Design of an Experimental System for the Assessment of the Drug Loss in Drug-Coated Balloons Due to Washing Off During Tracking
Abstract
1. Introduction
2. Materials and Methods
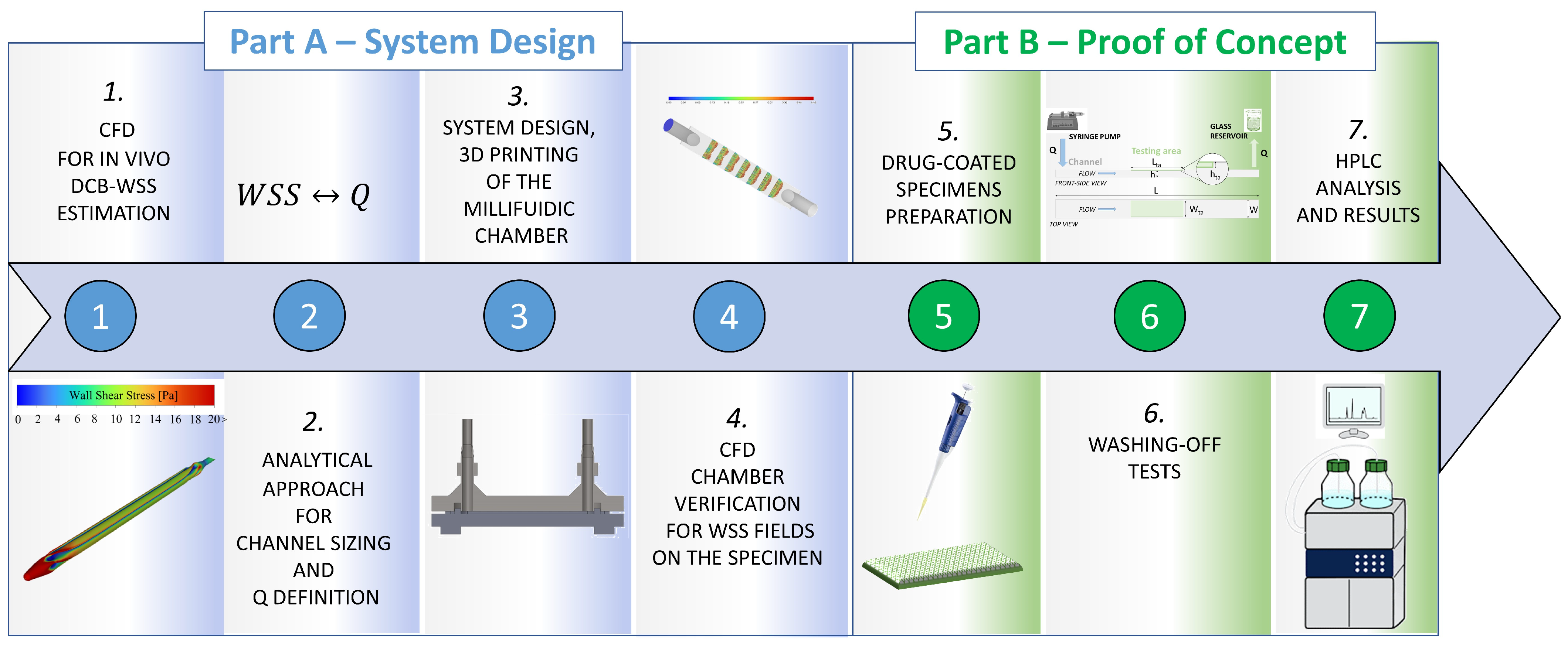
2.1. General Concept and Workflow of the Study
2.2. Part A—System Design
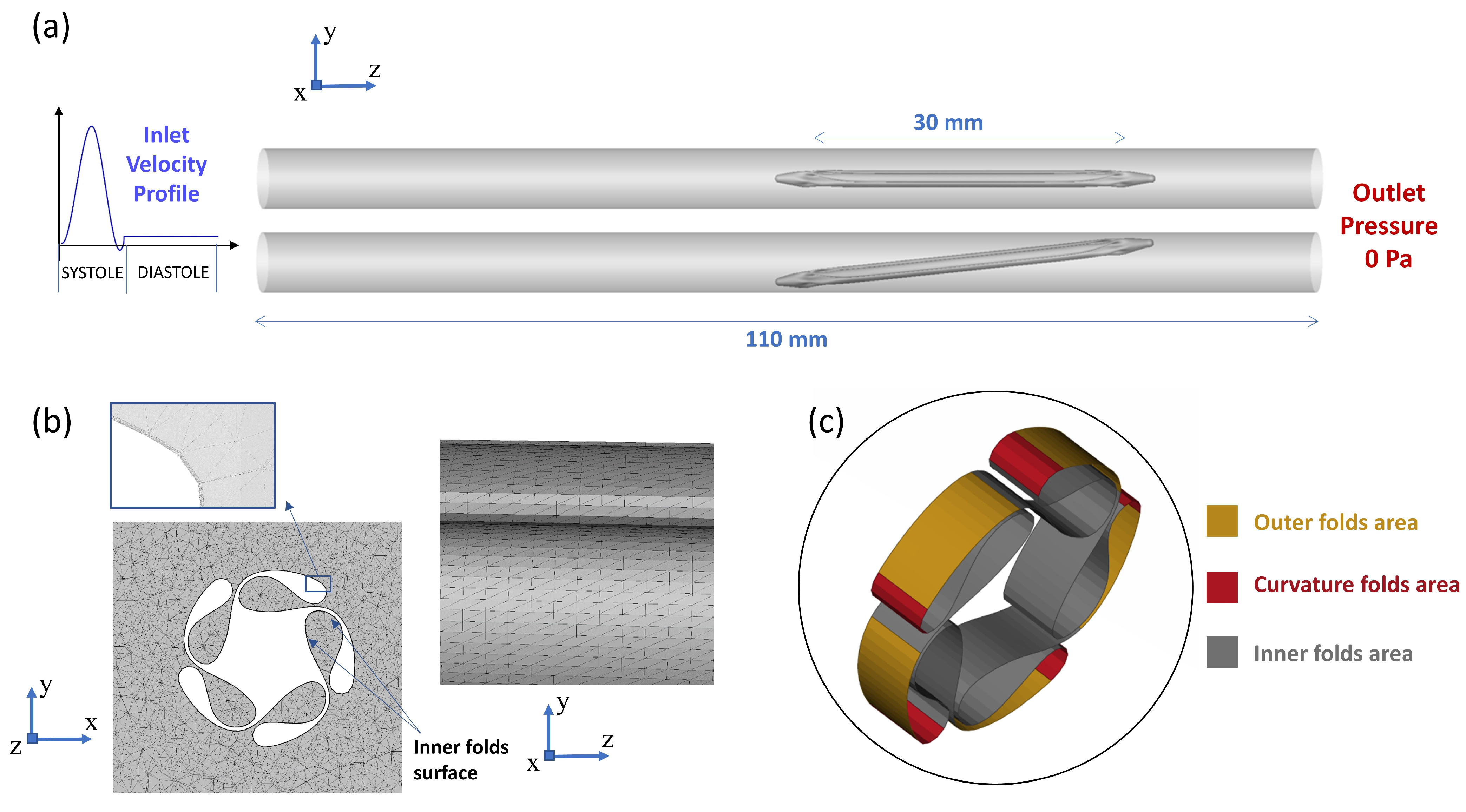
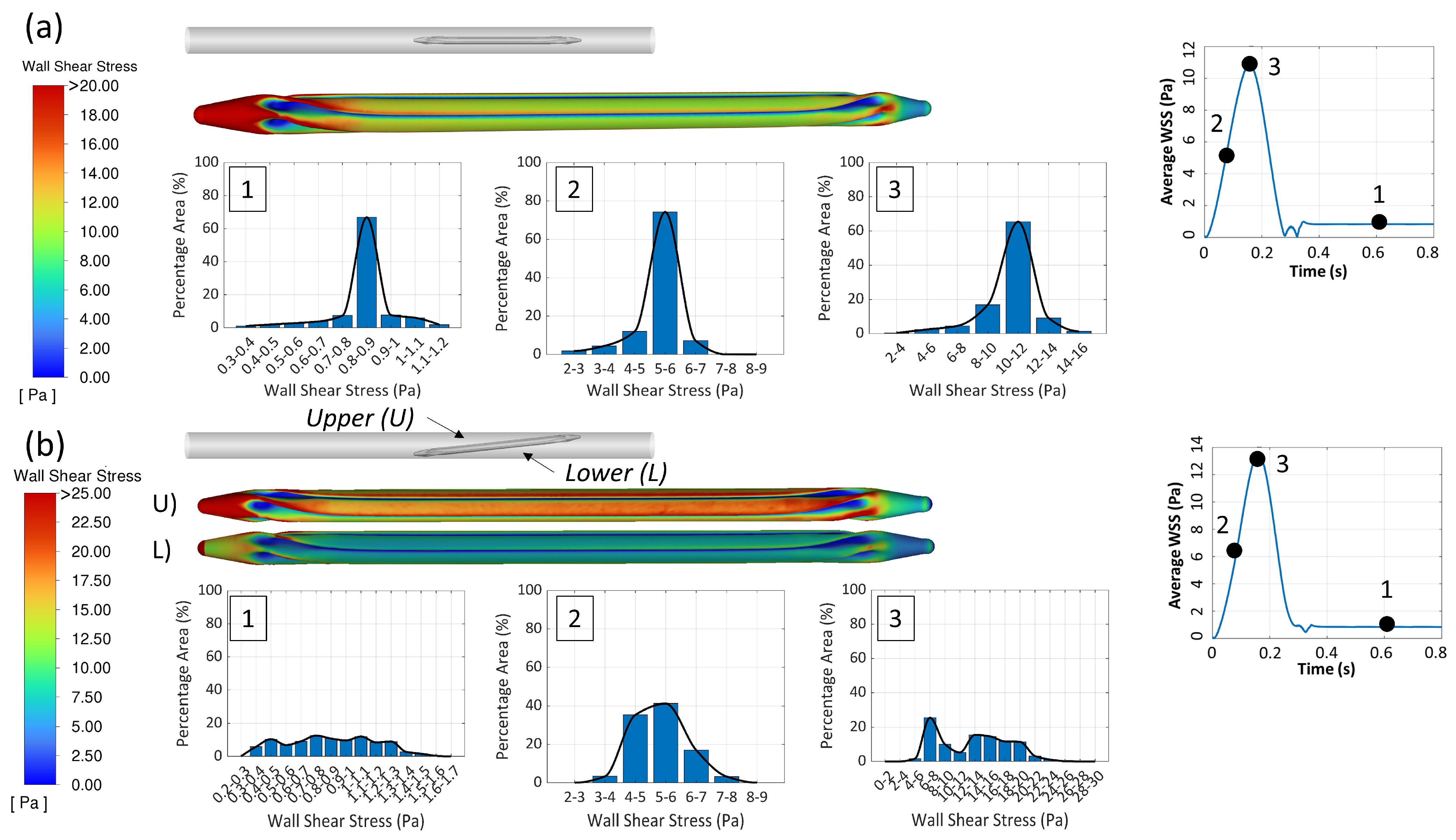
2.2.1. The 3D CFD Simulation of the Folded Balloon During Tracking
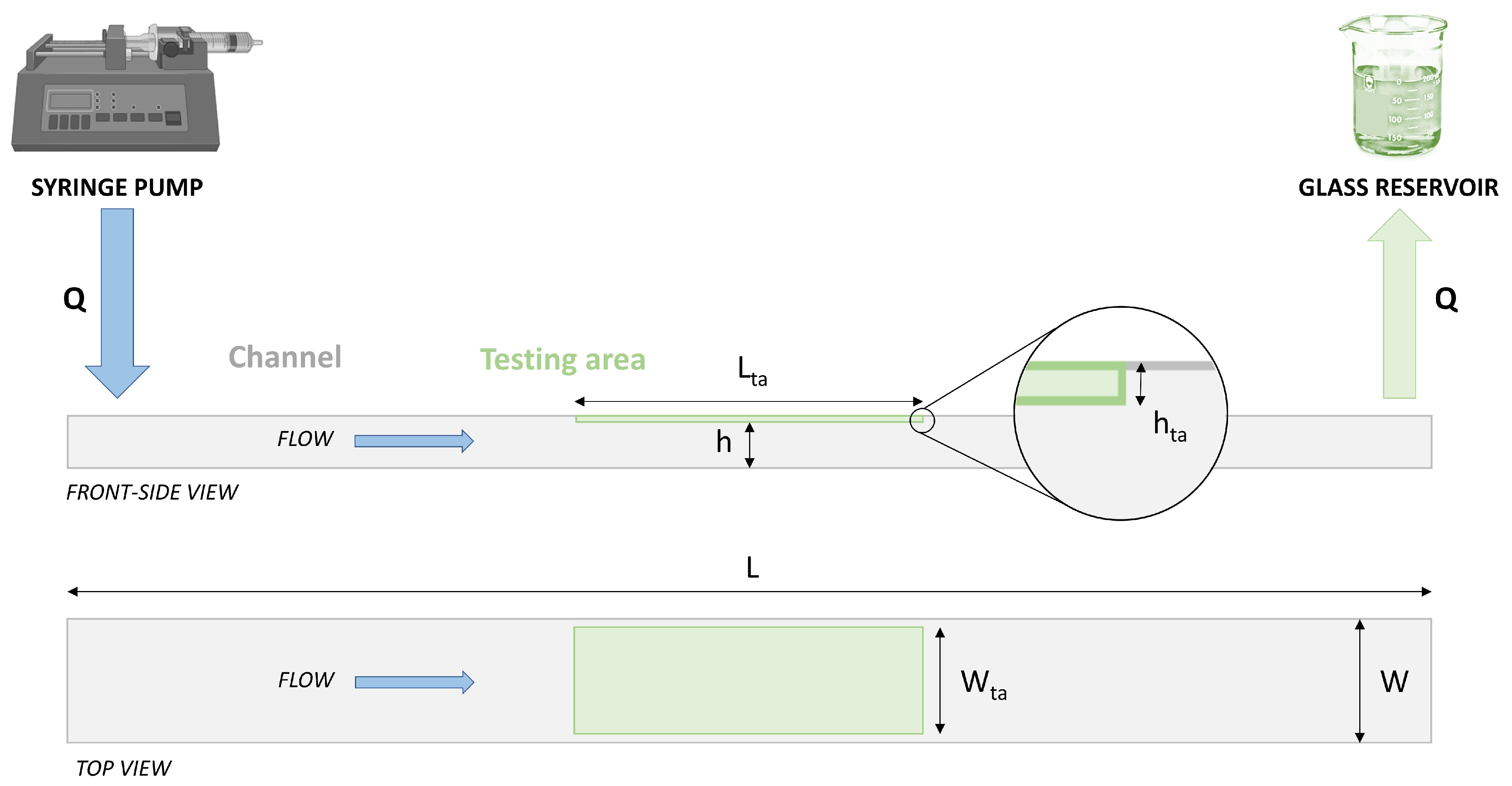
2.2.2. Analytical Approach for Channel Sizing and Q Definition
- h was set to 0.7–0.8 mm: this range is a reasonable compromise between (i) the need to use small flow rates Q to generate the WSS field (WSS ≈ Q/h2) on coated specimens (to minimize the fluid to be collected in the vial to be analyzed by HPLC) and (ii) the possibility to neglect the variability of the specimen thickness (h much larger than the flat-coated specimen thickness variation, expected to be about 20 μm) and the hta step (see Figure 1);
- W was set to 5 mm: this value is a reasonable compromise between (i) the need to test sufficiently large coated areas (to have measurable amounts of drug loss) and (ii) the need to use small flow rates to generate the WSS field (WSS ≈ Q/W) on coated specimens; moreover, this W guarantees a W/h ratio high enough to ensure almost homogeneous WSS along the specimen width W [29];
- L was set to 40 mm: this value is a reasonable compromise between (i) the need to ensure adequate flow control on the testing region and (ii) the need to limit overall dimensions for easy manual assembly and handling; the specimen to test is planned to occupy the full length of the chamber, whereas the coated area covers only a central part of 20 mm, and two uncoated 10 mm lengths are located close to the inlet and the outlet, where WSS values cannot be controlled.
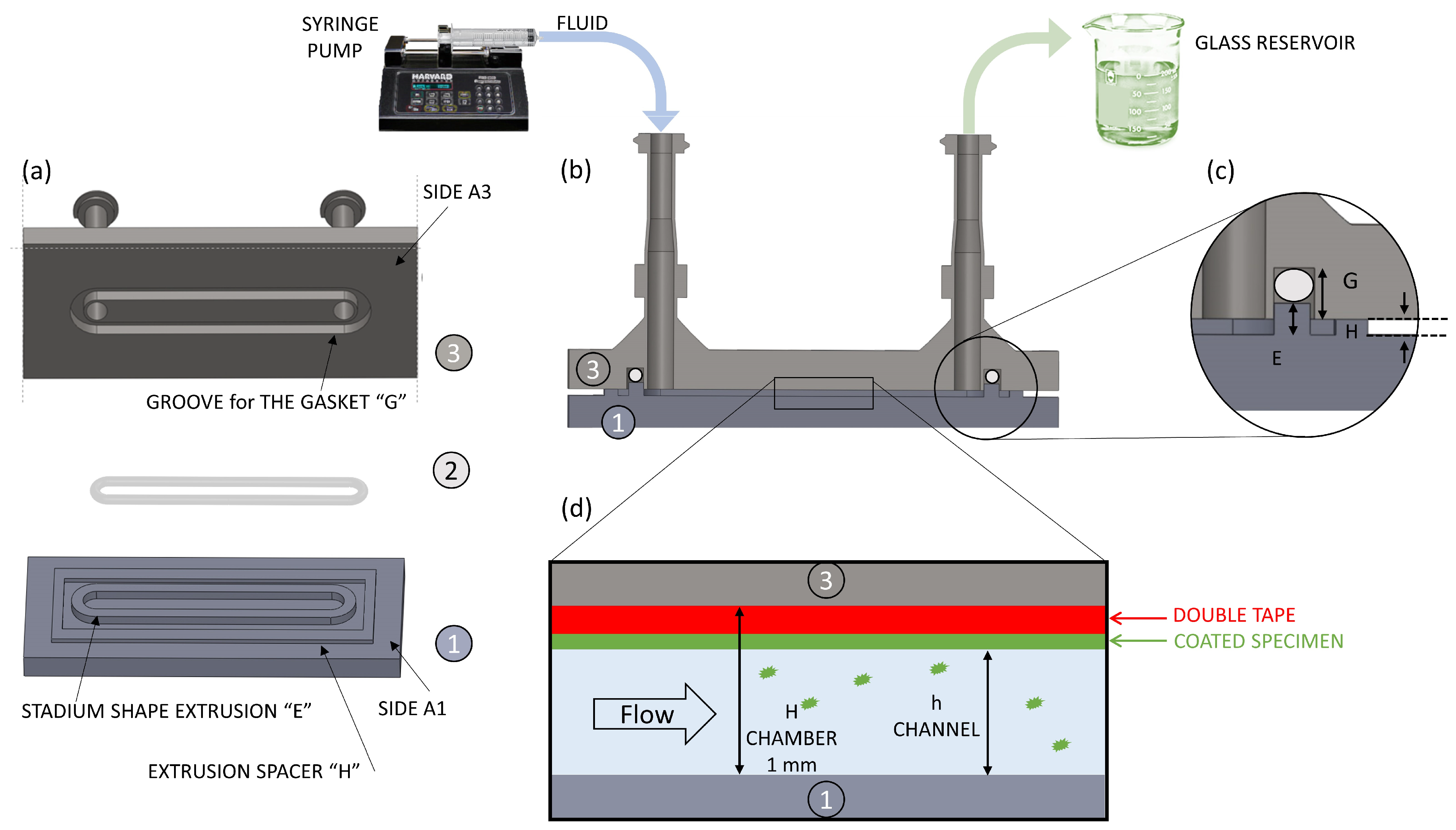
2.2.3. System Design, 3D Printing of a Prototype, and Sealing Testing
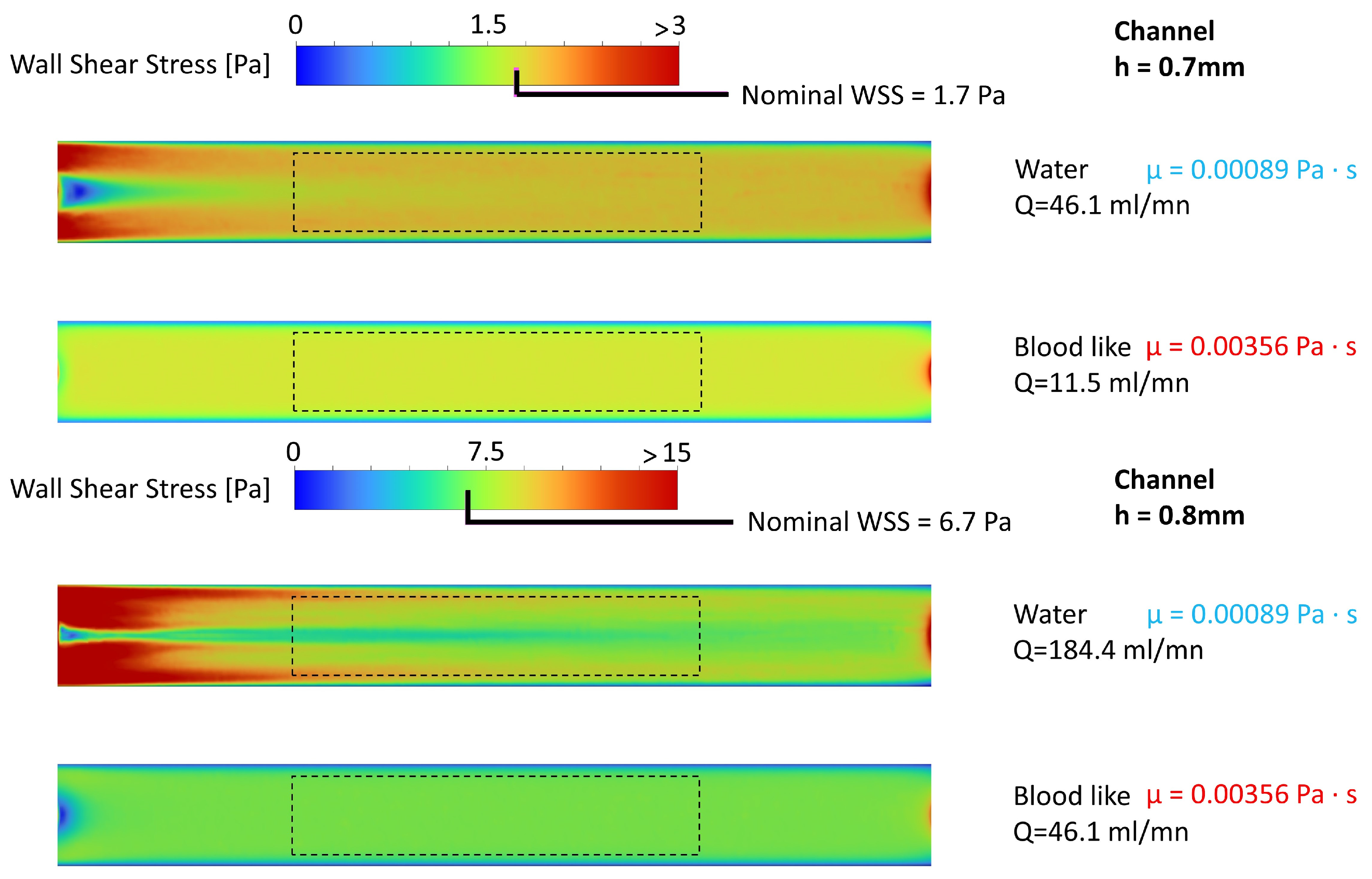
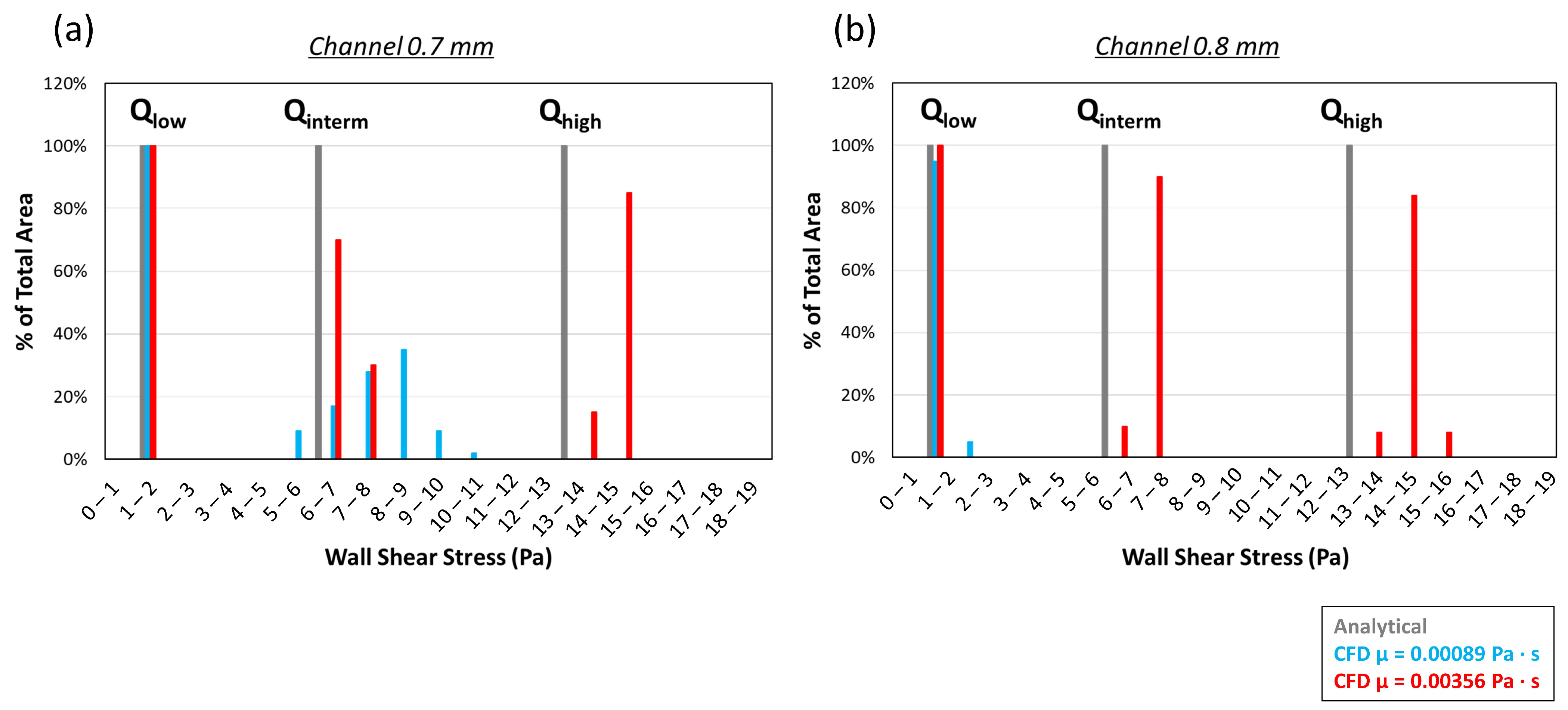
2.2.4. CFD Chamber Verification
2.3. Part B—Proof of Concept
2.3.1. Drug-Coated Specimen Preparation
2.3.2. Experiments
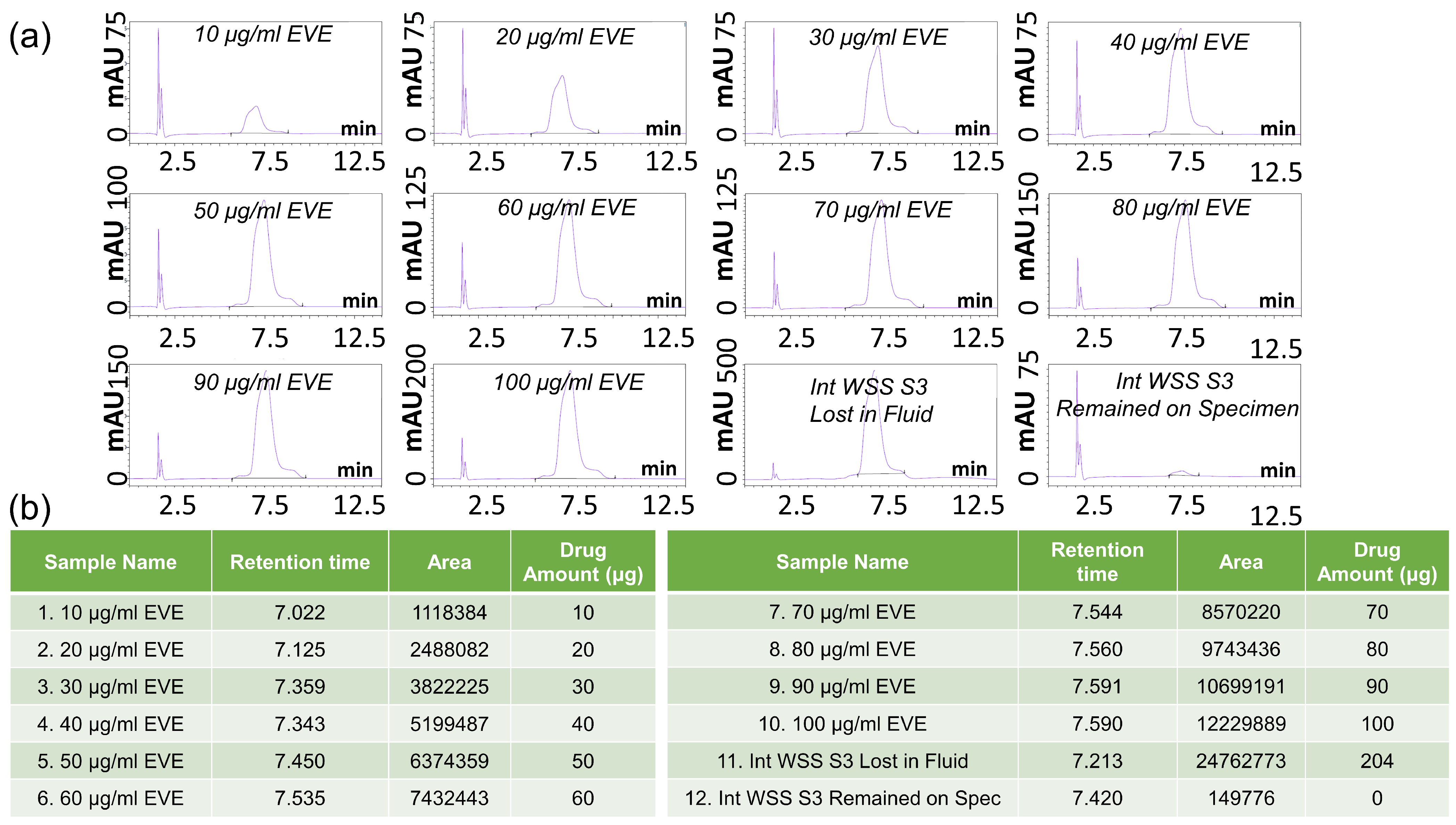
2.3.3. HPLC-UV Measurements and Data Processing
3. Results and Discussion
3.1. Part A—System Design
3.1.1. The 3D CFD Simulation of the Balloon Tracking
3.1.2. Analytical Equations Results
3.1.3. CFD Chamber Verification
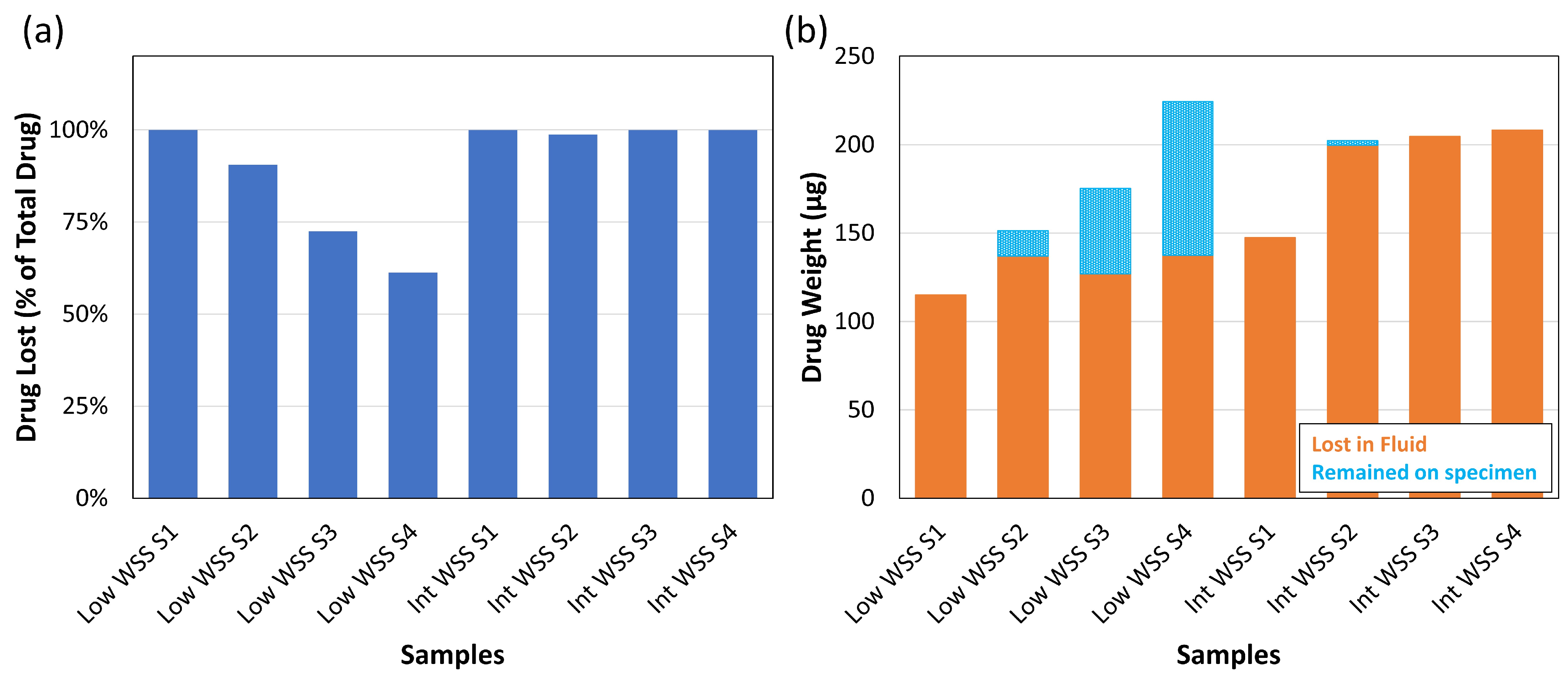
3.2. Part B—Proof of Concept
Experiments and HPLC Analysis
4. Conclusions
- This setup, by testing coated flat patches resembling DCBs, is the first to isolate the effects of blood flow on drug loss, distinguishing it from the losses that arise due to device–vessel interactions;
- The millifluidic approach allowed performing multiple tests on small specimens, hence reducing the amounts of reagents needed;
- The system is highly versatile in replicating different in vivo environments that must be required by several configurations of balloons, arteries, and DCBs by simply adjusting parameters such as flow rate or fluid properties;
- This open-loop system allows for the collection of fluid at multiple time points, providing insights into how the coating deteriorates over time. The use of HPLC analysis allowed direct measurements of the eluted drug, increasing the reliability of the pipeline.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimchak, A.C.; Patel, M.Y.; Iorga, Ş.R.; Kulkarni, N.; Wong, N.D. Lipid treatment and goal attainment characteristics among persons with atherosclerotic cardiovascular disease in the United States. Am. J. Prev. Cardiol. 2020, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar]
- Laird, J.R.; Loja, M.N. Drug-eluting stents in the superficial femoral artery: Seeing is believing. EuroIntervention 2016, 12, 1443–1445. [Google Scholar] [CrossRef]
- Giacoppo, D.; Cassese, S.; Harada, Y.; Colleran, R.; Michel, J.; Fusaro, M.; Kastrati, A.; Byrne, R.A. Drug-Coated Balloon Versus Plain Balloon Angioplasty for the Treatment of Femoropopliteal Artery Disease. JACC Cardiovasc. Interv. 2016, 9, 1731–1742. [Google Scholar]
- Shishehbor, M.H.; Scheinert, D.; Jain, A.; Brodmann, M.; Tepe, G.; Ando, K.; Krishnan, P.; Iida, O.; Laird, J.R.; Schneider, P.A.; et al. Comparison of Drug-Coated Balloons vs Bare-Metal Stents in Patients With Femoropopliteal Arterial Disease. J. Am. Coll. Cardiol. 2023, 81, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.M.; Ang, H.; Lin, J.; Lui, Y.S.; Phua, J.L.; Chan, J.N.; Venkatraman, S.; Foin, N.; Huang, Y. Materials technology in drug eluting balloons: Current and future perspectives. J. Control. Release 2016, 239, 92–106. [Google Scholar] [CrossRef]
- Bukka, M.; Rednam, P.J.; Sinha, M. Drug-eluting balloon: Design, technology and clinical aspects. Biomed. Mater. 2018, 13, 032001. [Google Scholar] [CrossRef]
- Heinrich, A.; Engler, M.S.; Güttler, F.V.; Matthäus, C.; Popp, J.; Teichgräber, U.K.M. Systematic evaluation of particle loss during handling in the percutaneous transluminal angioplasty for eight different drug-coated balloons. Sci. Rep. 2020, 10, 17220. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J. Am. Hear. Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef]
- Bandomir, J.; Kaule, S.; Schmitz, K.P.; Sternberg, K.; Petersen, S.; Kragl, U. Usage of different vessel models in a flow-through cell: In vitro study of a novel coated balloon catheter. RSC Adv. 2015, 5, 11604–11610. [Google Scholar] [CrossRef]
- Kaule, S.; Minrath, I.; Stein, F.; Kragl, U.; Schmidt, W.; Schmitz, K.P.; Sternberg, K.; Petersen, S. Correlating coating characteristics with the performance of drug-coated balloons—A comparative in vitro investigation of own established hydrogel- and ionic liquid-based coating matrices. PLoS ONE 2015, 10, e0116080. [Google Scholar]
- Speck, U.; Häckel, A.; Schellenberger, E.; Kamann, S.; Löchel, M.; Clever, Y.P.; Peters, D.; Scheller, B.; Trog, S.; Bettink, S. Drug Distribution and Basic Pharmacology of Paclitaxel/Resveratrol-Coated Balloon Catheters. Cardiovasc. Interv. Radiol. 2018, 41, 1599–1610. [Google Scholar]
- Bienek, S.; Kusmierczuk, M.; Mittag, A.; Bettink, S.; Scheller, B. Novel, vessel anatomy adjusting drug-coated balloon—Preclinical evaluation in peripheral porcine arteries. Catheter. Cardiovasc. Interv. 2020, 95, 319–328. [Google Scholar] [CrossRef]
- Bettink, S.; Löchel, M.; Peters, D.; Haider, W.; Speck, U.; Scheller, B. Efficacy and safety of a magnesium stearate paclitaxel coated balloon catheter in the porcine coronary model. Int. J. Cardiol. 2021, 331, 46–56. [Google Scholar] [CrossRef]
- ASTM F3320-18; Standard Guide for Coating Characterization of Drug Coated Balloons. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Stratakos, E.; Tscheuschner, L.; Vincenzi, L.; Pedrinazzi, E.; Sigala, F.; D’Andrea, L.; Gastaldi, D.; Berti, F.; Tzafriri, A.R.; Pennati, G. A Novel In Silico–Ex Vivo Model for Correlating Coating Transfer to Tissue with Local Drug-Coated Balloon-Vessel Contact Pressures. Ann. Biomed. Eng. 2024, 53, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Akrami-Hasan-Kohal, M.; Chouchou, A.; Blanquer, S.; Sharkawi, T. Everolimus-encapsulation in Pluronic P123 self-assembled micelles as drug delivery systems for drug-coated balloons. Int. J. Pharm. X 2024, 7, 100230. [Google Scholar] [CrossRef]
- Zheng, Q.; Chu, Z.; Li, X.; Kang, H.; Yang, X.; Fan, Y. The Effect of Fluid Shear Stress on the In Vitro Release Kinetics of Sirolimus from PLGA Films. Polymers 2017, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Campos, O.A.; Garcia-Herreros, A.; Sánchez, A.L.; Fineman, J.R.; Pawlak, G. A Multichamber Pulsating-Flow Device with Optimized Spatial Shear Stress and Pressure for Endothelial Cell Testing. J. Biomech. Eng. 2024, 147, 011006. [Google Scholar] [CrossRef]
- Nile, M.; Folwaczny, M.; Kessler, A.; Wichelhaus, A.; Rankovic, M.J.; Baumert, U. Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells. Cells 2024, 13, 1751. [Google Scholar] [CrossRef]
- Molteni, R.; Bianchi, E.; Patete, P.; Fabbri, M.; Baroni, G.; Dubini, G.; Pardi, R. A novel device to concurrently assess leukocyte extravasation and interstitial migration within a defined 3D environment. Lab Chip 2015, 15, 195–207. [Google Scholar]
- Stratakos, E.; Antonini, L.; Poletti, G.; Berti, F.; Tzafriri, A.R.; Petrini, L.; Pennati, G. Investigating Balloon-Vessel Contact Pressure Patterns in Angioplasty: In Silico Insights for Drug-Coated Balloons. Ann. Biomed. Eng. 2023, 51, 2908–2922. [Google Scholar]
- Shchetynska-Marinova, T.; Gerdes, L.; Hohneck, A.L.; Winter, L.; Amendt, K.; Schwenke, K.; Gerken, A.L.H.; Du, Y.; Dürschmied, D.; Sigl, M. First experiences of ultrasound vector flow imaging at the femoropopliteal artery in peripheral arterial disease. Vasa-Eur. J. Vasc. Med. 2023, 52, 394–401. [Google Scholar]
- Ninno, F.; Chiastra, C.; Colombo, M.; Dardik, A.; Strosberg, D.; Aboian, E.; Tsui, J.; Bartlett, M.; Balabani, S.; Díaz-Zuccarini, V. Modelling lower-limb peripheral arterial disease using clinically available datasets: Impact of inflow boundary conditions on hemodynamic indices for restenosis prediction. Comput. Methods Programs Biomed. 2024, 251, 108214. [Google Scholar]
- Bos, R.; Van Der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions—Its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Sun, R.; Zhang, D.; Su, G.H.; Tian, W.; Qiu, S. Experimental study on flow characteristics of rectangular narrow channel. Int. J. Adv. Nucl. React. Des. Technol. 2020, 2, 60–68. [Google Scholar] [CrossRef]
- Han, L.S. Hydrodynamic Entrance Lengths for Incompressible Laminar Flow in Rectangular Ducts. J. Appl. Mech. 1960, 27, 403–409. [Google Scholar] [CrossRef]
- Fröhlich, C.; Grabiger, S.; Schwefel, D.; Faelber, K.; Rosenbaum, E.; Mears, J.; Rocks, O.; Daumke, O. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. EMBO J. 2013, 32, 1280–1292. [Google Scholar]
- Detry, J.G.; Deroanne, C.; Sindic, M. Hydrodynamic systems for assessing surface fouling, soil adherence and cleaning in laboratory installations. Biotechnol. Agron. Soc. Environ. 2009, 13, 427–439. [Google Scholar]
- Alshimaysawee, S.; Fadhel Obaid, R.; Al-Gazally, M.E.; Alexis Ramírez-Coronel, A.; Bathaei, M.S. Recent Advancements in Metallic Drug-Eluting Implants. Pharmaceutics 2023, 15, 223. [Google Scholar] [CrossRef]
| Low WSS (1.7 Pa) | Int WSS (6.7 Pa) | High WSS (13.4 Pa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h (mm) | μ (Pa·s) | Q | Re | Le | Q | Re | Le | Q | Re | Le |
| 0.7 | 0.00089 | 46.1 | 303 | 9 | 184.4 | 1212 | 37 | 368.9 ** | 2424 | 74 |
| 0.00356 | 11.5 | 19 | 1 | 46.1 | 76 | 2 | 92.2 | 151 | 5 | |
| 0.8 | 0.00089 | 60.2 | 389 | 13 | 240.9 * | 1556 | 54 | 481.8 ** | 3111 | 107 |
| 0.00356 | 15.1 | 24 | 1 | 60.2 | 97 | 3 | 120.4 | 194 | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zantzas, D.; Bianchi, E.; Berti, F.; Akrami-Hasan-Kohal, M.; Sharkawi, T.; Pennati, G. Design of an Experimental System for the Assessment of the Drug Loss in Drug-Coated Balloons Due to Washing Off During Tracking. Designs 2025, 9, 37. https://doi.org/10.3390/designs9020037
Zantzas D, Bianchi E, Berti F, Akrami-Hasan-Kohal M, Sharkawi T, Pennati G. Design of an Experimental System for the Assessment of the Drug Loss in Drug-Coated Balloons Due to Washing Off During Tracking. Designs. 2025; 9(2):37. https://doi.org/10.3390/designs9020037
Chicago/Turabian StyleZantzas, Dimitrios, Elena Bianchi, Francesca Berti, Mohammad Akrami-Hasan-Kohal, Tahmer Sharkawi, and Giancarlo Pennati. 2025. "Design of an Experimental System for the Assessment of the Drug Loss in Drug-Coated Balloons Due to Washing Off During Tracking" Designs 9, no. 2: 37. https://doi.org/10.3390/designs9020037
APA StyleZantzas, D., Bianchi, E., Berti, F., Akrami-Hasan-Kohal, M., Sharkawi, T., & Pennati, G. (2025). Design of an Experimental System for the Assessment of the Drug Loss in Drug-Coated Balloons Due to Washing Off During Tracking. Designs, 9(2), 37. https://doi.org/10.3390/designs9020037









