Photocrosslinking of Adventitial Collagen in the Porcine Abdominal Aorta: A Preliminary Approach to a Strategy for Prevention of Aneurysmal Rupture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
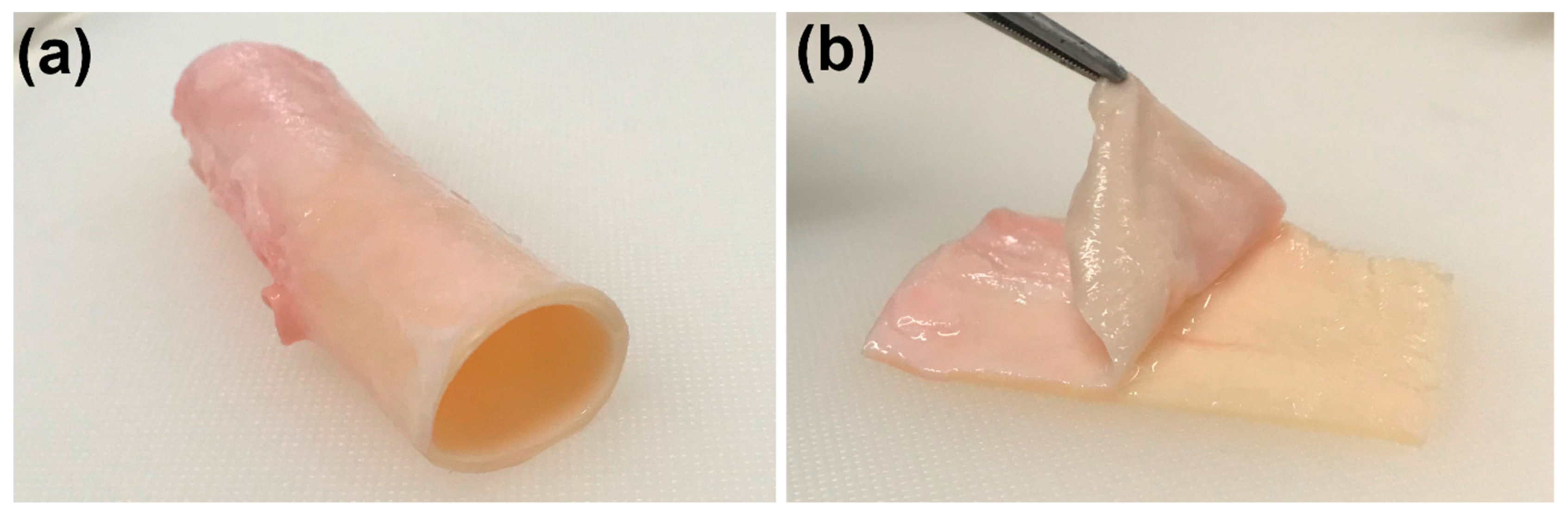
2.2. Sample Preparation
2.3. Treatment with Formalin
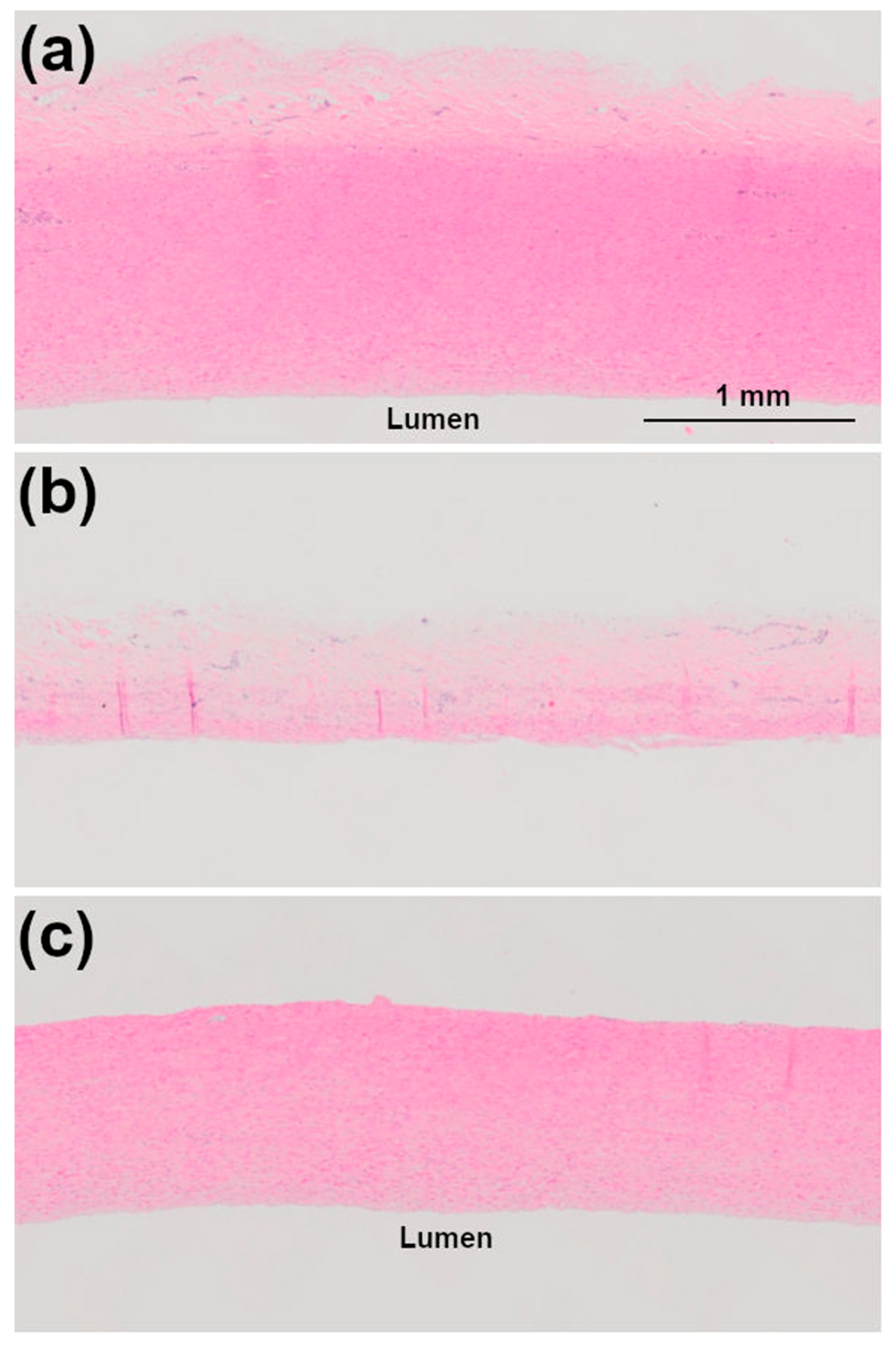
2.4. Histology
2.5. Irradiation Procedure
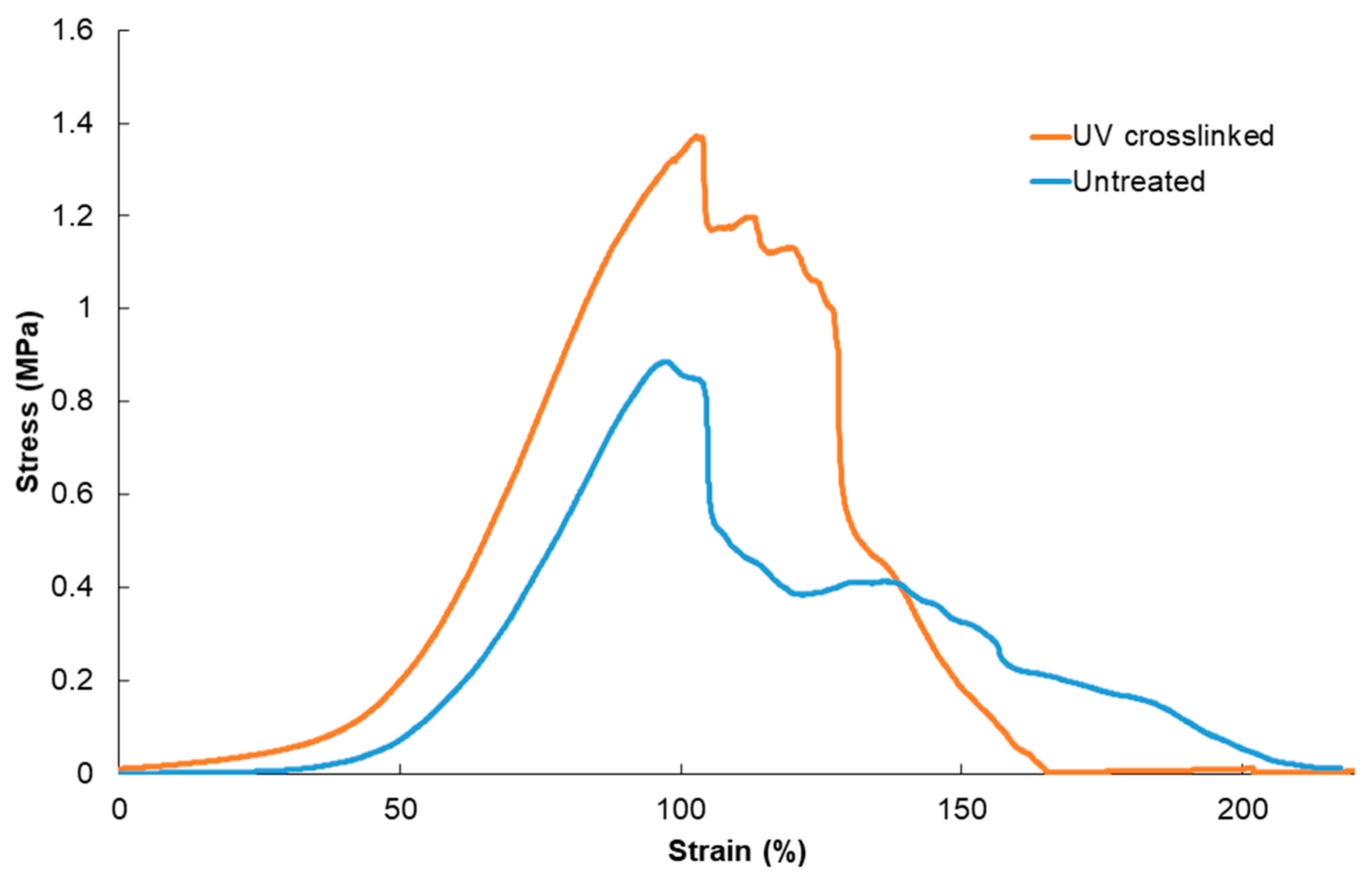
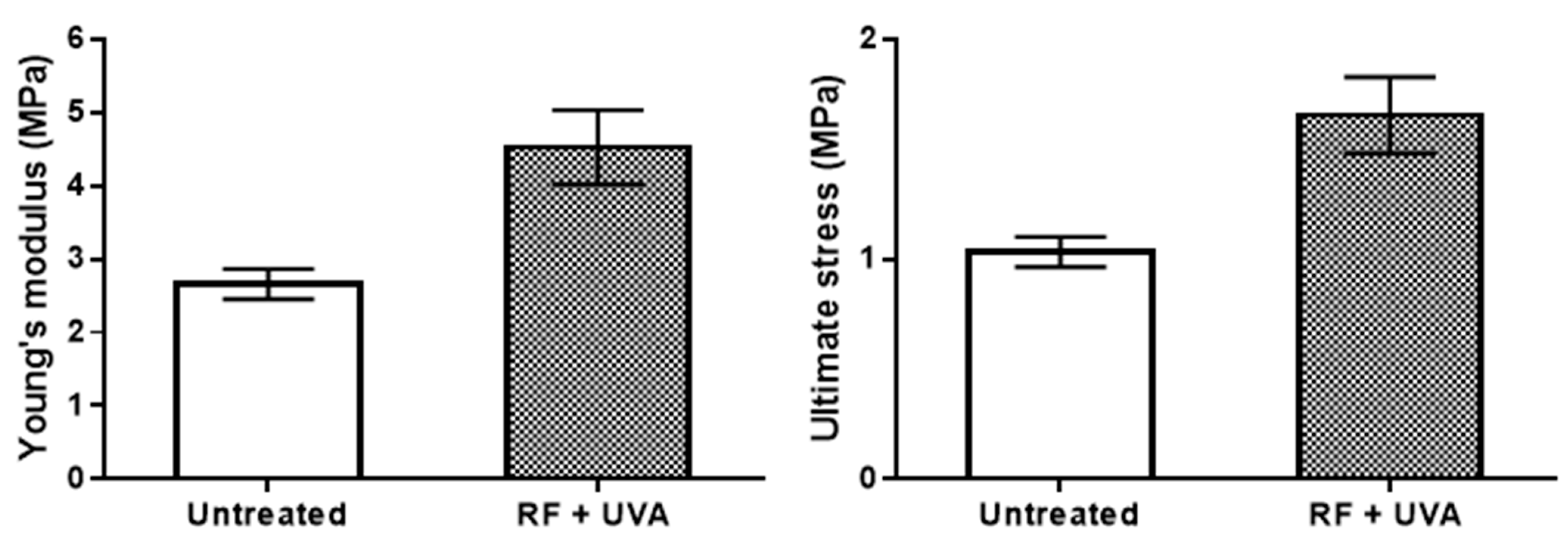
2.6. Mechanical Testing
2.7. Experimental Collagenolysis
2.8. Statistical Processing
3. Results
4. Discussion
- (a)
- The level of crosslinking of the collagen in aneurysmal wall tissues was shown to be elevated [46,47,48], which suggests that the remodelled repair collagen itself is in a crosslinked state. This natural, enzyme-controlled crosslinking process of the remodelled adventitial collagen could be aimed at providing the mechanical reinforcement needed to withstand the excessive pressure load induced by the loss of elastin and SMCs in the media. In fact, the terminal failure of the aneurysmal wall is believed to be a consequence of changes in the microarchitecture of the collagen network [49], rather than being caused by an ineffective natural crosslinking.
- (b)
- In diabetic experimental animals and post-mortem diabetic human tissue, it was found that arterial stiffness and remodelling were related to the non-enzymatic crosslinking of collagen through a natural process based on the advanced glycation end products (AGEs). The level of glycation-induced crosslinking of collagen was significantly higher in the aortic collagen when compared to other regions of the body, and it was associated with the stiffening of the vessel’s wall [50,51,52,53,54,55,56]. At the same time, a statistically significant negative association between diabetes and AAA has been demonstrated in a number of studies [57,58,59,60]. A legitimate conclusion would be that the stiffening of the aortic wall in diabetic patients due to crosslinking of collagen is one of the reasons for a delayed rupture of their aneurysms. This is another supporting premise for our proposed strategy.
- (c)
- An additional justification for selecting adventitia as the primary target in the method we report here comes from vascular surgery. Eversion endarterectomy consists of the debridement and removal of the intima and the media (or part of it) in order to treat lesions (e.g., atheromatous plaques or blockages) inside arteries and reduce their recurrence. Although it was mainly adventitia left onto the lumen side, the extant studies [61,62,63,64] have shown that no “false” aneurysms were formed postoperatively, and the strength level of endarterectomized walls was similar to that of full walls or even enhanced. The observed increased collagen content after endarterectomy suggests a deposition process of repair collagen. These findings may confirm an important contributory role of the adventitia to the mechanical performance of the aortic wall. While the knowledge of AAA is being continually re-evaluated and enriched, the prominent role of adventitial collagen in the evolution of the disease appears to be a safe presupposition underlying our investigation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.W.; Geraghty, P.J.; Lee, J.K. Abdominal aortic aneurysms: Basic mechanisms and clinical implications. Curr. Probl. Surg. 2002, 39, 110–230. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Brewster, D.C.; Dalman, R.L.; Makaroun, M.S.; Illig, K.A.; Sicard, G.A.; Timaran, C.H.; Upchurch, G.R., Jr.; Veith, F.J. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J. Vasc. Surg. 2009, 50, S2–S49. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.C. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef]
- Nordon, I.M. Abdominal aortic aneurysms: Fundamental concepts. In Oxford Textbook of Vascular Surgery; Thompson, M., Ed.; Oxford University Press: Oxford, UK, 2016; pp. 469–484. [Google Scholar]
- Golledge, J.; Norman, P.E.; Murphy, M.P.; Dalman, R.L. Challenges and opportunities in limiting abdominal aortic aneurysm growth. J. Vasc. Surg. 2017, 65, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Daugherty, A.; Lu, H.S. Updates of recent aortic aneurysm research. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e83–e90. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Beil, T.L.; Senger, C.A.; Coppola, E.L. Primary care screening for abdominal aortic aneurysm: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019, 322, 2219–2238. [Google Scholar] [CrossRef]
- Bains, P.; Oliffe, J.L.; Mackay, M.H.; Kelly, M.T. Screening older adult men for abdominal aortic aneurysm: A scoping review. Am. J. Mens. Health 2021, 15, 15579883211001204. [Google Scholar] [CrossRef]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological and mechanical properties. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef]
- Montes, G.S. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol. Int. 1996, 20, 15–27. [Google Scholar] [CrossRef]
- Sorkin, N.; Varssano, D. Corneal collagen crosslinking: A systematic review. Ophthalmologica 2014, 332, 10–27. [Google Scholar] [CrossRef]
- Randleman, J.B.; Khandelwal, S.S.; Hafezi, F. Corneal cross-linking. Surv. Ophthalmol. 2015, 60, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Lim, E.W.L. A review of corneal collagen cross-linking—current trends in practice applications. Open Ophthalmol. J. 2018, 12 (Suppl. 1), 181–213. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Karimian, F.; Esfandiari, H. Corneal crosslinking for the treatment of infectious keratitis: A review. Expert Rev. Ophthalmol. 2021, 16, 287–295. [Google Scholar] [CrossRef]
- Smith, T.M.; Suzuki, S.; Cronin, B.G.; Haghighatpanah, M.; Petcu, E.B.; Philippa, C.J.; Chirila, T.V. Photochemically induced crosslinking of tarsal collagen as a treatment for eyelid laxity: Assessing potentiality in animal tissue. Ophthal. Plast. Reconstr. Surg. 2018, 34, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Suzuki, S.; Sabat, N.; Rayner, C.L.; Harkin, D.G.; Chirila, T.V. Further investigations on the crosslinking of tarsal collagen as a treatment for eyelid laxity: Optimizing the procedure in animal tissue. Ophthal. Plast. Reconstr. Surg. 2019, 35, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Akella, S.S.; Liu, J.; Miao, Y.; Chuck, R.S.; Barmettler, A.; Zhang, C. Collagen structural changes in rat tarsus after crosslinking. Transl. Vis. Sci. Technol. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Ugradar, S.; Le, A.; Lesgart, M.; Goldberg, R.A.; Rootman, D.; Demer, J.L. Biomechanical and morphologic effects of collagen cross-linking in human tarsus. Transl. Vis. Sci. Technol. 2019, 8, 25. [Google Scholar] [CrossRef]
- Ugradar, S.; Karlin, J.; Le, A.; Park, J.; Goldberg, R.A. Photochemical collagen cross-linking reverses elastase-induced mechanical degradation of upper eyelid tarsus. Ophthal. Plast. Reconstr. Surg. 2020, 36, 562–565. [Google Scholar] [CrossRef]
- LaMuraglia, G.M.; ChandraSekar, N.R.; Flotte, T.J.; Abbott, W.M.; Michaud, N.; Hasan, T. Photodynamic therapy inhibition of experimental intimal hyperplasia: Acute and chronic effects. J. Vasc. Surg. 1994, 19, 321–331. [Google Scholar] [CrossRef]
- LaMuraglia, G.M.; Schiereck, J.; Heckenkamp, J.; Nigri, G.; Waterman, P.; Leszczynski, D.; Kossodo, S. Photodynamic therapy induces apoptosis in intimal hyperplastic arteries. Am. J. Pathol. 2000, 157, 867–875. [Google Scholar] [CrossRef]
- Overhaus, M.; Heckenkamp, J.; Kossodo, S.; Leszczynski, D.; LaMuraglia, G.M. Photodynamic therapy generates a matrix barrier to invasive vascular cell migration. Circ. Res. 2000, 86, 334–340. [Google Scholar] [CrossRef]
- Waterman, P.R.; Overhaus, M.; Heckenkamp, J.; Nigri, G.R.; Fungaloi, P.F.C.; Landis, M.E.; Kossodo, S.C.; LaMuraglia, G.M. Mechanisms of reduced human vascular cell migration after photodynamic therapy. Photochem. Photobiol. 2002, 75, 46–50. [Google Scholar] [CrossRef]
- Oskoui, P.; Stadler, I.; Lanzafame, R.J. A preliminary study of laser tissue soldering as arterial wall reinforcement in an acute experimental aneurysm model. Lasers Surg. Med. 2003, 32, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Heckenkamp, J.; Luebke, T.; Theis, T.; Schumacher, L.; Gawenda, M.; Thul, R.; Fries, J.W.U.; Brunkwall, J. Effects of vascular photodynamic therapy in a newly adapted experimental rat aortic aneurysm model. Interact. CardioVasc. Thorac. Surg. 2012, 15, 69–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayashi, T.; Sasaki, N.; Yamashita, T.; Mizoguchi, T.; Emoto, T.; Amin, Z.A.; Yodoi, K.; Matsumoto, T.; Kasahara, K.; Yoshida, N.; et al. Ultraviolet B exposure inhibits angiotensin II–induced abdominal aortic aneurysm formation in mice by expanding CD4+Foxp3+ regulatory T cells. J. Am. Heart Assoc. 2017, 6, e007024. [Google Scholar] [CrossRef] [PubMed]
- Gavish, L.; Rubinstein, C.; Bulut, A.; Berlatzky, Y.; Beeri, R.; Gilon, D.; Gavish, L.; Harlev, M.; Reissman, P.; Gertz, S.D. Low-level laser irradiation inhibits abdominal aortic aneurysm progression in apolipoprotein E-deficient mice. Cardiovasc. Res. 2009, 83, 785–792. [Google Scholar] [CrossRef]
- Gavish, L.; Rubinstein, C.; Berlatzky, Y.; Gavish, L.Y.; Beeri, R.; Gilon, D.; Bulut, A.; Harlev, M.; Reissman, P.; Gertz, S.D. Low level laser arrests abdominal aortic aneurysm by collagen matrix reinforcement in apolipoprotein E-deficient mice. Lasers Surg. Med. 2012, 44, 664–674. [Google Scholar] [CrossRef]
- Gavish, L.; Beeri, R.; Gilon, D.; Rubinstein, C.; Berlatzky, Y.; Bulut, A.; Reissman, P.; Gavish, L.Y.; Gertz, S.D. Arrest of progression of pre-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by low level laser phototherapy. Lasers Surg. Med. 2014, 46, 781–790. [Google Scholar] [CrossRef]
- Gavish, L.; Beeri, R.; Gilon, D.; Rubinstein, C.; Berlatzky, Y.; Gavish, L.Y.; Bulut, A.; Harlev, M.; Reissman, P.; Gertz, S.D. Inadequate reinforcement of transmedial disruptions at branch points subtends aortic aneurysm formation in apolipoprotein-E-deficient mice. Cardiovasc. Pathol. 2014, 23, 152–159. [Google Scholar] [CrossRef]
- Gavish, L.; Gilon, D.; Beeri, R.; Zuckerman, A.; Nachman, D.; Gertz, S.D. Photobiomodulation and estrogen stabilize mitochondrial membrane potential in angiotensin-II challenged porcine aortic muscle cells. J. Biophotonics 2021, 14, e202000329. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Gasser, T.C.; Regitnig, P.; Auer, M.; Holzapfel, G.A. Dissection properties of the human aortic media: An experimental study. J. Biomech. Eng. 2008, 130, 021007. [Google Scholar] [CrossRef]
- Tavares Monteiro, J.A.; da Silva, E.S.; Raghavan, M.L.; Puech-Leão, P.; de Lourdes Higuchi, M.; Pinhata Otoch, J. Histologic, histochemical, and biomechanical properties of fragments isolated from the anterior wall of abdominal aortic aneurysms. J. Vasc. Surg. 2014, 59, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Dobrin, P.B.; Baker, W.H.; Gley, W.C. Elastolytic and collagenolytic studies of arteries. Arch. Surg. 1984, 119, 405–409. [Google Scholar] [CrossRef]
- Dobrin, P.B.; Schwartz, T.H.; Baker, W.H. Mechanisms of arterial and aneurysmal tortuosity. Surgery 1988, 104, 568–571. [Google Scholar]
- Dobrin, P.B. Pathophysiology and pathogenesis of aortic aneurysms. Current concepts. Surg. Clin. N. Am. 1989, 69, 687–703. [Google Scholar] [CrossRef]
- White, J.V.; Haas, K.; Phillips, S.; Comerota, A.J. Adventitial elastolysis is a primary event in aneurysm formation. J. Vasc. Surg. 1993, 17, 371–381. [Google Scholar] [CrossRef]
- White, J.V. Aneurysm formation in vivo by the topical degradation of adventitial elastin. J. Vasc. Surg. 1994, 20, 153–155. [Google Scholar] [CrossRef]
- White, J.V.; Mazzacco, S.L. Formation and growth of aortic aneurysms induced by adventitial elastolysis. Ann. N. Y. Acad. Sci. 1996, 800, 97–120. [Google Scholar] [CrossRef]
- Choke, E.; Cockerill, G.; Wilson, W.R.W.; Sayed, S.; Dawson, J.; Loftus, I.; Thompson, M.M. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Vorp, D.A. Biomechanics of abdominal aortic aneurysm. J. Biomech. 2007, 40, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.L.; da Silva, E.S. Mechanical properties of AAA tissue. Stud. Mechanobiol. Tissue Eng. Biomater. 2011, 7, 139–162. [Google Scholar]
- Sumner, D.S.; Hokanson, D.E.; Strandness, D.E., Jr. Stress-strain characteristics and collagen-elastin content of abdominal aortic aneurysms. Surg. Gynecol. Obstret. 1970, 130, 459–466. [Google Scholar]
- Sobolewski, K.; Wolańska, M.; Bańkowski, E.; Gacko, M.; Głowiński, S. Collagen, elastin and glycosaminoglycans in aortic aneurysms. Acta Biochim. Pol. 1995, 42, 301–308. [Google Scholar] [CrossRef]
- Carmo, M.; Colombo, L.; Bruno, A.; Corsi, F.R.M.; Roncoroni, L.; Cuttin, M.S.; Radice, F.; Mussini, E.; Settembrini, P.G. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Villard, C.; Eriksson, P.; Hanemaaijer, R.; Lindeman, J.H.; Hultgren, R. The composition of collagen in the aneurysm wall of men and women. J. Vasc. Surg. 2017, 66, 579–585. [Google Scholar] [CrossRef]
- Lindeman, J.H.N.; Ashcroft, B.A.; Beenakker, J.-V.M.; van Es, M.; Koekkoek, N.B.R.; Prins, F.A.; Tielemans, J.F.; Abdul-Hussien, H.; Bank, R.A.; Oosterkamp, T.H. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 862–865. [Google Scholar] [CrossRef]
- Mikšik, I.; Deyl, Z. Change in the amount of ε-hexosyllisine, UV absorbance, and fluorescence of collagen with age in different animal species. J. Gerontol. 1991, 46, B111–B116. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Nagaraj, R.H.; Grandhee, S.K.; Odetti, P.; Lapolla, A.; Fogarty, J.; Monnier, V.M. Pentosidine: A molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab. Rev. 1991, 7, 239–251. [Google Scholar] [CrossRef]
- Takahashi, M.; Ohishi, T.; Aoshima, H.; Kawana, K.; Kushida, K.; Inoue, T.; Horiuchi, K. The Maillard protein cross-link pentosidine in urine from diabetic patients. Diabetologia 1993, 36, 664–667. [Google Scholar] [CrossRef][Green Version]
- Hoshino, H.; Takahashi, M.; Kushida, K.; Ohishi, T.; Kawana, K.; Inoue, T. Quantitation of the crosslinks, pyridinoline, deoxypyridinoline, and pentosidine, in human aorta with dystrophic calcification. Atherosclerosis 1995, 112, 39–46. [Google Scholar] [CrossRef]
- Sims, T.J.; Rasmussen, L.M.; Oxlund, H.; Bailey, A.J. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 1996, 39, 946–951. [Google Scholar] [CrossRef]
- Reddy, K.C. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc. Res. 2004, 68, 132–142. [Google Scholar] [CrossRef]
- Schram, M.T.; Henry, R.M.A.; van Dijk, R.A.J.M.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Westerhof, N.; Stehouwer, C.D.A. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes. Hypertension 2004, 43, 176–181. [Google Scholar] [CrossRef]
- Åstrand, H.; Rydén-Ahlgren, Å.; Sundkvist, G.; Sandgren, T.; Länne, T. Reduced aortic wall stress in diabetes mellitus. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 592–598. [Google Scholar] [CrossRef]
- Le, M.T.Q.; Jamrozik, K.; Davis, T.M.E.; Norman, P.E. Negative association between infra-renal aortic diameter and glycaemia: The Health in Men Study. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 599–604. [Google Scholar] [CrossRef]
- Norman, P.E.; Davis, T.M.E.; Le, M.T.Q.; Golledge, J. Matrix biology of abdominal aortic aneurysms in diabetes: Mechanisms underlying the negative association. Connect. Tissue Res. 2007, 48, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Karan, M.; Moran, C.S.; Muller, J.; Clancy, P.; Dear, A.E.; Norman, P.E. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur. Heart J. 2008, 29, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Butcher, H.R., Jr. The elastic properties of human aortic intima, media and adventitia: The initial effect of thromboendarterectomy. Ann. Surg. 1960, 151, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, D.E.; Smith, R.F.; Whitney, D.G. The durability of aorto-iliac endarteriectomy: A roentgenographic and pathologic study of late recurrence. Arch. Surg. 1964, 89, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D.S.; Hokanson, D.E.; Strandness, D.E., Jr. Arterial wall before and after endarterectomy. Stress-strain characteristics and collagen-elastin content. Arch. Surg. 1969, 99, 606–611. [Google Scholar] [CrossRef]
- Inahara, T. Eversion endarterectomy for aortoiliofemoral occlusive disease: A 16 year experience. Am. J. Surg. 1979, 138, 196–204. [Google Scholar] [CrossRef]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1996, 18, 95–105. [Google Scholar] [CrossRef]
- Friess, W. Collagen—biomaterial for drug delivery. Eur. J. Pharmac. Biopharmac. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Chan, B.P.; So, K.-F. Photochemical crosslinking improves the physicochemical properties of collagen scaffolds. J. Biomed. Mater. Res. 2005, 75, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials 2005, 26, 1237–1245. [Google Scholar] [CrossRef]
- Mercuri, J.J.; Lovekamp, J.J.; Simionescu, D.T.; Vyavahare, N.R. Glycosaminoglycan-targeted fixation for improved bioprosthetic heart valve stabilization. Biomaterials 2007, 28, 496–503. [Google Scholar] [CrossRef]
- Tedder, M.E.; Liao, J.; Weed, B.; Stabler, C.; Zhang, H.; Simionescu, A.; Simionescu, D.T. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng. A 2009, 15, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-linking of extruded collagen fibers–A biomimetic three-dimensional scaffold for tissue engineering application. J. Biomed. Mater. Res. 2009, 89, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, X.; Schwend, T.; Littlechild, S.; Conrad, G.W. Resistance of corneal RFUVA–Cross-linked collagens and small leucine-rich proteoglycans to degradation by matrix metalloproteinases. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Rich, H.; Odlyha, M.; Cheema, U.; Mudera, V.; Bozec, L. Effects of photochemical riboflavin-mediated crosslinks on the physical properties of collagen constructs and fibrils. J. Mater. Sci. Mater. Med. 2014, 25, 11–21. [Google Scholar] [CrossRef]
- Deborde, C.; Simionescu, D.T.; Wright, C.; Liao, J.; Sierad, L.N.; Simionescu, A. Stabilized collagen and elastin-based scaffolds for mitral valve tissue engineering. Tissue Eng. A 2016, 22, 1241–1251. [Google Scholar] [CrossRef]
- Tam, H.; Zhang, W.; Infante, D.; Parchment, N.; Sacks, M.; Vyavahare, N.R. Fixation of bovine pericardium-based tissue biomaterial with irreversible chemistry improves biochemical and biomechanical properties. J. Cardiovasc. Trans. Res. 2017, 10, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Krasselt, K.; Frommelt, C.; Brunner, R.; Rauscher, F.G.; Francke, M.; Körber, N. Various cross-linking methods inhibit the collagenase I degradation of rabbit scleral tissue. BMC Ophthalmol. 2020, 20, 488. [Google Scholar] [CrossRef]
- Reiser, K.; McCormick, R.J.; Rucker, R.B. Enzymatic and non-enzymatic cross-linking of collagen and elastin. FASEB J. 1992, 6, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Vrhovski, B.; Weiss, A.S. Biochemistry of tropoelastin. Eur. J. Biochem. 1998, 258, 1–18. [Google Scholar] [CrossRef]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Baurain, R.; Larochelle, J.F.; Lamy, F. Photolysis of desmosine and isodesmosine by ultraviolet light. Eur. J. Biochem. 1976, 67, 155–164. [Google Scholar] [CrossRef]
- Dhital, B.; Durlik, P.; Rathod, P.; Gul-E-Noor, F.; Wang, Z.; Sun, C.; Chang, E.J.; Itin, B.; Boutis, G.S. Ultraviolet radiation reduces desmosine cross-links in elastin. Biochem. Biophys. Rep. 2017, 10, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharmac. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Piskin, S.; Pillalamarri, N.R.; Romero, G.; Escobar, G.P.; Sprague, E.; Finol, E.A. Biomechanical restoration potential of pentagalloyl glucose after arterial extracellular matrix degeneration. Bioengineering 2019, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Elastin stabilization in cardiovascular implants: Improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials 2004, 25, 3293–3302. [Google Scholar] [CrossRef]
- Isenburg, J.C.; Karamchandani, N.V.; Simionescu, D.T.; Vyavahare, N.R. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006, 27, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Vyavahare, N.R.; Isenburg, J.C.; Simionescu, D.T. Elastin Stabilization of Connective Tissue. U.S. Patent 8435553, 24 January 2012. [Google Scholar]
- Isenburg, J.C.; Simionescu, D.T.; Starcher, B.C.; Vyavahare, N.R. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 2007, 115, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [CrossRef]
- Spencer, C.M.; Cai, Y.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Magnolato, D.; Lilley, T.H.; Haslam, E. Polyphenol complexation―some thoughts and observations. Phytochemistry 1988, 27, 2397–2409. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Sinha, A.; Nosoudi, N.; Vyavahare, N. Elasto-regenerative properties of polyphenols. Biochem. Biophys. Res. Commun. 2014, 444, 205–211. [Google Scholar] [CrossRef]
- Sinha, A.; Shaporev, A.; Nosoudi, N.; Lei, Y.; Vertegel, A.; Lessner, S.; Vyavahare, N. Nanoparticle targeting to diseased vasculature for imaging and therapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1003–1012. [Google Scholar] [CrossRef]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Parasaram, V.; Karamched, S.; Vyavahare, N. Systemic delivery of nanoparticles loaded with pentagalloyl glucose protects elastic lamina and prevents abdominal aortic aneurysm in rats. J. Cardiovasc. Trans. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Simionescu, D.T.; Goergen, C.J.; Hoyt, K.; Sirsi, S.; Finol, E.A. Pentagalloyl glucose and its functional role in vascular health: Biomechanics and drug-delivery characteristics. Ann. Biomed. Eng. 2019, 47, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandam, M.; Simionescu, D.T.; Escobar, P.G.; Sprague, E.; Goins, B.; Clarke, G.D.; Han, H.-C.; Amezcua, K.L.; Adeyinka, O.R.; Goergen, C.J.; et al. The effect of pentagalloyl glucose on the wall mechanics and inflammatory activity of rat abdominal aortic aneurysms. J. Biomech. Eng. 2018, 140, 084502. [Google Scholar] [CrossRef]
- Simionescu, D.; Casco, M.; Turner, J.; Rierson, N.; Yue, J.; Ning, K. Chemical stabilization of the extracellular matrix attenuates growth of experimentally induced abdominal aorta aneurysms in a large animal model. JVS Vasc. Sci. 2020, 1, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Vyavahare, N.R. Nanoparticle-based targeted delivery of pentagalloyl glucose reverses elastase-induced abdominal aortic aneurysm and restores aorta to the healthy state in mice. PLoS ONE 2020, 15, e0227165. [Google Scholar] [CrossRef] [PubMed]
- Schack, A.S.; Stubbe, J.; Steffensen, L.B.; Mahmoud, H.; Laursen, M.S.; Lindholt, J.S. Intraluminal infusion of penta-galloyl glucose reduces abdominal aortic aneurysm development in the elastase rat model. PLoS ONE 2020, 15, e0234409. [Google Scholar] [CrossRef]
- Anderson, J.L.; Niedert, E.E.; Patnaik, S.S.; Tang, R.; Holloway, R.L.; Osteguin, V.; Finol, E.A.; Goergen, C.J. Animal model dependent response to pentagalloyl glucose in murine abdominal aortic injury. J. Clin. Med. 2021, 10, 219. [Google Scholar] [CrossRef]
- Golledge, J.; Thanigaimani, S.; Phie, J. A systematic review and meta-analysis of the effect of pentagalloyl glucose administration on aortic expansion in animal models. Biomedicines 2021, 9, 1442. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirila, T.V.; Suzuki, S. Photocrosslinking of Adventitial Collagen in the Porcine Abdominal Aorta: A Preliminary Approach to a Strategy for Prevention of Aneurysmal Rupture. Designs 2022, 6, 5. https://doi.org/10.3390/designs6010005
Chirila TV, Suzuki S. Photocrosslinking of Adventitial Collagen in the Porcine Abdominal Aorta: A Preliminary Approach to a Strategy for Prevention of Aneurysmal Rupture. Designs. 2022; 6(1):5. https://doi.org/10.3390/designs6010005
Chicago/Turabian StyleChirila, Traian V., and Shuko Suzuki. 2022. "Photocrosslinking of Adventitial Collagen in the Porcine Abdominal Aorta: A Preliminary Approach to a Strategy for Prevention of Aneurysmal Rupture" Designs 6, no. 1: 5. https://doi.org/10.3390/designs6010005
APA StyleChirila, T. V., & Suzuki, S. (2022). Photocrosslinking of Adventitial Collagen in the Porcine Abdominal Aorta: A Preliminary Approach to a Strategy for Prevention of Aneurysmal Rupture. Designs, 6(1), 5. https://doi.org/10.3390/designs6010005






