3.1. Characterization of Citronella Oil and the Fractions
Fractional distillation under vacuum conditions was performed to obtain the fractions of citronella oil, FA and FB, based on an immediate difference in boiling point in a vacuum at 30 mmHg pressure with a reflux ratio of 20:1. It referred to the optimum condition presented in previous work highlighting the pressure of 30 mmHg, which shows that the best reflux ratio for separating citronella oil is 20:1 [
20]. A quick separation without any other chemicals required in the process is beneficial for fractionation [
21]. In addition, the reduced pressure of the system to be less than 1 atm (760 mmHg) gives the reducing vapor point of the distillate without any excessive chemical change. Calculation of the vapor point of the solution in this condition can be completed through the Clausius–Clapeyron equation at a pressure of 30 mmHg. The enthalpy of vaporization of citronellal is 44.22 kJ/mol, citronellol 63.50 kJ/mol, and geraniol 54.61 kJ/mol. The results of calculations using the Clausius–Clapeyron equation obtained that the boiling points of citronellal, citronellol, and geraniol at a pressure of 30 mmHg were 97.86, 138.91, and 129.57 °C, respectively.
Physico-chemical characteristics of citronella oil and the fractionation results (FA and FB) were carried out based on SNI-06-3953-1995. Color is one of the parameters for beginning fractionation, and its measurement was carried out organoleptically or by direct eye observation at a distance of 30 cm. The results presented in
Table 4 represent that the color of citronella oil and the fractions are pale yellow, which met the SNI standard stating the parameter of “no color difference”, namely pale yellow-brown. Another organoleptic parameter is odor. Odor testing was carried out with the sense of smell at a distance of 5 cm. The test results show that citronella oil has a fresh smell typical of lemongrass. FA fraction has a high citronellal content and smells of pungent lemon. FA fraction has a strong odor because it has more citronellal content compared to citronella oil. All odor test results follow SNI of citronella oil.
Density characteristics were carried out using a pycnometer. The results showed that the densities of citronella oil, FA, and FA, respectively, were 0.882; 0.867; 0.878 g/mL. In its pure state, citronellal (C10H18O BM 154.25 g/mol) has a density of 0.855 g/mL, citronellol (C10H20O BM 156.27 g/mol) is 0.855 g/mL, and geraniol (C10H18O BM 154.25 g/mol) is 0.889 g/mL. Compared to the SNI standard, the results are valued as good results within the range of 0.880 to 0.922 g/mL. Refractive index measurement was conducted on a refractometer, giving the values of 1.475; 1.449; 1,467 for citronella oil, FA, and FB, respectively. They are represented to be qualified within the acceptable range of refractive index of 1.466–1.375. Conclusively, citronella oil and its fractions are met following SNI standard from the four parameters.
The chemical components of citronella oil and their fractions were determined by GC-MS, and the compared chromatograms are presented in
Figure 2. The data in
Table 5 represent the identification results of citronellal, citronellol and geraniol. Citronellal is identified at 6.38 min of retention time with the percentage of peak area of 19.01%, citronellol is identified at 7.46 min and a peak area of 20.48%, and at 7.86 min geraniol was identified at 18.81%. Other minor components were identified at a percentage below 5%. It can be concluded that in terpenoid groups (monoterpenoid C
10) are found many essential oils, and they are composed as secondary metabolites and are characteristic of each essential oil [
22,
23]. The percentage is acceptable as it is similar to what was reported by [
24], reporting the component of citronellal (36.11%), geraniol (20.07%) and citronellol (20.82%). Meanwhile, regarding citronella oil (Javanese type), ISO 3848:2016 states the range of citronellal content (31.00–40.00%), citronellol content (8.00–14.00%) and geraniol content (20.00–25.00%).
Citronellal, citronellol and geraniol compounds tend to be semipolar compounds. In separation, the interaction of the three compounds with the GC column (nonpolar) is only affected by the boiling point of the compound. The three main compounds have similar polarity and interact weakly with the GC column so that the three compounds have relatively short retention times. Citronellal has a lower boiling point than citronellol and geraniol. The -OH group (alcohol) in citronellol and geraniol can form strong hydrogen bonds so that the boiling point becomes higher. Citronellal is a type of monoterpenoid aldehyde (-CHO). Therefore, citronellal has a faster retention time than citronellol and geraniol.
Physically, each fraction has a different color; FA is pale yellow, and FB 4 is yellow-brown. The increasing citronellal content was achieved in FA, as it is about 89.37% compared to citronella oil which is 19.01%. Likewise, FB contains more citronellol and geraniol—in the range of 27.36–31.71%—compared to citronella oil (18–20%). According to previous work [
24], isolation of citronella oil by vacuum fractionation distillation was able to increase the levels of citronellal up to 90%, citronellol up to 30% and geraniol up to 45%. The density of citronella oil and its fraction is shown in
Table 6 and the refractive index in
Table 7.
Referring to [
9], a good bio-additive must contain oxygenate (O atoms), which can increase the oxygen content in the fuel so that efficient combustion occurs in diesel engines. FA and FB have potential to be diesel fuel bio-additives. Both citronellol and geraniol have similar physicochemical properties, such as boiling points of 225 °C and 226 °C, respectively. It was not easy to separate them through fractional vacuum distillation (physics) which relies on differences in the boiling points of the compounds. In addition, citronellol is a functional group isomer of geraniol.
3.2. Utilization of Citronella Oil Fractions as Bio-Additives
The use of citronella oil fractions as bio-additives was tested based on previous research, which mentioned that the optimum percentage of at 0.1–1.0% can save fuel up to 50% [
15,
25]. There were 36 formulas used, with three different types of diesel fuel, two types of bio-additives, and four variations of concentration of bio-additives.
The physical characteristics of blending were determined to see the changes in physical properties after the addition of bio-additives. Density characteristics were carried out using a pycnometer and kinematic viscosity was carried out using an Oswald viscometer according to SNI 8220:2017. Viscosity indicates the ability of a fluid to flow through an area per unit of time. This is important related to the mechanism of fuel atomization shortly after leaving the nozzle into the combustion chamber [
15].
The Effect of adding citronella oil and the fractions to density and viscosity of fuel is represented by the data in
Table 8 and
Table 9. Based on the Indonesian standard (SNI 8220:2017) regarding the specifications for type 48 diesel fuel, the density of at least 815 kg/m
3 is a maximum of 860 kg/m
3 and the viscosity is a minimum of 2.0 m
2/s, a maximum of 4.5 m
2/s. This shows that after the addition of bio-additives, there is no significant change in the physical properties of diesel fuel. The maintaining viscosity values in the addition of the bio-additives are related to the similar response of the volume restricting the movement of the lon- chain hydrocarbon molecules. The viscosity of DI fuel is also related to temperature and pressure dependency, which are important for fuel performance [
20].
3.3. Characterization of Citronella Oil and the Fractions
The Effect of bio-additive on fuel consumption is the most crucial parameter in this research. Fuel consumption occurs in the combustion process due to air compression in the engine combustion chamber. The amount of fuel consumed is measured in weight units per unit of time. Mathematically, the fuel consumption is shown in the following equation (Equation (1)):
where
fc is fuel consumption expressed in kg/h,
b is the volume of fuel consumption expressed in milliliters (mL),
t is the time used, which is expressed in seconds (s), and
f is the density of fuel expressed in kg/m
3.
The efficiency of diesel fuel consumption is carried out volumetrically, namely by calculating the fuel economy consumption when the engine produces power within a certain time frame. The purpose of determining consumption efficiency is to determine the optimum formula to improve fuel quality. The diesel engine used is a one-cylinder compression ignition (CI) type with a maximum speed of 2500 rpm for 7 min. Measurements were made by comparing the consumption volume of blending with the control. The calculation of fuel consumption results shows a decrease in fuel consumption when bio-additives are added. The decrease in fuel consumption is due to the compounds in bio-additives acting as oxygen providers, also called oxygenate compounds. The more oxygen content is contained in fuel; the easier and more completely the combustion will occur [
26,
27].
The results of experiments are presented in
Figure 3. It is seen that at all varied concentrations, citronella oil and the fractions significantly affect the efficiencies towards tested fuels; dexlite, pertamina-dex, and biosolar. In more detail, the increasing efficiency of biosolar is most intensive compared to pertamina-dex and dexlite. In addition, the effect of concentration is not linear with the efficiency for all tested fuels, particularly for biosolar; the trends are increasing efficiency at increasing concentration. A specific pattern was expressed by the use of FA and FB in the diesel fuel which showed the optimum concentration of 0.2%. Still, the efficiency decreased at the increasing concentration, representing the possible chemical or oxidative effect of the bio-additives.
Generally, by comparing FA, FB and citronella oil, it was found that FA had the most influence. This phenomenon is closely related to the greater abundance of oxygen in the fraction. This is also associated with more oxygen in biosolar, which presented better efficiency. The alcohol (-OH) and aldehyde (-CHO) groups (containing oxygen) in blending will react with CO gas and charcoal (C) to form CO
2, which causes fewer CO and green emissions [
28]. In the combustion chamber, there are three main reactions: initiation, propagation, and termination. The availability of oxygen is important to produce an efficient, constant chemical reaction in the combustion chamber.
When burning in a diesel engine, two possible combustion reactions will occur, a complete combustion reaction (Equation (2)) or an incomplete combustion (Equation (3)). Complete combustion occurs when there is sufficient oxygen in the engine combustion chamber. Incomplete combustion occurs when there are insufficient oxygen molecules to burn one complex hydrocarbon molecule in diesel fuels completely. In this study, diesel fuel, pertamina-dex, dexlite, and biosolar were mixed with bio-additives from fractions FA and FB of citronella oil. The diesel fuels-bio-additive mixture is expected to reduce emissions from CO gas and charcoal (soot). This is because the FA and FB fractions contain citronellal and citronellol-geraniol, which have oxygenated aldehyde (-CHO) and alcohol (-OH) functional groups to contribute to the availability of oxygen in the combustion chamber when the diesel engine is running. According to previous research, it was found that a mixture of citronella-diesel oil was able to reduce CO gas emissions by 23–30%, NOx 31–36%, SOx 12–22%, and particulates 30–33% compared to that without the addition of bio-additives [
11,
12].
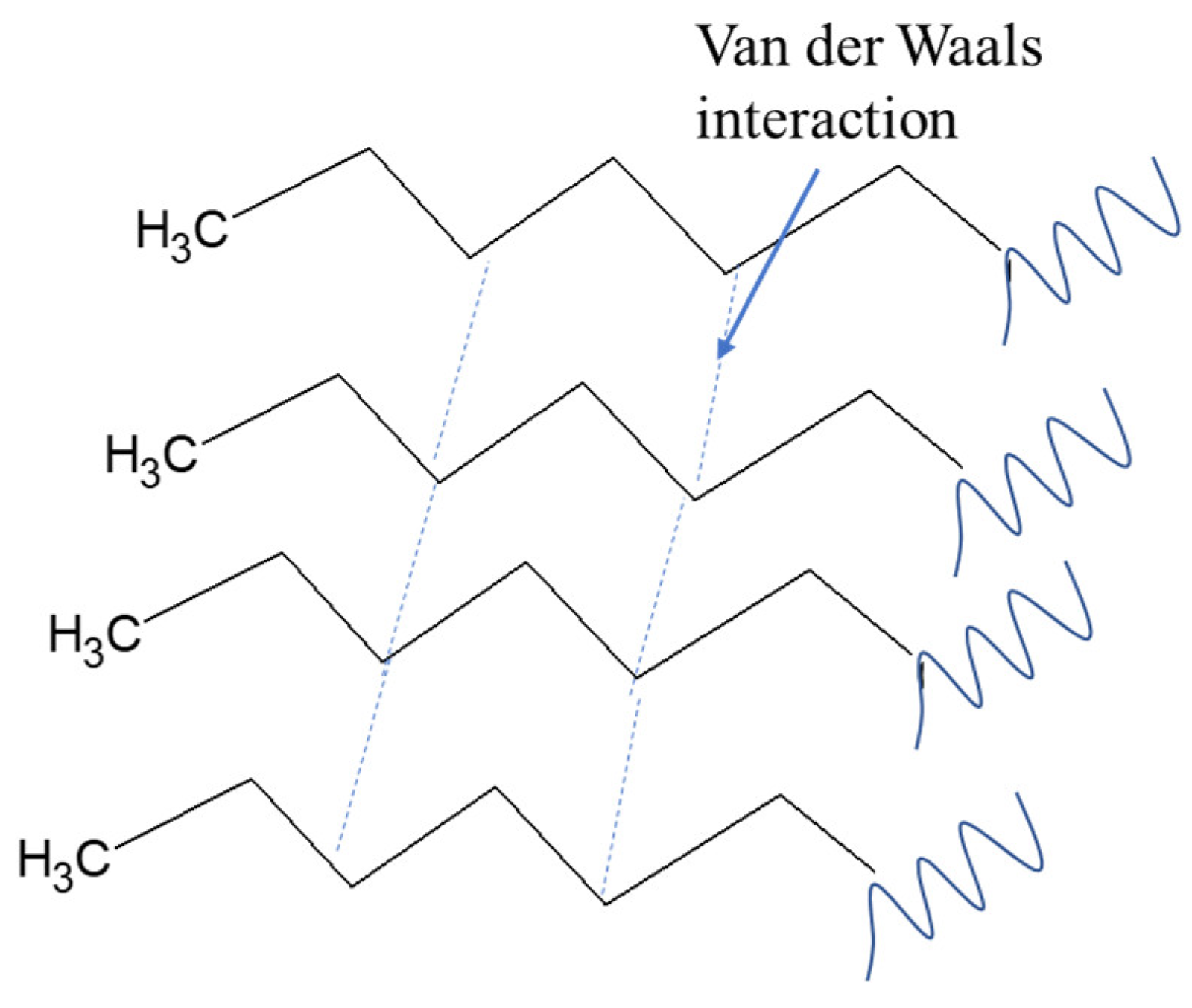
The bulk structure of the compounds contained in essential oils can reduce the strength of the van der Waals bonds of the compounds that make up the fuel. The bond between the fuel molecules is a fragile dispersion force [
9,
14]. Meanwhile, citronellal, citronellol, and geraniol compounds have dipole–dipole interactions between their molecules. Dipole–dipole interactions, which are stronger than dispersion forces, can facilitate the breaking of intermolecular bonds. Therefore, this causes the fuel molecules to break up easily to achieve more efficient combustion process. The interactions of compounds in diesel fuel after adding bio-additives are shown in
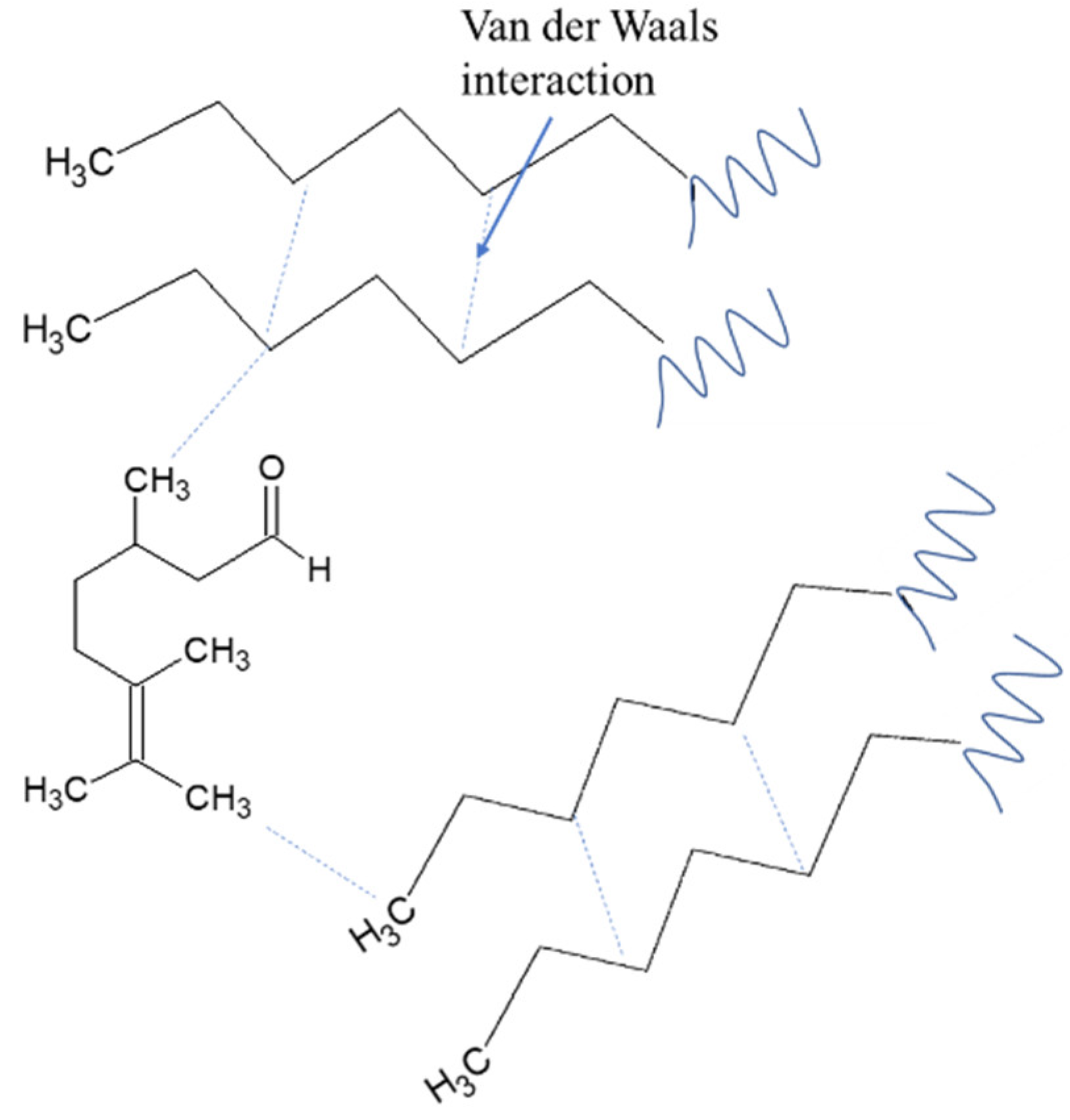
Figure 4,
Figure 5 and
Figure 6. The interactions that occur are induced dipole forces, namely interactions between compounds with permanent dipole moments that influence non-polar compounds. The presence of oxygen and the volatility of the citronellal, citronellol and geraniol supplied easier oxidation for faster compression in DI fuel. Oxidation reactivity of DI fuel is supplied by the combination of chemical interactions including the van der Waals and hydrogen bonding in the fuel mixture with additive. Similar results were reported by the influence of additive and oxygen-rich fuel [
29,
30].
Based on the volume of fuel consumption obtained, the fuel consumption efficiency can be calculated. Consumption efficiency shows an increase in fuel quality after adding bio-additives. Based on the fuel consumption test, FA with citronellal as the main component has a higher consumption efficiency compared to FB (citronellol and geraniol as the main components) and citronella oil. The lessened flash point is probably the main reason for this as citronellal, citronellol, and geraniol have flash points of 86 °C, 99 °C, and 108 °C, respectively. The lessened flash point represents the ease of being burnt and oxidized.
Another factor is the viscosity, which affects liquid fuel properties. Viscosity is related to the flow rate and characteristics of the spray or mist of fuel into the combustion chamber [
20]. The higher the viscosity, the more difficult the fuel is to move and spray or atomize into the combustion chamber, so the combustion process is not optimal. Low viscosity helps in atomization, evaporation, and diffusion, and increases the interaction of fuel with air. Based on the viscosity test of fuel that has been mixed with bio-additives, the fuel that has been mixed with the citronellal fraction (FA) gives the lowest viscosity value compared to the citronellol-geraniol (FB) fraction and citronella oil.
Generally speaking, the results from this work show the potential of the use of essential oil as a sustainable bio-additive in minimizing energy consumption, especially DI fuel. More exploration for other fuel as well as techno-economic studies are required.