Introduction of Hybrid Additive Manufacturing for Producing Multi-Material Artificial Organs for Education and In Vitro Testing
Abstract
1. Introduction
- Bio-Inkjet Printing: Deposition of bioink containing cells onto a substrate, cured to form living tissue;
- Electrospinning: Electric field production of nanofiber scaffolds supporting living cells;
- Microfluidic Printing: Droplet deposition of bioink with cells through a microfluidic device;
- Stereolithography with Cell Encapsulation: Encapsulating cells in a cured hydrogel for tissue formation.
2. Materials and Methods
- PLA: Used for printing the liver mold to ensure mechanical integrity. Additionally, this material is flexible in the way that it is printed, ensuring that no special conditions are needed;
- PVA: Employed as the mold for veins and arteries, allowing easy removal by washing with hot water. This was crucial as the delicate veins’ geometry could not be extracted from a PLA mold without damage;
- Polysiloxane: Medical-grade silicone used to fill the PLA molds. This silicone is harmless to living organisms, and its density is close to the density of organ tissue (~1.1 g/cm3). For this reason, this material could also be a base in future applications to accommodate living cell populations.
| Material | Nozzle Temperature (°C) | Bed Temperature (°C) | Water Temperature While Dissolving | Filament Diameter | Wall Thickness | Infill | Layer High |
|---|---|---|---|---|---|---|---|
| PLA | 205 | 60 | - | 1.75 mm | 0.8 mm | 0% | 0.28 mm |
| PVA | 220 | 60 | 40–60 °C (with stirring) | 1.75 mm | 0.8 mm | 0% | 0.28 mm |
| Polysiloxane | 25 | 60 | - | - | - | - | - |
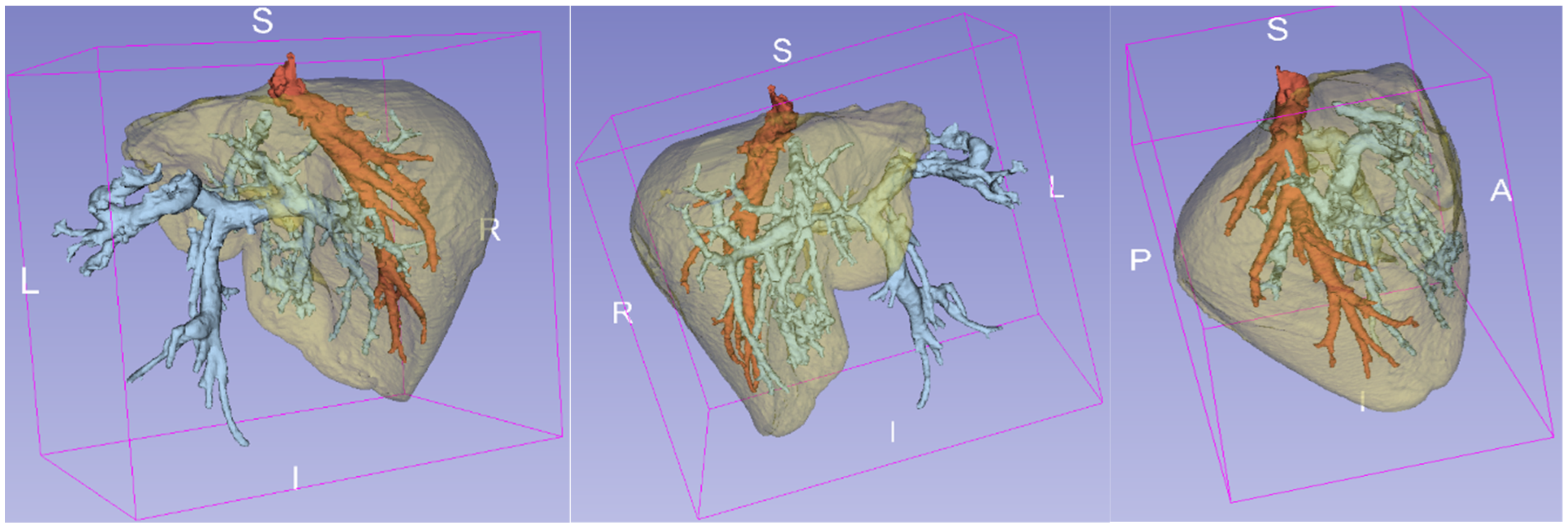
3. Results
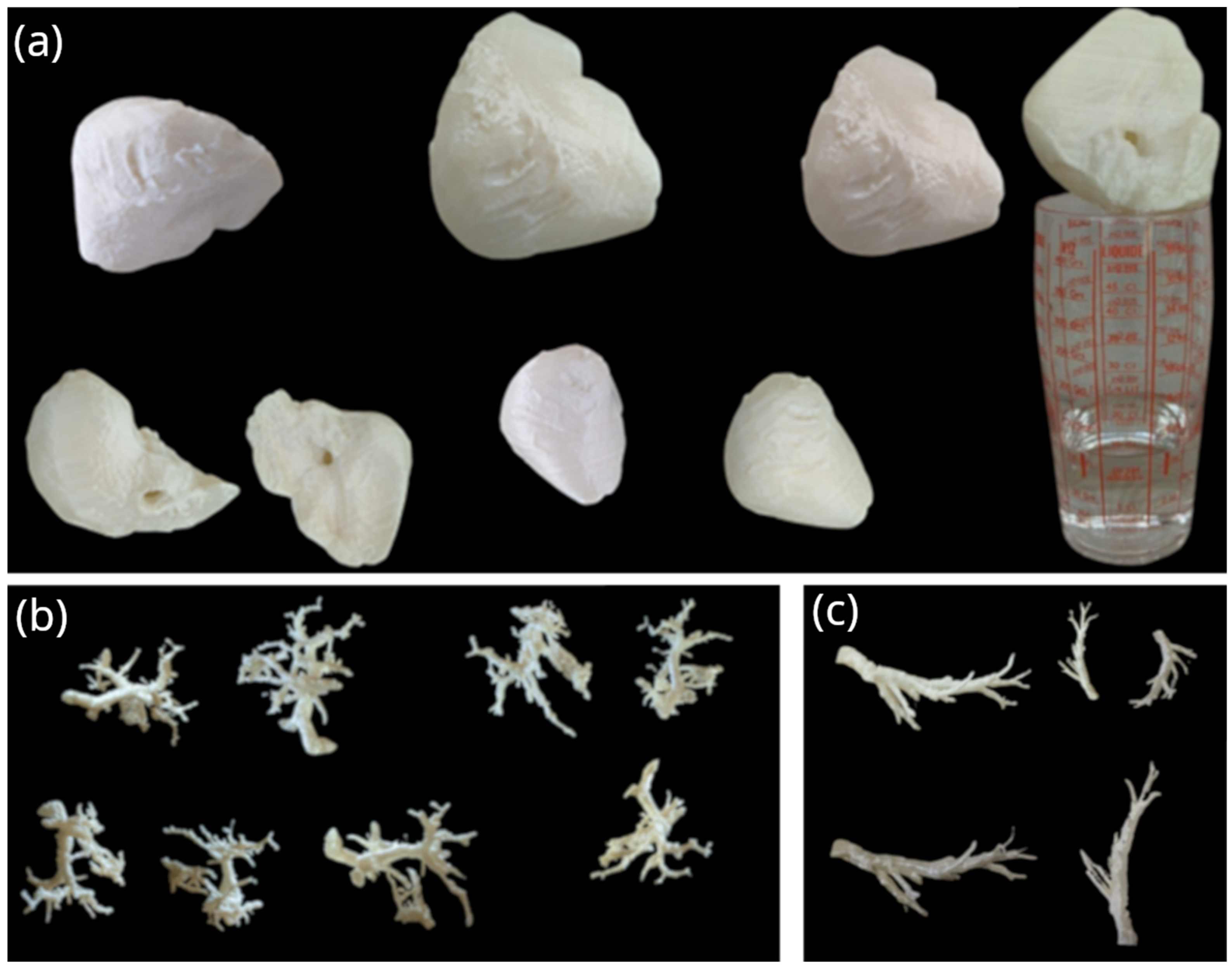

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Niu, Y.; Sun, F.; Huang, S.; Ding, P.; Wang, X.; Zhang, X.; Zhang, J. Three-dimensional printing and 3D slicer powerful tools in understanding and treating neurosurgical diseases. Front. Surg. 2022, 9, 1030081. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.; Minshall, T. Invited review article: Where and how 3D printing is used in teaching and education. Addit. Manuf. 2019, 25, 131–150. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Li, L. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int. J. Bioprint. 2016, 2, 54–66. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint. 2016, 2, 53–62. [Google Scholar] [CrossRef]
- Arifin, N.; Sudin, I.; Ngadiman, N.H.A.; Ishak, M.S.A. A Comprehensive Review of Biopolymer Fabrication in Additive Manufacturing Processing for 3D-Tissue-Engineering Scaffolds. Polymers 2022, 14, 2119. [Google Scholar] [CrossRef]
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 586406. [Google Scholar] [CrossRef]
- Hart, L.R.; Harries, J.L.; Greenland, B.W.; Colquhoun, H.M.; Hayes, W. Molecular design of a discrete chain-folding polyimide for controlled inkjet deposition of supramolecular polymers. Polym. Chem. 2015, 6, 7342–7352. [Google Scholar] [CrossRef]
- Hart, L.R.; Harries, J.L.; Greenland, B.W.; Colquhoun, H.M.; Hayes, W. Supramolecular Approach to New Inkjet Printing Inks. ACS Appl. Mater. Interfaces 2015, 7, 8906–8914. [Google Scholar] [CrossRef]
- Tolbert, J.W.; Hammerstone, D.E.; Yuchimiuk, N.; Seppala, J.E.; Chow, L.W. Solvent-Cast 3D Printing of Biodegradable Polymer Scaffolds. Macromol. Mater. Eng. 2021, 306, 2100442. [Google Scholar] [CrossRef]
- Trachtenberg, J.E.; Mountziaris, P.M.; Miller, J.S.; Wettergreen, M.; Kasper, F.K.; Mikos, A.G. Open-source three-dimensional printing of biodegradable polymer scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Taneja, H.; Salodkar, S.M.; Singh Parmar, A.; Chaudhary, S. Hydrogel based 3D printing: Bio ink for tissue engineering. J. Mol. Liq. 2022, 367, 120390. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Fazeli, N.; Arefian, E.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. 3D-Printed PCL Scaffolds Coated with Nanobioceramics Enhance Osteogenic Differentiation of Stem Cells. ACS Omega 2021, 6, 35284–35296. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Zhang, H.; Hu, Q.; Wang, Y.; Ramalingam, M. High-resolution combinatorial 3D printing of gelatin-based biomimetic triple-layered conduits for nerve tissue engineering. Int. J. Biol. Macromol. 2021, 166, 1280–1291. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; et al. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Düzgün, D.E.; Nadolny, K. Continuous liquid interface production (CLIP) method for rapid prototyping. J. Mech. Energy Eng. 2018, 2, 5–12. [Google Scholar] [CrossRef]
- Nisja, G.A.; Cao, A.; Gao, C. Short review of nonplanar fused deposition modeling printing. Mater. Des. Process. Commun. 2021, 3, e221. [Google Scholar] [CrossRef]
- Rogers, J.A. Techniques and applications for non-planar lithography. MRS Online Proc. Libr. 2002, 739, 121–128. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Farahani, R.D.; Chizari, K.; Therriault, D. Three-dimensional printing of freeform helical microstructures: A review. Nanoscale 2014, 6, 10470–10485. [Google Scholar] [CrossRef]
- O’Connell, J. Non-Planar 3D Printing: All You Need to Know. Available online: https://all3dp.com/2/non-planar-3d-printing-simply-explained/ (accessed on 21 March 2024).
- Zhang, H.; Liu, D.; Huang, T.; Hu, Q.; Lammer, H. 3D Printing Method of Spatial Curved Surface by Continuous Natural Fiber Reinforced Composite. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 022059. [Google Scholar] [CrossRef]
- Ahlers, D.; Wasserfall, F.; Hendrich, N.; Zhang, J. 3D Printing of Nonplanar Layers for Smooth Surface Generation. In Proceedings of the 2019 IEEE 15th International Conference on Automation Science and Engineering (CASE), Vancouver, BC, Canada, 22–26 August 2019; pp. 1737–1743. [Google Scholar]
- CNCKitchen. GCodeBending. Available online: https://github.com/CNCKitchen/GCodeBending (accessed on 21 March 2024).
- Etienne, J.; Ray, N.; Panozzo, D.; Hornus, S.; Wang, C.C.L.; Martínez, J.; McMains, S.; Alexa, M.; Wyvill, B.; Lefebvre, S. CurviSlicer: Slightly curved slicing for 3-axis printers. ACM Trans. Graph. 2019, 38, 81. [Google Scholar] [CrossRef]
- Gleadall, A. FullControl GCode Designer: Open-source software for unconstrained design in additive manufacturing. Addit. Manuf. 2021, 46, 102109. [Google Scholar] [CrossRef]
- Loepmeier, H. Nozzleboss. Available online: https://github.com/Heinz-Loepmeier/nozzleboss (accessed on 21 March 2024).
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: New York, NY, USA, 2014; pp. 451–474. [Google Scholar]
- Srivatsan, T.S.; Sudarshan, T.S. Additive Manufacturing: Innovations, Advances, and Applications; CRC Press: Boca Raton, FL, USA, 2015; pp. 390–420. [Google Scholar]
- Chen, E.P.; Toksoy, Z.; Davis, B.A.; Geibel, J.P. 3D Bioprinting of Vascularized Tissues for in vitro and in vivo Applications. Front. Bioeng. Biotechnol. 2021, 9, 664188. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, T. Regenerative medicine and 3D bioprinting for human space exploration and planet colonisation. J. Thorac. Dis. 2018, 10, S2363–S2375. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Chatzipapas, K.; Dordevic, M.; Zivkovic, S.; Tran, N.H.; Lampe, N.; Sakata, D.; Petrovic, I.; Ristic-Fira, A.; Shin, W.-G.; Zein, S.; et al. Geant4-DNA simulation of human cancer cells irradiation with helium ion beams. Phys. Medica 2023, 112, 102613. [Google Scholar] [CrossRef] [PubMed]
- Chatzipapas, K.P.; Papadimitroulas, P.; Emfietzoglou, D.; Kalospyros, S.A.; Hada, M.; Georgakilas, A.G.; Kagadis, G.C. Ionizing Radiation and Complex DNA Damage: Quantifying the Radiobiological Damage Using Monte Carlo Simulations. Cancers 2020, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Z.; Jiang, S. Tissue mimicking materials in image-guided needle-based interventions: A review. Mater. Sci. Eng. C 2018, 93, 1116–1131. [Google Scholar] [CrossRef] [PubMed]
- Filippou, V.; Tsoumpas, C. Recent advances on the development of phantoms using 3D printing for imaging with CT, MRI, PET, SPECT, and ultrasound. Med. Phys. 2018, 45, e740–e760. [Google Scholar] [CrossRef]
- Glick, S.J.; Ikejimba, L.C. Advances in digital and physical anthropomorphic breast phantoms for X-ray imaging. Med. Phys. 2018, 45, e870–e885. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A systematic review on 3D-printed imaging and dosimetry phantoms in radiation therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870208. [Google Scholar] [CrossRef]
- Xu, X.G. An exponential growth of computational phantom research in radiation protection, imaging, and radiotherapy: A review of the fifty-year history. Phys. Med. Biol. 2014, 59, R233. [Google Scholar] [CrossRef] [PubMed]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.P.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65, 23TR01. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.; Keenan, P.; Kirkwood, D.A.; Oji, S.; Webster, C.; Russell, K.A.; Koch, T.G. A Review of Recent Advances in 3D Bioprinting With an Eye on Future Regenerative Therapies in Veterinary Medicine. Front. Vet. Sci. 2021, 7, 584193. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.R.; Anupama Sekar, J.; Ajit, S.; Velayudhan, S.; Kasoju, N.; Anil Kumar, P.R. 5—Three-dimensional bioprinting of tissues and organs. In Biomedical Product and Materials Evaluation; Woodhead Publishing Series in Biomaterials; Mohanan, P.V., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 135–150. [Google Scholar] [CrossRef]
- Salaoru, I.; Maswoud, S.; Paul, S. Inkjet Printing of Functional Electronic Memory Cells: A Step Forward to Green Electronics. Micromachines 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bethers, B.; Chen, H.; Xiao, S.; Lin, S.; Tran, B.; Jiang, L.; Yang, Y. Recent Advancements in Biomimetic 3D Printing Materials With Enhanced Mechanical Properties. Front. Mater. 2021, 8, 518886. [Google Scholar] [CrossRef]
- Wasserthal, J.; Breit, H.-C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust Segmentation of 104 Anatomic Structures in CT Images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef]
- Wasserthal, J. Dataset with segmentations of 117 important anatomical structures in 1228 CT images (2.0.1). Available online: https://zenodo.org/records/10047292 (accessed on 27 March 2024). [CrossRef]
- Dental-Co. Available online: https://dental-co.gr/product/protolast-pf-150ml/ (accessed on 21 March 2024).
- RTsafe. RTsafe. Available online: https://rt-safe.com/ (accessed on 21 March 2024).
- Langer, L.; Schmitt, M.; Schlick, G.; Schilp, J. Development of an Automated Process Chain for Hybrid Additive Manufacturing using Laser Powder Bed Fusion. Procedia CIRP 2022, 112, 358–363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzipapas, K.; Nika, A.; Krimpenis, A.A. Introduction of Hybrid Additive Manufacturing for Producing Multi-Material Artificial Organs for Education and In Vitro Testing. Designs 2024, 8, 51. https://doi.org/10.3390/designs8030051
Chatzipapas K, Nika A, Krimpenis AA. Introduction of Hybrid Additive Manufacturing for Producing Multi-Material Artificial Organs for Education and In Vitro Testing. Designs. 2024; 8(3):51. https://doi.org/10.3390/designs8030051
Chicago/Turabian StyleChatzipapas, Konstantinos, Anastasia Nika, and Agathoklis A. Krimpenis. 2024. "Introduction of Hybrid Additive Manufacturing for Producing Multi-Material Artificial Organs for Education and In Vitro Testing" Designs 8, no. 3: 51. https://doi.org/10.3390/designs8030051
APA StyleChatzipapas, K., Nika, A., & Krimpenis, A. A. (2024). Introduction of Hybrid Additive Manufacturing for Producing Multi-Material Artificial Organs for Education and In Vitro Testing. Designs, 8(3), 51. https://doi.org/10.3390/designs8030051








