar, W.V., III. On the Theoretical CO2 Sequestration Potential of Pervious Concrete. Infrastructures 2019, 4, 12. https://doi.org/10.3390/infrastructures4010012
Ellingboe E, Arehart JH, Srubar WV III. On the Theoretical CO2 Sequestration Potential of Pervious Concrete. Infrastructures. 2019; 4(1):12. https://doi.org/10.3390/infrastructures4010012
Chicago/Turabian StyleEllingboe, Ethan, Jay H. Arehart, and Wil V. Srubar, III. 2019. "On the Theoretical CO2 Sequestration Potential of Pervious Concrete" Infrastructures 4, no. 1: 12. https://doi.org/10.3390/infrastructures4010012
APA StyleEllingboe, E., Arehart, J. H., & Srubar, W. V., III. (2019). On the Theoretical CO2 Sequestration Potential of Pervious Concrete. Infrastructures, 4(1), 12. https://doi.org/10.3390/infrastructures4010012